Abstract
Combined thrombophilia represents 7.8–8.3% of the patients with thrombophilia and confers a higher risk for thrombosis development and recurrence. Here, we present a 17-year-old boy carrier of three congenital thrombophilias, two severe (type I antithrombin deficiency and type I protein S deficiency) and one prothrombotic polymorphism (prothrombin G20210A), all in heterozygosis. He developed an extensive deep venous thrombosis in lower left limb, reaching proximal inferior vena cava and contralateral iliac vein, in the setting of prolonged rest. Endovascular therapy with local thrombolytic agent infusion followed by mechanical thrombectomy was performed, achieving a favorable clinical and radiological evolution. Antithrombin replacement to achieve levels between 80% and 120% with heparin administration was used during the endovascular procedure. The patient is currently asymptomatic and maintains indefinite anticoagulation with warfarin, keeping an appropriate anticoagulation range (international normalized range between 2.5 and 3.5).
Introduction
Thrombophilia is defined as the disruption of the procoagulant and anticoagulant normal balance, leading to an abnormal coagulation tendency to clot formation.Citation1,Citation2 Thrombophilia can be inherited or acquired.
Inherited prothrombotic disorders include common but mild prothrombotic polymorphisms: factor V Leiden and prothrombin G20210A, which require a trigger for thrombosis developments, such as prolonged immobilization, cancer, pregnancy or obesity.Citation2 On the other hand, rare and severe deficiencies of natural anticoagulants, including antithrombin (AT), protein S (PS) and protein C, are associated with a high risk of thrombosis. Other much rare severe congenital thrombophilias are plasminogen deficiency, hypo/dysfibrinogenemia, FIX Padua or prothrombin Yukuhashi.Citation1,Citation3–5
Among acquired thrombophilias, antiphospholipid syndrome is the most prevalent disorder.Citation6
Thrombophilia can be identified in as many as 50% of the patients with venous thrombosis (VT) (35% of them with inherited thrombophilia).Citation1 Although all these thrombophilias play a role in thrombus development, the risk for initial thromboembolism varies, being hereditary AT deficiency the one with the highest thrombotic risk, although also with considerable clinical heterogeneity.Citation7
The estimated prevalence of inherited AT deficiency in the general population lies between 0.02–0.2% and 1–5% in patients with thrombosis. The annual recurrence risk without long-term anticoagulation is 8.8% (95 CI 4.6–14.1) in AT-deficient VT patients.Citation8 The risk of thrombosis is related to the type of AT deficiency. Quantitative (type I) defects, when antigen and activity are similarly reduced, are usually associated with a high risk of thrombosis and recurrence. Different genetic defects cause type I deficiency: single nucleotide variants (SNVs) with non-sense, missense or splicing consequences, small insertions/deletions or structural variants. In contrast, qualitative (type II) defects, when antigen levels are normal but activity is reduced, are in general milder. Most genetic defects causing type II deficiencies are missense variants that depending on the functional consequences, define three subgroups: 1) type IIa or reactive site: mainly located in reactive center loop affecting the reactivity of AT; 2) type IIb or heparin-binding site, which impair heparin affinity; 3) type IIc or pleiotropic effect, if the mutation affects both the reactivity and heparin affinity.Citation9
Mutations in PROS1, most of them SNVs, cause three types of hereditary PS deficiency: qualitative PS deficiency (type II), and two types of quantitative PS deficiencies: type I implies a reduction in both total and free levels, and type III when only free levels are reduced. In contrast to AT deficiency, the risk of thrombosis, which ranged 1- to 10-fold, did not change dependently on the type of PS deficiency.Citation1
Thrombophilia is usually performed in patients with VT, particularly if they are under 50 years old, have recurrent episodes, thrombosis at unusual sites, or in asymptomatic subjects with a positive family hereditary thrombophilia.Citation10
Combined thrombophilia is defined by the presence of more than one inherited defect in the same patient, constitutes 7.8–8.3% of the patients with thrombophilia, and confers a higher risk for thrombosis development and recurrence at an early age than single thrombophilia.Citation11,Citation12
Thus, patients with combined thrombophilia usually require long-term anticoagulation. Unfortunately, the rarity of severe combined thrombophilia had not allowed giving recommendations for the management of these patients. Another conflictive issue concerning patients with severe thrombophilia that affects carriers of combined thrombophilia is the anticoagulation to be used. Anticoagulation with vitamin K antagonists (VKA), heparin or direct oral anticoagulants (DOACs) is available. Although some published data suggest DOACs as an alternative to VKA in inherited thrombophilia,Citation1 the concerns for DOACs in patients with antiphospholipid syndrome, a severe acquired thrombophilia, strongly encourage further studies.Citation13
Thrombolytic treatment, also known as fibrinolytic treatment (streptokinase, alteplase and urokinase are the drugs most used in clinical practice), dissolves pathologic thrombi to prevent ischemic damage and to improve blood flow.Citation14 Fibrinolytics can be administered systemically (intravenously) or locally (via catheter) and they have been mainly used in acute myocardial infarction and acute stroke, although they also have other less common indications including pulmonary embolism (PE) and deep venous thrombosis (DVT).Citation14–17
Catheter-based endovascular techniques (either with local fibrinolytic agents or mechanical thrombectomy or the combination of both) improve clinical results in extensive DVT and preserves valvular vein competence, preventing or at least reducing post- thrombotic syndrome (PTS). The extent and location of the thrombus may alter the natural history of the DVT and should be considered when choosing the treatment. Anticoagulation prevents thrombus propagation. However, thrombus removal is achieved by the body´s own endogenous lytic capacity, which can be easily overcome by the large burden of an ilio-femoral DVT. The presence of massive proximal acute DVT in lower extremities accompanied by severe symptomatic leg swelling can be a feasible option for endovascular treatment and could be helpful in relieving PTS symptoms.Citation18,Citation19 Enden et al included 189 patients with iliofemoral DVT, and the use of catheter-directed thrombolysis (CDT) was associated with a 26% reduction of PTS risk development.Citation20
The ultrasound-accelerated CDT (USACDT) combines high frequency and low power ultrasound energy with thrombolytic therapy to achieve clot dissolution. Farrokhi et al in a meta-analysis of 18 studies included 597 participants and compared USACDT versus conventional CDT for DVT. They concluