Abstract
The β-thalassemias are a group of hereditary hematological diseases caused by over 300 mutations of the adult β-globin gene. Together with sickle cell anemia, thalassemia syndromes are among the most impactful diseases in developing countries, in which the lack of genetic counseling and prenatal diagnosis have contributed to the maintenance of a very high frequency of these genetic diseases in the population. Gene therapy for β-thalassemia has recently seen steadily accelerating progress and has reached a crossroads in its development. Presently, data from past and ongoing clinical trials guide the design of further clinical and preclinical studies based on gene augmentation, while fundamental insights into globin switching and new technology developments have inspired the investigation of novel gene-therapy approaches. Moreover, human erythropoietic stem cells from β-thalassemia patients have been the cellular targets of choice to date whereas future gene-therapy studies might increasingly draw on induced pluripotent stem cells. Herein, we summarize the most significant developments in β-thalassemia gene therapy over the last decade, with a strong emphasis on the most recent findings, for β-thalassemia model systems; for β-, γ-, and anti-sickling β-globin gene addition and combinatorial approaches including the latest results of clinical trials; and for novel approaches, such as transgene-mediated activation of γ-globin and genome editing using designer nucleases.
Introduction
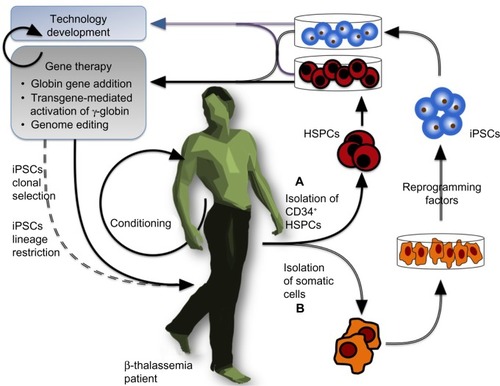
The β-thalassemias are a group of hereditary hematological diseases caused by over 300 mutations of the adult β-globin gene,Citation1 with excellent reviews providing background information outlining genetics,Citation2–Citation4 pathophysiology,Citation5,Citation6 and therapeuticsCitation7 of β-thalassemia that is beyond the scope of this review. In brief, β-thalassemia is brought about by mutations reducing or abrogating β-globin expression, which thus lead to reduced adult hemoglobin ([HbA] an α2β2 heterotetramer) and excess α-globin content in erythroid cells, in turn resulting in ineffective erythropoiesis and apoptosis in the erythroid lineage.Citation3,Citation8,Citation9 Most β-thalassemia patients therefore require lifelong clinical management by blood transfusion and chelation therapy,Citation10–Citation12 with a few having the option of curative but potentially hazardous allogeneic transplantation of hematopoietic stem and progenitor cells (HSPCs) instead.Citation13,Citation14 This indicates the need for alternative therapies, and the observation that high levels of the fetal β-globin-like γ-globin chain result in an ameliorated β-thalassemia phenotypeCitation15 has prompted the search for γ-globin-inducing chemical agents.Citation16–Citation21 Patient response to known γ-globin inducers, however, is varied,Citation22 and the search continues for reagents with higher efficiency, consistency, and tolerability in chronic application,Citation23 if not to cure the disease, then to reduce transfusion requirements and the significant cost of disease management. Of note, hemoglobinopathies, such as the thalassemia syndromes and sickle cell anemia (SCA; caused by the toxic β-globinE6V mutation), most severely affect low-income countries, where the lack of prevention programs and an underlying high carrier rate bring about high disease frequencies,Citation24 although global migration has now turned hemoglobinopathies into a concern for many nonendemic countries as well.Citation25 Globally, β-thalassemia mutations introducing gene deletions, aberrant splicing, or premature stop codons have the greatest impact in terms of global disease burden and clinical severity.Citation26,Citation27 Recent progress in the research of disease modifiers,Citation28 chemical modulation of gene expression,Citation22,Citation29 and tools and approaches for DNA-based therapiesCitation30,Citation31 have opened new avenues toward novel and more personalized strategies to manage or cure β-thalassemia, as we have reviewed recently.Citation23,Citation32 Particularly with regard to curative approaches by gene therapy, the field has come to a crossroads, with the initiation of clinical trials, the possible plateauing off of incremental improvements to gene augmentation therapy, and the increasing preclinical application of novel genome-editing tools. The objective of the present manuscript is to review the most relevant findings published in the period 2005–2014 concerning the preclinical and clinical application of gene therapy for β-thalassemia. To this end, we will describe the pertinent model systems, β-like-globin gene-addition strategies, gene addition in combination with chemical inducers of γ-globin, transgene-mediated activation of endogenous γ-globin, and the emerging use of designer nucleases for β-thalassemia gene therapy. The general flow of gene-therapy-based approaches for β-thalassemia is outlined in .
Figure 1 General view of a gene-therapy approach for β-thalassemia.
Experimental model systems
Several experimental systems have been developed to establish the suitability of and provide proof of principle for gene- therapy approaches to β-thalassemia. Erythroid cell lines, such as human and murine erythroleukemia cells, allow cost-effective high-throughput assessments in the erythroid lineage,Citation33–Citation36 and cancer-prone mouse models have been instrumental in gauging the genotoxicity of integration and genome-modification events for vector classes applied to β-thalassemia.Citation37–Citation39 The most informative functional studies of candidate therapies toward their clinical application, however, instead rely on thalassemic human stem cells for in vitro assessments of authentic human responses and on thalassemic murine models for long-term systemic assessments in vivo.
In vitro experimental systems: erythroid precursor cells from β-thalassemia patients
HSPCs are the substrate for clinical gene-therapy application, so that in vitro assessment of HSPC-derived erythroid precursor cells (ErPCs) is highly informative for toxicity and efficacy of any therapeutic intervention (). ErPCs from peripheral blood are widely used, while access to bone marrow and mobilized blood,Citation40,Citation41 which, incidentally, contain the cells preferentially used in clinical applications,Citation42,Citation43 is more restricted. Using peripheral-blood-derived ErPCs, it is possible to obtain large cultures of relatively pure and synchronized erythroid cell populations in which compounds can be added at specific stages of maturation. In the procedure developed by Fibach et al,Citation44,Citation45 the culture is divided into two phases: first, an erythropoietin (EPO)-independent proliferation phase, in which peripheral blood cells are first cultured in the presence of a combination of growth factors, but in the absence of EPO; and, second, a differentiation phase, when the culture, supplemented with EPO, generates orthochromatic normoblasts and enucleated erythrocytes, with cells decreasing in size and accumulating hemoglobin (Hb) and large cellular clusters assuming a reddish color and giving brown-red pellets upon centrifugation.Citation45,Citation46 This system recapitulates many aspects of in vivo erythropoiesis, including globin RNA metabolism, cell cycle kinetics, expression of cell surface antigens, iron and ferritin metabolism, and recruitment of transcription factors,Citation45 and allows analysis of Hb content by a variety of techniques, such as alkaline denaturation, benzidine staining, capillary electrophoresis, cation-exchange high-performance liquid chromatography for hemoglobins, and reversed-phase high-performance liquid chromatography for globin chains.Citation45,Citation46
In vitro experimental systems: human embryonic stem cells and induced pluripotent stem cells
Human embryonic stem cells (hESCs) have been used extensively to study the early phases of hematopoietic and erythroid development.Citation47 In this approach, after 5 to 7 days of in vitro cell culture, a blastocyst is generated, showing a clearly visible and easily accessible inner cell mass, from which pluripotent stem cells can be isolated, giving rise to in vitro hESC lines. From these cell lines, embryoid bodies can be developed and used for further tissue-specific differentiation. hESCs themselves have only a minor role in the preclinical study of therapies for hemoglobinopathies,Citation48,Citation49 and their clinical application would suffer due to the ethical repugnance of their origin and from the same incompatibilities seen for allogeneic HSPC transplantations. However, the underlying hESC methodology is being reemployed in the culture of induced pluripotent stem cells (iPSCs),Citation50–Citation53 which closely mimic hESCs and represent a potential cornucopia for cell-based therapies in general. The creation of iPSCs from somatic cells with the use of reprogramming factors (originally Oct3/4, Sox2, c-Myc, and Klf4Citation50) represented a paradigm shift in our understanding of developmental biology and in the conception of novel therapeutic approaches, not least because their use avoids the ethical concerns associated with hESCs and creates a patient-specific, histocompatible substrate for cell therapy. Human iPSCs retain embryonic and fetal characteristics of gene expression even upon erythroid differentiation in vitro, so that the hope arose that patient-derived iPSCs for β-thalassemia or SCA might be therapeutic in their own right via the maintenance of high levels of γ-globin expression ().Citation54,Citation55 However, according to recent in vivo findings after transplantation into immunodeficient mice, in which a gradual switch to the adult β-globin gene was observed,Citation56,Citation57 this hope appears to be unfounded. Notwithstanding this apparent setback, iPSCs are a promising substrate for gene therapy, as they can be amplified in vitro indefinitely (where they are, alas, still subject to the same mutation rates and potentially undesirable changes as any other cell type) and thus allow the clonal selection of rare events of therapeutic interest. Since its inception, iPSC technology has been used extensively in innovative studies on β-thalassemia and other hemoglobinopathies, as will be detailed for specific gene-addition and genome-editing approaches.
In vivo experimental systems: mouse models
Thalassemic mouse models provide the most economical option for gauging the putative and systemic effects of gene-therapy approaches in thalassemic patients. Of note, the regulation of β-like globin chains in humans comprises a switch in utero from the embryonic (ε) to the fetal (γ) chain, followed by an HbA switch perinatally up to 6 months after birth,Citation58,Citation59 which also allows the birth and early postnatal development of homozygous β0 patients without disease management. In contrast, the murine β-globin locus encodes four functional β-like globin genes: βh1 and εy (transcribed only during the embryonic phase of development up to E14–E15 of a total gestation period of approximately 21 days), and the b1 (βmajor) and b2 (βminor) genes, which are transcriptionally activated in utero around 11 days after conception.Citation59 Accordingly, mice homozygous for (β0) mutations that prevent expression of the adult β-globin genes die perinatally, owing to a complete lack of expression of any Hb.Citation59 The most widely used, non-humanized adult murine models of β-thalassemia therefore need to retain some β-globin expression and thus show features similar to those observed for β-thalassemia intermedia patients, who carry moderate to mild (β+) mutations,Citation60 although a β0 surgical model of murine β-thalassemia major has also been developed.Citation60,Citation61
In order to test the activity of novel mutation-specific approaches in vivo, humanized mouse models needed to be developed,Citation58 with those combining absence of murine β-like globin genes with the presence of a human β-globin gene cluster and mutated β-globin gene being of the greatest utility. For instance, Vadolas et alCitation62 reported generation of a humanized mouse model carrying the common β+ IVSI-110 splicing mutation on a bacterial artificial chromosome carrying the human β-globin locus. Comparison of heterozygous β-globin knockout mice carrying either the IVSI-110 or the normal human β-globin locus showed a 90% decrease in human β-globin chain synthesis in the IVSI-110 mouse model. The model, moreover, accurately recapitulates the splicing defect found in β-thalassemia patients and is thus a suitable platform on which to test approaches for the restoration of normal splicing. Similarly, a humanized mouse model carrying the common G26A (HbE) mutation, frequently co-inherited with β-thalassemia in Southeast Asia, has been developed, which allows in vivo analysis in mouse of therapies for HbE/β-thalassemia.Citation63 Mouse models (whether of a wild-type or thalassemic background) carrying all or parts of the human β-globin locus have also proven an essential resource for the analysis of globin switching and therapeutic approaches for β-thalassemia.Citation64–Citation66 Finally, a keen interest in the study of developmental gene regulation, γ-globin induction, and therapies for β-thalassemia major has prompted the development of further humanized transgenic mice as models for β-thalassemia major.Citation67 These mice carry a mutated human β-globin gene and are born viable due to the prolonged expression of human fetal hemoglobin (HbF), but require chronic transfusion for survival and are not yet widely available in the community.Citation67–Citation69
Globin gene addition
Over the last 2 decades, major efforts have been made to achieve therapeutic levels of exogenous β-like globin chains in β-thalassemia and SCA. These finally came to fruition when a switch from γ-retroviral vectors to lentiviral vectors allowed the efficient transduction of nondividing cells with a sufficiently large expression cassette,Citation70 encouraging numerous research groups to work toward vectors expressing β-globin, anti-sickling variants of β-globin and γ-globin.
Lentiviral expression of exogenous β-globin
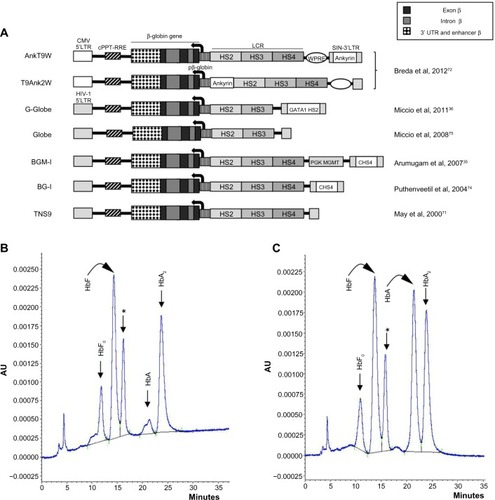
The efforts of the groups working in this field have been dedicated to achieving highly efficient and stable transduction of HSPCs, to optimizing transgene expression (erythroid- and stage-specific, elevated, position-independent, and sustained over time), and to correcting the β-thalassemia phenotype in preclinical models with minimal genotoxicity.Citation35,Citation36,Citation40,Citation42,Citation71–Citation75 While the field has reached a high level of optimization, incremental improvements to procedures and vectors continue to be made. These include the use of rapamycin to enhance LV transductionCitation76 and the recent inclusion of chromatin opening elementsCitation77–Citation79 or an ankyrin insulatorCitation72 for improved vector-derived expression, with an ongoing search for and evaluation of alternative insulatorsCitation80 to prevent transgene silencing and minimize host gene perturbation while avoiding the reduction of vector titer during production that is associated with the most widely used chicken HS4 insulator.Citation81 It has also been demonstrated that, in order to avoid insertional mutagenesis, it is possible to select suitable clones with insertions in inert (“safe harbor”) genome sites, in combination with iPSC technology.Citation82 Several recent reviews on gene therapy of thalassemia and related hemoglobinopathies point out the state of the art with respect to the structure of β-globin-carrying lentiviral vectors,Citation70,Citation83,Citation84 and depicts a number of therapeutic lentiviral vectors that produce high levels of β-globin in human or murine β-thalassemic erythroid cells.Citation35,Citation36,Citation40,Citation42,Citation71–Citation75 illustrates how one such vector, T9W, generates high-level HbA production in ex vivo culture of HSPC-derived cells isolated from a β0-thalassemia patient upon differentiation.Citation46
Figure 2 Lentiviral vectors expressing exogenous β-globin.
Abbreviations: Ankyrin, ankyrin insulator; AU, absorbance units; cHS4, chicken β-globin hypersensitive site 4 insulator; CMV, Cytomegalovirus; GATA1 HS2, globin transcription factor 1 hypersensitive site 2 enhancer; HIV-1, human immunodeficiency virus; HS, hypersensitive site; LCR, locus control region; LTR, long terminal repeat; pβ-globin, β-globin promoter; PGK MGMT, murine phosphoglycerate kinase-1 promoter and OCitation6-methylguanine DNA methyltransferase gene; SIN, self-inactivating; UTR, untranslated region; WPRE, woodchuck posttranscriptional regulatory element; cPPT, central polypurine track; RRE, Rev response element.
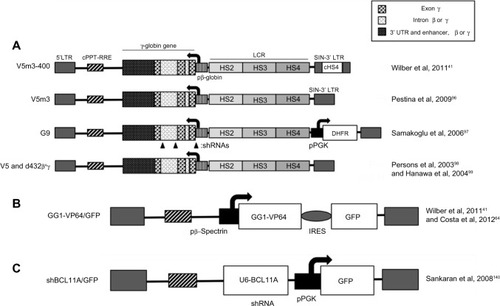
Lentiviral expression of anti-sickling β-globin and exogenous γ-globin
With a view to applying the same vector for β-thalassemia and SCA, β-globin vectors have also been modified to approach or even exceed the anti-sickling activity shown by γ- and δ-globin. Of note are the HPV569 and BB305 LentiGlobin® vectors,Citation85,Citation86 which feature in βE/β0 clinical trialsCitation86–Citation89 (see Clinical trials below) and carry β-globinT87Q, and which are expected to provide some anti-sickling activityCitation90 and thus be suitable for SCA therapy. Particularly important in this context, however, are the anti-sickling β(AS3) β-globin designed by Townes et alCitation91,Citation92 and pertaining lentiviral vectors.Citation93 The combination of three amino acid changes (creating the artificial β-globinG16D, E22A, T87Q variant) confers anti-sickling activity exceeding that of γ-globin to β(AS3) and therefore renders the mutant transgene particularly suitable for the therapy of SCA and β-thalassemia/β-globinE6V compound heterozygotes. Independently, and primarily with clinical application for SCA in mind, numerous groups have also developed retroviral vectors encoding γ-globin instead of β-globin ().Citation41,Citation94–Citation101 Of note, Wilber et alCitation41 used lentiviral vectors encoding the human γ-globin gene with or without an insulator, which were tested on erythroid progeny of normal CD34+ cells and resulted in high levels of HbF production, suggesting that lentiviral-mediated treatments have the potential to provide therapeutic HbF levels to patients. These findings are corroborated by several independent research groups that work on γ-globin-based lentiviral (and γ-retroviral) therapy of SCA and β-thalassemia.Citation41,Citation94–Citation101 shows the structure of some of the corresponding vectors.Citation41,Citation96–Citation99 All vectors intended for gene augmentation described here, be it for the expression of β-globin, anti-sickling β-globin, or γ-globin, have overlapping fields of application. Further preclinical and clinical studies will show which vector may be most suitable for specific disease conditions, with the vector itself as a key factor, but with all components of the treatment protocol, including conditioning, HSPC source and isolation, transduction protocol, and general culture conditions, playing a critical role in the outcome and in the comparison of vector performance.
Figure 3 Lentiviral vectors expressing exogenous γ-globin or inducing endogenous γ-globin.
Abbreviations: cHS4, chicken β-globin hypersensitive site 4 insulator; HS, hypersensitive site; LCR, locus control region; LTR, long terminal repeat; pβ-globin, β-globin promoter; shRNA, short hairpin RNA; SIN, self-inactivating; cPPT-RRE, viral sequences harboring the central polypurine tract and the rev response element; pPGK, PGK promoter; DHFR, the DHFR gene, providing partial resistance to myelosuppression and thus potentially in vivo selection for transduced cells; U6-BCL11A, an shRNA expressed from the U6 promoter and targeting BCL11A mRNA; IRES, internal ribosome entry site; GFP, green fluorescent protein.
Combination therapy of gene addition with HbF inducers
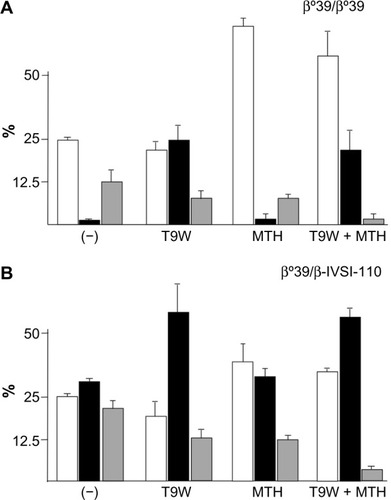
Induction of endogenous HbF is one of the most widely applied therapeutic strategies for β-thalassemia and SCA, as indicated by several recent studies and reviews.Citation102–Citation110 Lending additional significance to preclinical studies, it has been shown that the level of γ-globin mRNA and in vitro induction of HbF in primary ErPCs isolated from β-thalassemia patients is predictive of the hydroxyurea response in vivo.Citation111,Citation112 While most of the recent studies in the field still focus on low-molecular-weight HbF inducers,Citation102–Citation110,Citation113 the innovative strategy of combining them with vector-derived β-globin has lately been investigated and reviewed.Citation30,Citation114 The combined treatment induces an increase of both HbA (by gene addition) and HbF (by chemical HbF induction) with important therapeutic implications, given that β-like globin transfer in some β-thalassemia major ErPCs has been unable to reach physiological levels of Hb in vitro and might thus only lead to partial phenotypic correction in vivo as well. Since increased production of HbF in β-thalassemia is undoubtedly beneficial, the one-off application of gene therapy combined with chronic application of HbF inducers appears to be a pertinent strategy to achieve clinical benefits not achievable with either strategy alone. Representative results for this approach are depicted in on ErPCs from a β0-thalassemia patient carrying the codon-39 stop codon mutation (β039) () and a β039/β-IVSI-110-thalassemia patient (). The results demonstrate that this combination strategy achieves high levels of functional Hb in β-thalassemic cells and a concomitant sharp decrease of excess α-globin, with significant scope for further improvements for what is as yet a nascent field of research.
Figure 4 Combination therapy using lentiviral-vector-derived β-globin expression and chemical γ-globin induction.
Clinical trials
To date, there are a total of seven patients who have been treated successfully or for whom longer follow-up is pending in three clinical trials for β-thalassemia, all of which have used β-globin-expressing lentiviral vectors. The first successful gene therapy trial for β-thalassemiaCitation85 was reported in the manuscript published by Cavazzana-Calvo et al in 2010Citation87 and commented on by Kaiser.Citation115 The pertaining β-globinT87Q vector (LentiGlobin® HPV569) holds a tandem copy of the 250-bp cHS4 insulator in its 3′ long terminal repeat (LTR) as a safety feature and bears a T87Q amino acid, which, besides its conferring anti-sickling activity,Citation90 makes it distinguishable from transfusion-derived β-globin and thus allows the quantification of vector-derived β-globin during follow-up. Three patients with severe βE/β0-thalassemia have been treated to date. In the first patient, engraftment of treated bone marrow failed after full myeloablation, requiring reinfusion of backup bone marrow. For the second patient, however, transfusion independence was achieved at 12 months after treatment and continues to date. At 36-month follow-up, of 24 detectable clones in peripheral blood, one clone with cross-lineage dominance held a proviral integration in the high mobility group AT-hook 2 (HMGA2) gene, whose expression is associated with tumor metastasis and proliferation,Citation116,Citation117 in a position that removed posttranscriptional control elements and thus increased HMGA2 mRNA stability. This clone, moreover, showed a recombination event that had removed one of the cHS4 copies and possibly exacerbated transcriptional enhancement of HMGA2 from the proviral β-globin locus control region (LCR), with transcriptional and posttranscriptional effects combined resulting in 10,000-fold HMGA2 expression. Clonal dominance of this clone (peaking at 22% of nucleated cells after 48 months) dropped to 6.8% 7 years after treatment. Notably, at 36 months, only one-third of the total Hb was vector derived, with endogenous HbE and unexpectedly high HbF constituting the other two-thirds, so that the patient might have failed to become transfusion independent in the absence of endogenous HbE and elevated γ-globin expression and if mild conditioning instead of full myeloablation had been applied. Finally, engraftment with HPV569-treated cells of the third adult patient for this trial was also successful. However, the patient remains transfusion dependent, with a low vector copy number (VCN) in the originally engrafted cell material (VCN 0.3) and a low VCN in nucleated cells (VCN in neutrophils 0.016), and with vector-derived Hb accounting for only approximately 5% of total Hb more than 2 years after engraftment.Citation88 Engraftment failure for the first patient, a low VCN for the third patient, and oligoclonal reconstitution, vector recombination, and low vector-derived gene expression for the second patient provide important pointers for necessary improvements in future trials and vectors and, moreover, call for ex vivo preclinical assessment in cells from prospective trial participants, as we argue elsewhere.Citation72
A second clinical study (HGB-205) and follow-up to the trial described above has been initiated by bluebird bio Inc. in France and utilizes the third-generation lentiviral LentiGlobin® BB305 vector.Citation86 Compared to HPV569, BB305 holds a cytomegalovirus (CMV) promoter instead of the U3 promoter/enhancer in its 5′ LTR and no longer bears cHS4 insulator elements in its 3′ LTR. Preliminary results obtained for two βE/β0-thalassemia patients, who had both been transfusion-dependent for most of their lives, were encouraging, with a VCN of 1.5 and 2.1, respectively, in the engrafted material and with a reported transfusion independence at 3.5 and 6.5 months, respectively, after treatment.Citation88 This success has most recently also prompted the application of BB305 for gene therapy of SCA.Citation89
Finally, an independent trial for globin gene transfer in adult patients with β-thalassemia major has been initiated (NCT01639690) by the Sadelain group and associatesCitation118,Citation119 to study safety and efficacy, representing the first US trial for β-thalassemia. The β-globin vector used for the trial, TNS9.3.55, holds the cHS4 insulator and minor unpublished modifications compared to TNS9.Citation60,Citation71 A preclinical study testing TNS9.3.55 in patient HSPCs in vitro, by BFU-E assays, and, in vivo, using NOD-scid IL2rγnull mice, indicated high vector-derived expression (73% to 100% of normal hemizygous levels) and long-term repopulation potential (69% retention after 7 months) for vector-positive cells.Citation42 In the ongoing clinical trial, five patients have been enrolled and three treated to date, using G-CSF-mobilized CD34+ cells and mild conditioning (8 mg/kg busulfan). Possibly owing to the latter, which reduces the risk for patients but also the level of donor chimerism and thus the overall efficiency of the approach, transfusion independence had not been reached 12 months after treatment in the first three patients, albeit with an ongoing rise of the average VCN in peripheral blood mononuclear cells (from, initially, 1% to 7%–9%) and without the emergence of clonal dominance. As of this writing, treatment of additional patients has been postponed until fuller evaluation of the first three patients can indicate whether dose escalation of the conditioning treatment might be required.
Transgene-mediated activation of endogenous γ-globin genes
Inspired by chemical induction of HbF as a therapeutic approach,Citation102–Citation109 and enabled by the burgeoning fields of engineered transcription factors and RNA interference,Citation23,Citation32 a relatively novel approach to the therapy of β-thalassemia is the transgenic activation of γ-globin, either by the over-expression of γ-globin-activating transcription factors or by the stable knockdown of γ-globin repressors.
Overexpression of γ-globin-activating transcription factors
The β-type globin genes are activated through dynamic interactions with a distal upstream enhancer, the LCR. The LCR physically contacts the developmental stage-appropriate globin gene via chromatin looping, a process partially dependent on the protein Ldb1. Deng et al showed that tethering Ldb1 to the murine β-globin promoter with a custom-designed zinc finger protein (ZF-Ldb1) can induce loop formation and β-globin transcription in an erythroid cell line.Citation120 Further work using a similar approach showed that forced chromatin looping can be exploited to potently reactivate fetal globin gene expression in adult human erythroid cells.Citation121 For this work, a fusion protein that brings together a zinc finger protein, which recognizes a specific sequence at the γ-globin promoter, and Ldb1 was created.Citation120,Citation122 Insertion of a lentiviral vector carrying this fusion protein into adult primary human erythroid cells strongly activated the γ-globin gene, whose transcription accounted for nearly 90% of total β-like globins and led to concomitant reduction of β-globin. This approach would therefore be particularly suitable for the therapy of SCA, by increasing anti-sickling γ-globin, while at the same time reducing βS expression (see alsoCitation97).
Alternatively, engineered zinc-finger-based transcription factors can be used to reactivate developmentally silenced γ-globin genes in adult cells. shows the structure of a lentiviral vector expressing the artificial zinc finger protein GG1-VP64, which was designed to interact with the -117 region of the Aγ-globin gene proximal promoter and led to a significant increase in γ-globin gene expression in K562 cells.Citation123 Moreover, Wilber et alCitation124 and Costa et alCitation64 reported increased γ-globin gene expression following transfection with GG1-VP64 constructs, with significantly increased HbF levels in CD34+ erythroid progenitor cells from normal human donors and β-thalassemia patients. These results provide new insights into the mechanism of γ-globin silencing and may translate into mechanism-based, improved therapies for β-thalassemia and related SCA.
Transgene-mediated silencing of β-thalassemia modifiers
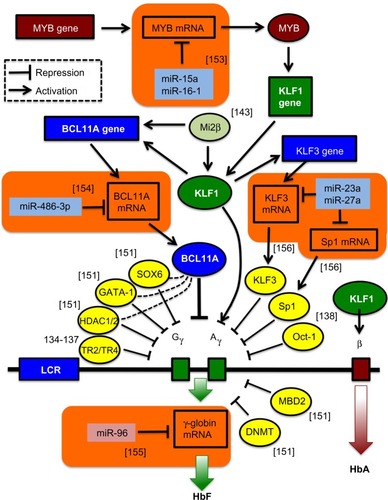
With the advent of the concept of RNA interference, efforts began to utilize short interfering RNAs (siRNAs) and short hairpin RNAs (shRNAs) in the therapy of β-thalassemia. Early efforts, mindful of the amelioration of β-thalassemia pathology by a reduction in α-globin excess, knocked down α-globin mRNA to achieve a moderate but significant reduction in disease parameters,Citation34,Citation125–Citation127 an approach superior to the alternative strategy of sequestering excess α-globin protein by overexpression of its private chaperone, AHSP.Citation128 Recently, regulation by RNA interference has also become an option for the activation of endogenous γ-globin expression. This was made possible through a growing understanding of the regulation of globin switching, also by regulatory microRNAs (miRNAs), and of transcriptional repressors of γ-globin as therapeutic targets (see ).Citation129–Citation144 Among the candidate target genes for knockdown is the zinc finger transcription factor Krüppel-like factor 1 (KLF1, also known as the erythroid Krüppel-like factor, EKLF), which acts as an erythroid-specific master switch of globin gene expressionCitation145 and whose autonomy in directing globin gene expression is underlined by the observation that the mere insertion of a KLF1 binding site into the human δ-globin promoter confers developmental inducibility and a reduction of the thalassemia phenotype in mice.Citation146 Besides KLF1, Oct-1,Citation138 MYB,Citation139 and BCL11ACitation129,Citation133,Citation140 have been identified as repressors of γ-globin gene transcription. For instance, the zinc finger transcription factor BCL11A has recently been shown to function as a repressor of HbF expression, with transgenic deactivation of BCL11A reactivating HbF and correcting a humanized sickle Hb mouse modelCitation147 and with BCL11A knockdown leading to significant HbF induction in human cells,Citation129,Citation133,Citation140,Citation144 similar to knockdown of its positive regulator KLF1.Citation132 Moreover, compound Klf1::Bcl11a mutant mice that carry the human β-globin locus showed further enhanced γ-globin expression compared to single-mutant animals,Citation148 indicating that a strategy targeting both genes together (without affecting non-erythroid functions of BCL11A) might have additional therapeutic benefits in β-thalassemia. In order to move transgene-mediated activation of γ-globin from concept to therapeutic application, shRNA expression from constitutive RNA polymerase III promoters, such as the commonly used U6 promoter (see ), needs to be avoided. To this end, Renella et al has surrounded a BCL11A-specific shRNA with the flanking sequences of a naturally occurring miRNA (miR223), allowing its (potentially regulated) expression from RNA polymerase II-driven promoters.Citation149 Using lentiviral vectors for spleen focus-forming virus (SFFV)-promoter-driven BCL11A shRNAmiR expression in murine erythroleukemia cells, approximately 50% of control embryonic εy levels were achieved compared to the equivalent positive U6 shRNA control,Citation150 so that controlled and stable shRNA-mediated HbF induction has achieved an efficiency of potential clinical relevance. , in addition to transcription factors negatively regulating the expression of the γ-globin genes,Citation151,Citation152 shows examples of miRNAs validated as regulators of γ-globin gene expression,Citation153–Citation156 either directlyCitation155 or through interactions with relevant target transcription-factor mRNA,Citation153,Citation154,Citation156 such as miR-15a and miR-16-1 (targeting MYB),Citation153 miR-23 and miR-27a (targeting KLF3 and Sp1, respectively),Citation156 and miRNA-486-3p (targeting BCL11A).Citation154 Lentiviral vectors carrying sequences of these miRNAs are expected to lead to inhibition of γ-globin gene transcription-factor repressors and induction of HbF.Citation41
Figure 5 Novel targets for γ-globin gene transcriptional activation.
Abbreviation: LCR, locus control region; HbF, fetal hemoglobin; HbA, adult hemoglobin.
Genome editing
In contrast to gene-augmentation approaches, the direct DNA-level repair of primary mutations would achieve physiological levels of gene expression for each corrected cell and, in the absence of off-target activity, would altogether avoid the risk of insertional mutagenesis inherent to integrating vectors. Genome-editing approaches, however, still suffer from low efficiencies in HSPCs, which, without selection (eg, of iPSC clones) or enrichment steps, mostly precludes their clinical application for gene therapy. However, Genovese et alCitation157 have recently achieved high-efficiency targeted DNA replacement in HSPC from controls and patients with X-linked severe combined immunodeficiency, reaching efficiencies of 3%–11% depending on the subpopulation, thus moving homology-directed gene repair of HSPCs into the realm of clinical application.
Repair of causative mutations
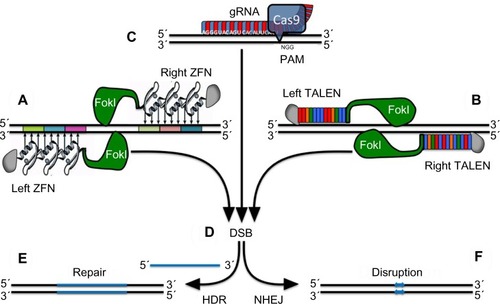
Endogenous genomic loci can be altered efficiently and specifically using engineered zinc finger nucleases (ZFN)Citation158–Citation163 and transcription activator-like effector nucleases (TALENs), as recently reported by Voit et al for the human globin locus.Citation164 Moreover, besides ZFN and TALEN, clustered regularly interspaced short palindromic repeats (CRISPR) linked to Cas9 nuclease are now also being investigated for their utility in modifying β-globin.Citation165,Citation166 ZFN (), ΤALEN (), and CRISPR () comprise a specifically engineered DNA binding domain fused to a nuclease.Citation31 Binding of a ZFN or TALEN pair at contiguous sequences flanking a target site leads to the dimerization of the FokI (a double-stranded DNA nickase) domain, resulting in a targeted DNA double-strand break, while CRISPR/Cas9 introduces double-strand breaks as a monomer.Citation31 To increase target-site specificity and thus reduce off-target activity, CRISPR linked to nickases and thus requiring dimerization for genome modification are also being investigated.Citation167 The resulting double-strand break () can be repaired by error-prone nonhomologous end joining or by high-fidelity homology-directed repair in the presence of a homologous DNA donor template (). In their study, Voit et al engineered a pair of highly active TALENs that induce modification of about 50% of human β-globin alleles near the site of the sickle mutation. These TALENs stimulate targeted integration of therapeutic, full-length β-globin complementary DNA to the endogenous β-globin locus in about 20% of K562 erythroleukemia cells.Citation164
Figure 6 Alternative strategies for targeted gene repair.
Abbreviations: DSB, double-strand break; gRNA, guide RNA; HDR, homology-directed repair; NHEJ, nonhomologous end joining; PAM, protospacer adjacent motif; TALEN, transcription activator-like effector nuclease; ZFN, zinc finger nuclease.
Using patient-specific iPSCs, Ma et alCitation168 have recently applied this technology to β-thalassemia, with Sun and ZhaoCitation169 likewise applying it to SCA patient-specific iPSCs, both groups following the idea that correction of disease-causing mutations offers an ideal therapeutic solution when iPSCs are available. In the β-thalassemia study, Ma et al described a robust process combining efficient generation of integration-free patient-specific β-thalassemia iPSCs and TALEN-based universal correction of HBB mutations in situ. Integration-free and gene-corrected iPSC lines from two patients carrying different types of homozygous mutations were generated. These iPSCs are pluripotent, have normal karyotype, and, more importantly, can be induced to differentiate into hematopoietic progenitor cells and then further to erythroblasts expressing normal β-globin. Interestingly, and of importance for any clinical application of genome-editing tools, the correction process did not generate TALEN-induced off-target mutations.Citation168
HbF activation by genome editing
In contrast to SCA, wherein a single mutation is present in all patients, β-thalassemia is caused by a large variety of mutations, each of which would have to be corrected by an individually validated designer nuclease. The alternative and universally applicable approach of using designer nucleases to induce HbF instead is therefore an attractive option. While results for this strategy as a gene-therapy approach have, to our knowledge, not yet been published in peer-reviewed journals, it is already being employed, as patent applications for corresponding ZFN and TALEN indicate.Citation170,Citation171 Intriguingly, and depending on the target (such as a γ-globin repressor or its binding site), this approach might use nonhomologous end joining to disrupt the target sequence in HSPCs and thus achieve high levels of efficiency that would allow a direct translation to clinical applications for β-thalassemia.
Toward personalized therapy of thalassemia
With hundreds of primary mutations, disease modifiers, and polymorphisms linked to hereditary persistence of HbF,Citation1 β-thalassemia patients can be stratified into clinically distinct subgroups. It is expected, therefore, that the management of β-thalassemia patients will increasingly be customized for stratified classes of β-thalassemia patients, which will also hold for intervention by gene therapy. For instance, with the objective of reaching therapeutic levels of hemoglobins, an optimized gene-therapy protocol might differ between patients with β0 genotypes (without endogenous β-globin expression, such as β039 and β0-IVSI-1 homozygotes or compound heterozygotes) and those with β+ genotypes (with residual β-globin expression, such as β+-IVSI-110 and β+-IVSI-6 homozygotes or compound heterozygotes). In this respect, the response of patients with compound heterozygote β0/β+ genotypes to exogenous β-globin expression might need careful study. Moreover, in the case of mutation-specific genome editing, considerations of personalization are inherent in the approach, while, for other approaches, these considerations might be less obvious but similarly critical. For instance, the efficiency of gene therapy based on exogenous γ-globin gene expression or on the activation of endogenous γ-globin by any of the means discussed above may be in doubt in cases where the patients involved are already expressing high endogenous levels of HbF. These considerations also hold for the combination of gene therapy and pharmacological induction of HbF detailed in the section titled “Combination therapy of gene addition with HbF inducers”, because the individual genetic composition is an important cause of variations in the response and tolerance to drug treatment, as recently reviewed.Citation30 Pharmacogenomic-based studies have clearly demonstrated that several genomic variations (not restricted to the human β-globin gene cluster) are significantly associated with differential responses of β-globinopathy patients to treatment with chemical HbF inducers, such as hydroxyurea.Citation172 This insight renders the use of genomic/transcriptomic analysis to predict the in vivo response and to guide the personalization of any such therapy a logical conclusion.Citation111,Citation112 With the same rationale, the analysis of patient-specific responses in cell culture before therapy, and, in particular, before permanent therapeutic intervention, is strongly recommendedCitation83 and will become increasingly common. This trend, combined with the ex vivo approach used for the therapy itself and with an increasing use and creation of patient-specific iPSCs (in particular for gene-correction approaches), is expected to lead to a dramatic increase in biobanking of patient-derived cells, with all the regulatory, management, and ethical issues involved.Citation173
Conclusion
In summary, gene therapy is one of the most promising approaches for the future treatment of β-thalassemia patients and comprises several, at times complementary, strategies. The clinically most advanced approach, that of substituting nonfunctional endogenous β-globin genes with a normal β-globin gene carried by lentiviral vectors, leads to de novo production of HbA. This approach can be enhanced, as in vitro evidence indicates, by additional treatment with inducers of endogenous HbF, which is firmly established as clinically beneficial. In the same vein, numerous gene-therapy approaches also draw on HbF as a positive disease modifier, either by expressing exogenous HbF from a lentiviral vector or by inducing endogenous HbF with a variety of approaches, including the expression of exogenous artificial transcription factors or the disruption of γ-globin repressors or their binding sites. This latter approach has been made possible by an increasingly detailed understanding of globin gene regulation and by the development of rationally designed artificial nucleases for genome editing. Designer nucleases in turn now also allow gene editing of the human globin locus and thus the correction of altered β-globin genes as the most direct gene-therapy approach. As for the cellular targets of gene therapy, human erythropoietic stem cells have been considered in most studies and are still the substrate of choice for clinical applications. However, it can be expected that iPSCs from β-thalassemia patients will play an increasing role in preclinical, and possibly clinical, gene-therapy studies in the future.
As a result of all these developments, and after decades in the making, gene therapy of β-thalassemia has reached a critical phase and is beginning to live up to its long-held promise. At this privileged moment in time, the model systems and protocols are in place to test gene-therapy approaches, and the first clinical trials show therapeutic efficiency and guide our decisions for future developments, such as the choice of conditioning regimen (full or mild), the HSPC source (bone-marrow-derived or mobilized), and the inclusion of insulators for gene augmentation. Ongoing optimization of extant gene-augmentation tools and combinatorial approaches with chemical reagents are approaching therapeutic efficiency, even for severe forms of the disease. At the same time, fundamental insights into globin switching and new tools for cellular reprogramming, transcriptional regulation, post-transcriptional silencing, and genome editing have opened up as-yet uncharted territory in what has become a fast-moving and highly competitive field of research. While there is no telling which approach will win out for widespread clinical application in the course of time, vigilance, widespread competence in shared methodology, and the availability of diametrically different treatment strategies will provide the pressure and scope for fast improving efficacy and safety, for the good of the field and for the benefit of the patients.
Acknowledgments
Funded by the EU Seventh Framework Programme for research, technological development, and demonstration under grant agreement number 306201 (THALAMOSS). This work was also supported by grants from MIUR (Italian Ministry of University and Research) (RG and AF), from the Fondazione Cariparo (Cassa di Risparmio di Padova e Rovigo) (RG and AF), by Telethon grant GGP10124 (RG and AF), by the Research Promotion Foundation of Cyprus (ΥΓΕΙΑ/ΒΙΟΣ/0311(BE)/20) (CWL and MK), by grant KL2TR000458 of the Clinical and Translational Science Center at Weill Cornell Medical College (LB), by the NIH-NKLBI 1R01HL102449 grant (SR) and the Daedalus grants (SR). This research was also supported by Associazione Veneta per la Lotta alla Talassemia (AVLT), Rovigo, Italy (RG and AF).
Disclosure
The authors report no conflicts of interest in this work.
References
- KountourisPLedererCWFanisPFelekiXOldJKleanthousMIthaGenes: an interactive database for haemoglobin variations and epidemiologyPLoS One20149e10302025058394
- OldJMScreening and genetic diagnosis of haemoglobin disordersBlood Rev200317435312490210
- GalanelloROrigaRBeta-thalassemiaOrphanet J Rare Dis201051120492708
- HiggsDREngelJDStamatoyannopoulosGThalassaemiaLancet201237937338321908035
- WeatherallDJPhenotype-genotype relationships in monogenic disease: lessons from the thalassaemiasNat Rev Genet2001224525511283697
- NienhuisAWNathanDGPathophysiology and clinical manifestations of the β-thalassemiasCold Spring Harb Perspect Med20122a01172623209183
- QuekLTheinSLMolecular therapies in beta-thalassaemiaBr J Haematol200713635336517129232
- RivellaSIneffective erythropoiesis and thalassemiasCurr Opin Hematol20091618719419318943
- TurbpaiboonCWilairatPAlpha-hemoglobin stabilizing protein: molecular function and clinical correlationFront Biosci (Landmark Ed)20101511120036801
- OlivieriNFBrittenhamGMManagement of the thalassemiasCold Spring Harb Perspect Med20133
- GossCGiardinaPDegtyaryovaDKleinertDShethSCushingMRed blood cell transfusions for thalassemia: results of a survey assessing current practice and proposal of evidence-based guidelinesTransfusion2014541773178124611697
- PoggialiECassinerioEZanaboniLCappelliniMDAn update on iron chelation therapyBlood Transfus20121041142222790257
- KingAShenoySEvidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemiaBlood201412330893094 quiz 321024511087
- AngelucciEMatthes-MartinSBaroncianiDEBMT Inborn Error and EBMT Paediatric Working PartiesHematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panelHaematologica20149981182024790059
- TheinSLMenzelSLathropMGarnerCControl of fetal hemoglobin: new insights emerging from genomics and clinical implicationsHum Mol Genet200918R216R22319808799
- FibachEBianchiNBorgattiMPrusEGambariRMithramycin induces fetal hemoglobin production in normal and thalassemic human erythroid precursor cellsBlood200310241276128112738678
- LamprontiIBianchiNBorgattiMFibachEPrusEGambariRAccumulation of gamma-globin mRNA in human erythroid cells treated with angelicinEur J Haematol20037118919512930320
- MischiatiCSereniALamprontiIRapamycin-mediated induction of gamma-globin mRNA accumulation in human erythroid cellsBr J Haematol200412661262115287957
- LamprontiIBianchiNZuccatoCMediciABergaminiPGambariREffects on erythroid differentiation of platinum(II) complexes of synthetic bile acid derivativesBioorg Med Chem2006145204521016709458
- ZuccatoCBianchiNBorgattiMEverolimus is a potent inducer of erythroid differentiation and gamma-globin gene expression in human erythroid cellsActa Haematol200711716817617148936
- LamprontiIBianchiNZuccatoCIncrease in gamma-globin mRNA content in human erythroid cells treated with angelicin analogsInt J Hematol20099031832719777196
- PourfarzadFvon LindernMAzarkeivanAHydroxyurea responsiveness in β-thalassemic patients is determined by the stress response adaptation of erythroid progenitors and their differentiation propensityHaematologica20139869670423100274
- FinottiAGambariRRecent trends for novel options in experimental biological therapy of β-thalassemiaExpert Opin Biol Ther2014141443145424934764
- ColahRGorakshakarANadkarniAGlobal burden, distribution and prevention of β-thalassemias and hemoglobin E disordersExpert Rev Hematol2010310311721082937
- LedererCWBasakANAydinokYAn electronic infrastructure for research and treatment of the thalassemias and other hemoglobinopathies: the Euro-mediterranean ITHANET projectHemoglobin20093316317619657830
- Traeger-SynodinosJHarteveldCLAdvances in technologies for screening and diagnosis of hemoglobinopathiesBiomark Med2014811913124325233
- IpHWSoCCDiagnosis and prevention of thalassemiaCrit Rev Clin Lab Sci20135012514124295057
- TheinSLGenetic association studies in β-hemoglobinopathiesHematology Am Soc Hematol Educ Program2013201335436124319204
- SuzukiMYamamotoMEngelJDFetal globin gene repressors as drug targets for molecular therapies to treat the β-globinopathiesMol Cell Biol2014343560356925022757
- BredaLRivellaSZuccatoCGambariRCombining gene therapy and fetal hemoglobin induction for treatment of beta-thalassemiaExpert Rev Hematol2013625526423782080
- GajTGersbachCABarbasCF3rdZFN, TALEN, and CRISPR/Cas-based methods for genome engineeringTrends Biotechnol20133139740523664777
- GambariRAlternative options for DNA-based experimental therapy of β-thalassemiaExpert Opin Biol Ther20121244346222413823
- RyuBYPersonsDAEvans-GaleaMVGrayJTNienhuisAWA chromatin insulator blocks interactions between globin regulatory elements and cellular promoters in erythroid cellsBlood Cells Mol Dis20073922122817601756
- VoonHPWardanHVadolasJsiRNA-mediated reduction of alpha-globin results in phenotypic improvements in beta-thalassemic cellsHaematologica2008931238124218556409
- ArumugamPIScholesJPerelmanNXiaPYeeJKMalikPImproved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator elementMol Ther2007151863187117622240
- MiccioAPolettiVTiboniFThe GATA1-HS2 enhancer allows persistent and position-independent expression of a β-globin transgenePLoS One20116e2795522164220
- MontiniECesanaDGenotoxicity assay for gene therapy vectors in tumor prone Cdkn2a−/− miceMethods Enzymol201250717118522365774
- CesanaDRanzaniMVolpinMUncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivoMol Ther20142277478524441399
- NowrouziACheungWTLiTThe fetal mouse is a sensitive genotoxicity model that exposes lentiviral-associated mutagenesis resulting in liver oncogenesisMol Ther20132132433723299800
- RoselliEAMezzadraRFrittoliMCCorrection of beta-thalassemia major by gene transfer in haematopoietic progenitors of pediatric patientsEMBO Mol Med2010231532820665635
- WilberAHargrovePWKimYSTherapeutic levels of fetal hemoglobin in erythroid progeny of β-thalassemic CD34+ cells after lentiviral vector-mediated gene transferBlood2011117102817282621156846
- BouladFWangXQuJSafe mobilization of CD34+ cells in adults with beta-thalassemia and validation of effective globin gene transfer for clinical investigationBlood20141231483148624429337
- YannakiEKarponiGZervouFHematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with β-thalassemia majorHum Gene Ther20132485286024001178
- FibachEBianchiNBorgattiMEffects of rapamycin on accumulation of alpha-, beta- and gamma-globin mRNAs in erythroid precursor cells from beta-thalassaemia patientsEur J Haematol200677543744116939628
- PopeSHFibachESunJChinKRodgersGPTwo-phase liquid culture system models normal human adult erythropoiesis at the molecular levelEur J Haematol20006429230310863975
- BredaLKleinertDACasuCA preclinical approach for gene therapy of beta-thalassemiaAnn N Y Acad Sci2010120213414020712784
- HuberTLDissecting hematopoietic differentiation using the embryonic stem cell differentiation modelInt J Dev Biol2010546–7991100220711977
- HonigGRLuSJFengQVidaLNLeeBSLanzaRalpha-Thalassemia-like globin gene expression by primitive erythrocytes derived from human embryonic stem cellsHemoglobin20103414515020353349
- VerlinskyYStrelchenkoNKukharenkoVHuman embryonic stem cell lines with genetic disordersReprod Biomed Online20051010511015705304
- TakahashiKYamanakaSInduction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factorsCell200612666367616904174
- CsobonyeiovaMPolakSKollerJDanisovicLInduced pluripotent stem cells and their implication for regenerative medicineCell Tissue Bank Epub7192014
- KimCDisease modeling and cell based therapy with iPSC: future therapeutic option with fast and safe applicationBlood Res20144971424724061
- FanYLuoYChenXLiQSunXGeneration of human β-thalassemia induced pluripotent stem cells from amniotic fluid cells using a single excisable lentiviral stem cell cassetteJ Reprod Dev20125840440922498813
- OchiKTakayamaNHiroseSNakahataTNakauchiHEtoKMulticolor staining of globin subtypes reveals impaired globin switching during erythropoiesis in human pluripotent stem cellsStem Cells Transl Med2014379280024873860
- DiasJGumenyukMKangHGeneration of red blood cells from human induced pluripotent stem cellsStem Cells Dev2011201639164721434814
- TubsuwanAAbedSDeichmannAParallel assessment of globin lentiviral transfer in induced pluripotent stem cells and adult hematopoietic stem cells derived from the same transplanted β-thalassemia patientStem Cells2013311785179423712774
- KobariLYatesFOudrhiriNHuman induced pluripotent stem cells can reach complete terminal maturation: in vivo and in vitro evidence in the erythropoietic differentiation modelHaematologica2012971795180322733021
- PásztyCTransgenic and gene knock-out mouse models of sickle cell anemia and the thalassemiasCurr Opin Hematol1997488939107524
- FarrellCMGrinbergAHuangSPA large upstream region is not necessary for gene expression or hypersensitive site formation at the mouse beta-globin locusProc Natl Acad Sci U S A200097145541455911121056
- RivellaSMayCChadburnARiviereISadelainMA novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transferBlood20031012932293912480689
- Weizer-SternOAdamskyKAmariglioNmRNA expression of iron regulatory genes in beta-thalassemia intermedia and beta-thalassemia major mouse modelsAm J Hematol20068147948316755567
- VadolasJNefedovMWardanHHumanized beta-thalassemia mouse model containing the common IVSI-110 splicing mutationJ Biol Chem20062817399740516421096
- JamsaiDZaibakFVadolasJA humanized BAC transgenic/knockout mouse model for HbE/beta-thalassemiaGenomics20068830931516631345
- CostaFCFedosyukHNeadesRde Los RiosJBBarbasCF3rdPetersonKRInduction of fetal hemoglobin in vivo mediated by a synthetic gamma-globin zinc finger activatorAnemia2012201250789422778925
- GetmanMEnglandSJMalikJPetersonKPalisJSteinerLAExtensively self-renewing erythroblasts derived from transgenic β-yac mice is a novel model system for studying globin switching and erythroid maturationExp Hematol201442536546 e8.24704162
- McCollBKaoBRLourthaiPAn in vivo model for analysis of developmental erythropoiesis and globin gene regulationFASEB J2014282306231724443374
- HuoYMcConnellSCLiuSHumanized mouse models of Cooley’s anemia: correct fetal-to-adult hemoglobin switching, disease onset, and disease pathologyAnn N Y Acad Sci20101202455120712771
- HuoYMcConnellSCRyanTMPreclinical transfusion- dependent humanized mouse model of beta thalassemia majorBlood20091134763477019258591
- HuoYMcConnellSCLiuSRHumanized mouse model of Cooley’s anemiaJ Biol Chem20092844889489619098001
- ArumugamPMalikPGenetic therapy for beta-thalassemia: from the bench to the bedsideHematology Am Soc Hematol Educ Program2010201044545021239833
- MayCRivellaSCallegariJTherapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globinNature2000406828610894546
- BredaLCasuCGardenghiSTherapeutic hemoglobin levels after gene transfer in β-thalassemia mice and in hematopoietic cells of β-thalassemia and sickle cells disease patientsPLoS One20127e3234522479321
- ImrenSFabryMEWestermanKAHigh-level beta-globin expression and preferred intragenic integration after lentiviral transduction of human cord blood stem cellsJ Clin Invest200411495396215467834
- PuthenveetilGScholesJCarbonellDSuccessful correction of the human beta-thalassemia major phenotype using a lentiviral vectorBlood20041043445345315292064
- MiccioACesariRLottiFIn vivo selection of genetically modified erythroblastic progenitors leads to long-term correction of beta-thalassemiaProc Natl Acad Sci U S A2008105105471055218650378
- WangCXSatherBDWangXRapamycin relieves lentiviral vector transduction resistance in human and mouse hematopoietic stem cellsBlood201412491392324914132
- PhaltaneRLachmannNBrennigSAckermannMModlichUMoritzTLentiviral MGMT(P140K)-mediated in vivo selection employing a ubiquitous chromatin opening element (A2UCOE) linked to a cellular promoterBiomaterials2014357204721324875758
- AckermannMLachmannNHartungSPromoter and lineage independent anti-silencing activity of the A2 ubiquitous chromatin opening element for optimized human pluripotent stem cell-based gene therapyBiomaterials2014351531154224290698
- DigheNKhouryMMattarCLong-term reproducible expression in human fetal liver hematopoietic stem cells with a UCOE-based lentiviral vectorPLoS One20149e10480525118036
- GrothACLiuMWangHLovelettEEmeryDWIdentification and characterization of enhancer-blocking insulators to reduce retroviral vector genotoxicityPLoS One20138e7652824098520
- ArumugamPIUrbinatiFVeluCSHigashimotoTGrimesHLMalikPThe 3′ region of the chicken hypersensitive site-4 insulator has properties similar to its core and is required for full insulator activityPLoS One20094e699519746166
- PapapetrouEPLeeGMalaniNGenomic safe harbors permit high β-globin transgene expression in thalassemia induced pluripotent stem cellsNat Biotechnol201129737821151124
- DongARivellaSBredaLGene therapy for hemoglobinopathies: progress and challengesTransl Res201316129330623337292
- YannakiEEmeryDWStamatoyannopoulosGGene therapy for β-thalassaemia: the continuing challengeExpert Rev Mol Med201012e3120883576
- BankADorazioRLeboulchPA phase I/II clinical trial of beta-globin gene therapy for beta-thalassemiaAnn N Y Acad Sci2005105430831616339679
- Bluebird bio IBluebird bio reports rapid transfusion independence in beta-thalassemia major patients treated with its lentiglobin product candidate [press release]Cambridge, MAbluebird bio Headquarters6142014 Available from: http://investor.bluebirdbio.com/phoenix.zhtml?c=251820&p=irol-newsArticle&ID=1939867&highlight=. Accessed November 26, 2014
- Cavazzana-CalvoMPayenENegreOTransfusion independence and HMGA2 activation after gene therapy of human β-thalassaemiaNature201046731832220844535
- CavazzanaMRibeilJ-APayenEOutcomes of gene therapy for beta-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral beta-globin vector (Abstract submission 3287, Abstract number S742)Presented at: European Hematology Association, 19th Annual CongressMilan, ItalyJune 14, 2014
- Bluebird Bio IncBluebird bio Announces First Patient with Sickle Cell Disease Transplanted with LentiGlobin Gene Therapy2014
- NagelRLBookchinRMJohnsonJStructural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin SProc Natl Acad Sci U S A197976670672284392
- McCuneSLReillyMPChomoMJAsakuraTTownesTMRecombinant human hemoglobins designed for gene therapy of sickle cell diseaseProc Natl Acad Sci U S A199491985298567937904
- LevasseurDNRyanTMReillyMPMcCuneSLAsakuraTTownesTMA recombinant human hemoglobin with anti-sickling properties greater than fetal hemoglobinJ Biol Chem2004279275182752415084588
- RomeroZUrbinatiFGeigerSβ-globin gene transfer to human bone marrow for sickle cell diseaseJ Clin Invest201312333173330
- NishinoTTubbJEmeryDWPartial correction of murine beta-thalassemia with a gammaretrovirus vector for human gamma-globinBlood Cells Mol Dis2006371716814578
- NishinoTCaoHStamatoyannopoulosGEmeryDWEffects of human gamma-globin in murine beta-thalassaemiaBr J Haematol200613410010816803575
- PestinaTIHargrovePWJayDGrayJTBoydKMPersonsDACorrection of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobinMol Ther20091724525219050697
- SamakogluSLisowskiLBudak-AlpdoganTA genetic strategy to treat sickle cell anemia by coregulating globin transgene expression and RNA interferenceNat Biotechnol200624899416378095
- PersonsDAAllayERSawaiNSuccessful treatment of murine beta-thalassemia using in vivo selection of genetically modified, drug-resistant hematopoietic stem cellsBlood200310250651312663444
- HanawaHHargrovePWKepesSSrivastavaDKNienhuisAWPersonsDAExtended beta-globin locus control region elements promote consistent therapeutic expression of a gamma-globin lentiviral vector in murine beta-thalassemiaBlood20041042281229015198957
- PerumbetiAHigashimotoTUrbinatiFA novel human gamma-globin gene vector for genetic correction of sickle cell anemia in a humanized sickle mouse model: critical determinants for successful correctionBlood20091141174118519474450
- ZhaoHPestinaTINasimuzzamanMMehtaPHargrovePWPersonsDAAmelioration of murine beta-thalassemia through drug selection of hematopoietic stem cells transduced with a lentiviral vector encoding both gamma-globin and the MGMT drug-resistance geneBlood20091135747575619365082
- ReidMEEl BeshlawyAInatiAA double-blind, placebo- controlled phase II study of the efficacy and safety of 2,2-dimethylbutyrate (HQK-1001), an oral fetal globin inducer, in sickle cell diseaseAm J Hematol20148970971324677033
- PerrineSPPaceBSFallerDVTargeted fetal hemoglobin induction for treatment of beta hemoglobinopathiesHematol Oncol Clin North Am20142823324824589264
- AhmadvandMNoruziniaMFardADThe role of epigenetics in the induction of fetal hemoglobin: a combination therapy approachInt J Hematol Oncol Stem Cell Res2014891424505546
- FardADHosseiniSAShahjahaniMSalariFJasebKEvaluation of novel fetal hemoglobin inducer drugs in treatment of β-hemoglobinopathy disordersInt J Hematol Oncol Stem Cell Res20137475424505535
- RahimFAllahmoradiHSalariFEvaluation of signaling pathways involved in γ-globin gene induction using fetal hemoglobin inducer drugsInt J Hematol Oncol Stem Cell Res20137414624505534
- QianXChenJZhaoDGuoLPlastrum testudinis induces γ-globin gene expression through epigenetic histone modifications within the γ-globin gene promoter via activation of the p38 MAPK signaling pathwayInt J Mol Med2013311418142823588991
- FibachEPrusEBianchiNResveratrol: antioxidant activity and induction of fetal hemoglobin in erythroid cells from normal donors and β-thalassemia patientsInt J Mol Med20122997498222378234
- FrancoSSDe FalcoLGhaffariSResveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic miceHaematologica20149926727523975182
- MaYNChenMTWuZKEmodin can induce K562 cells to erythroid differentiation and improve the expression of globin genesMol Cell Biochem201338212713623744534
- ItaliaKJijinaFMerchantRComparison of in-vitro and in-vivo response to fetal hemoglobin production and γ-mRNA expression by hydroxyurea in hemoglobinopathiesIndian J Hum Genet20131925125824019630
- PecoraroARiganoPTroiaAQuantification of HBG mRNA in primary erythroid cultures: prediction of the response to hydroxyurea in sickle cell and beta-thalassemiaEur J Haematol201492667224112139
- BianchiNZuccatoCLamprontiIBorgattiMGambariRFetal hemoglobin inducers from the natural world: a novel approach for identification of drugs for the treatment of β-thalassemia and sickle-cell anemiaEvid Based Complement Alternat Med2009614115118955291
- ZuccatoCBredaLSalvatoriFA combined approach for β-thalassemia based on gene therapy-mediated adult hemoglobin (HbA) production and fetal hemoglobin (HbF) inductionAnn Hematol2012911201121322460946
- KaiserJGene therapy. Beta-thalassemia treatment succeeds, with a caveatScience20093261468146920007873
- IkedaKMasonPJBesslerM3′UTR-truncated Hmga2 cDNA causes MPN-like hematopoiesis by conferring a clonal growth advantage at the level of HSC in miceBlood20111175860586921460244
- MorishitaAZaidiMRMitoroAHMGA2 is a driver of tumor metastasisCancer Res2013734289429923722545
- SadelainMGlobin gene transfer for the treatment of β-thalassemia and Sickle Cell DiseasePresented at: American Society for Gene and Cell Therapy 19th Annual CongressWashington, DCMay 24, 2014
- SadelainMRivièreIWangXStrategy for a multicenter phase I clinical trial to evaluate globin gene transfer in beta-thalassemiaAnn N Y Acad Sci20101202525820712772
- DengWLeeJWangHControlling long-range genomic interactions at a native locus by targeted tethering of a looping factorCell20121491233124422682246
- DengWRuponJWKrivegaIReactivation of developmentally silenced globin genes by forced chromatin loopingCell2014158484986025126789
- RuponJWDengWWangHUsing forced chromatin looping to overcome developmental silencing of embryonic and fetal β-type globin genes in adult erythroid cellsBlood2013122433
- GräslundTLiXMagnenatLPopkovMBarbasCF3rdExploring strategies for the design of artificial transcription factors: targeting sites proximal to known regulatory regions for the induction of gamma-globin expression and the treatment of sickle cell diseaseJ Biol Chem20052803707371415537646
- WilberATschulenaUHargrovePWA zinc-finger transcriptional activator designed to interact with the gamma-globin gene promoters enhances fetal hemoglobin production in primary human adult erythroblastsBlood20101153033304120190190
- VoonHPVadolasJControlling alpha-globin: a review of alpha-globin expression and its impact on beta-thalassemiaHaematologica2008931868187618768527
- XieSYRenZRZhangJZRestoration of the balanced alpha/beta-globin gene expression in beta654-thalassemia mice using combined RNAi and antisense RNA approachHum Mol Genet2007162616262517716993
- VoonHPWardanHVadolasJCo-inheritance of alpha- and beta-thalassaemia in mice ameliorates thalassaemic phenotypeBlood Cells Mol Dis20073918418817493845
- NasimuzzamanMKhandrosEWangXAnalysis of alpha hemoglobin stabilizing protein overexpression in murine beta-thalassemiaAm J Hematol20108582082220815047
- RoosjenMMcCollBKaoBGearingLJBlewittMEVadolasJTranscriptional regulators Myb and BCL11A interplay with DNA methyltransferase 1 in developmental silencing of embryonic and fetal β-like globin genesFASEB J2014281610162024371119
- ForgetBGProgress in understanding the hemoglobin switchN Engl J Med201136585285421879905
- SankaranVGXuJByronRA functional element necessary for fetal hemoglobin silencingN Engl J Med201136580781421879898
- ZhouDLiuKSunCWPawlikKMTownesTMKLF1 regulates BCL11A expression and gamma- to beta-globin gene switchingNat Genet20104274274420676097
- SankaranVGMenneTFXuJHuman fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11AScience20083221839184219056937
- TanabeOMcPheeDKobayashiSEmbryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4EMBO J2007262295230617431400
- TanabeOShenYLiuQThe TR2 and TR4 orphan nuclear receptors repress Gata1 transcriptionGenes Dev2007212832284417974920
- CuiSKolodziejKEObaraNNuclear receptors TR2 and TR4 recruit multiple epigenetic transcriptional corepressors that associate specifically with the embryonic β-type globin promoters in differentiated adult erythroid cellsMol Cell Biol2011313298331121670149
- CampbellADCuiSShiLForced TR2/TR4 expression in sickle cell disease mice confers enhanced fetal hemoglobin synthesis and alleviated disease phenotypesProc Natl Acad Sci U S A2011108188081881322042865
- XuXSHongXWangGInduction of endogenous gamma-globin gene expression with decoy oligonucleotide targeting Oct-1 transcription factor consensus sequenceJ Hematol Oncol200921519327156
- JiangJBestSMenzelScMYB is involved in the regulation of fetal hemoglobin production in adultsBlood20061081077108316861354
- SankaranVGXuJOrkinSHTranscriptional silencing of fetal hemoglobin by BCL11AAnn N Y Acad Sci20101202646820712774
- BorgJPapadopoulosPGeorgitsiMHaploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobinNat Genet20104280180520676099
- BorgJPhylactidesMBartsakouliaMKLF10 gene expression is associated with high fetal hemoglobin levels and with response to hydroxyurea treatment in β-hemoglobinopathy patientsPharmacogenomics2012131487150023057549
- AmayaMDesaiMGnanapragasamMNMi2β-mediated silencing of the fetal γ-globin gene in adult erythroid cellsBlood20131213493350123444401
- SankaranVGTargeted therapeutic strategies for fetal hemoglobin inductionHematology Am Soc Hematol Educ Program2011201145946522160074
- TallackMRPerkinsACThree fingers on the switch: Krüppel-like factor 1 regulation of γ-globin to β-globin gene switchingCurr Opin Hematol20132019320023474875
- ManchinuMFMarongiuMFPoddieDIn vivo activation of the human δ-globin gene: the therapeutic potential in β-thalassemic miceHaematologica201499768423872310
- XuJPengCSankaranVGCorrection of sickle cell disease in adult mice by interference with fetal hemoglobin silencingScience201133499399621998251
- EsteghamatFGillemansNBilicIErythropoiesis and globin switching in compound Klf1::Bcl11a mutant miceBlood20131212553256223361909
- RenellaRPerlovAHarrisCEHematopoietic SIN lentiviral micro RNA-mediated silencing of BCL11A: pre-clinical evidence for a sickle cell disease gene-therapy trialPresented at: American Society of Hematology 54th Annual MeetingAtlanta, GADecember 10, 2012
- GudaSPengDBauerDEOptimization of lentivirus vector RNA polymerase II driven microRNA embedded shRNAs for enhanced processing and efficient knockdown of Bcl11a for induction of fetal hemoglobin in erythroid cellsPresented at: American Society for Gene and Cell Therapy 19th Annual CongressWashington, DCMay 23, 2014
- XuJBauerDEOrkinSHTargeting regulators of hemoglobin FThe Hematologist201119
- XuJBauerDEKerenyiMACorepressor-dependent silencing of fetal hemoglobin expression by BCL11AProc Natl Acad Sci U S A20131101665182323576758
- SankaranVGMenneTFŠćepanovićDMicroRNA-15a and -16–1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13Proc Natl Acad Sci U S A20011081519152421205891
- LulliVRomaniaPMorsilliOMicroRNA-486–3p regulates γ-globin expression in human erythroid cells by directly modulating BCL11APLoS One201384e6043623593217
- AzzouziIMoestHWinklerJMicroRNA-96 directly inhibits γ-globin expression in human erythropoiesisPLoS One20116e2283821829531
- MaYWangBJiangFA feedback loop consisting of microRNA 23a/27a and the β-like globin suppressors KLF3 and SP1 regulates globin gene expressionMol Cell Biol201333203994400723918807
- GenovesePSchiroliGEscobarGTargeted genome editing in human repopulating haematopoietic stem cellsNature201451023524024870228
- ZouJMaliPHuangXDoweySNChengLSite-specific gene correction of a point mutation in human iPS cells derived from an adult patient with sickle cell diseaseBlood20111184599460821881051
- KatadaHKomiyamaMArtificial restriction DNA cutters to promote homologous recombination in human cellsCurr Gene Ther201111384521182465
- HockemeyerDSoldnerFBeardCEfficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleasesNat Biotechnol20092785185719680244
- PerezEEWangJMillerJCEstablishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleasesNat Biotechnol20082680881618587387
- UrnovFDMillerJCLeeYLHighly efficient endogenous human gene correction using designed zinc-finger nucleasesNature200543564665115806097
- VoitRAMcMahonMASawyerSLPorteusMHGeneration of an HIV resistant T-cell line by targeted “stacking” of restriction factorsMol Ther20132178679523358186
- VoitRAHendelAPruett-MillerSMPorteusMHNuclease-mediated gene editing by homologous recombination of the human globin locusNucleic Acids Res2014421365137824157834
- PatsaliPMussolinoCStephanouCTowards personalized gene therapy for β-thalassemia in CyprusPresented at: American Society for Gene and Cell Therapy 19th Annual CongressWashington, DCMay 23, 2014
- CradickTJFineEJAnticoCJBaoGCRISPR/Cas9 systems targeting β-globin and CCR5 genes have substantial off-target activityNucleic Acids Res2013419584959223939622
- TsaiSQWyvekensNKhayterCDimeric CRISPR RNA-guided FokI nucleases for highly specific genome editingNat Biotechnol20143256957624770325
- MaNLiaoBZhangHTranscription activator-like effector nuclease (TALEN)-mediated gene correction in integration-free beta-thalassemia induced pluripotent stem cellsJ Biol Chem2013288346713467924155235
- SunNZhaoHSeamless correction of the sickle cell disease mutation of the HBB gene in human induced pluripotent stem cells using TALENsBiotechnol Bioeng20141111048105323928856
- CostGJGregoryPDGuschinDHolmesMCMillerJCPaschonDMethods and compositions for treatment of a genetic condition (US20100093913)Sangamo Biosciences, Inc2014
- CostGJGregoryPDGuschinDHolmesMCMillerJCPaschonDMethods and compositions for treatment of a genetic condition (US20140080216)Sangamo Biosciences, Inc2014
- GraviaAChondrouVSgourouAIndividualizing fetal hemoglobin augmenting therapy for β-type hemoglobinopathies patientsPharmacogenomics201415101355136425155936
- PatelATissue banking for research – bench to bedside and back –myth, reality or fast fading reality at the dawn of a personalised healthcare eraCell Tissue Bank201112192120824353
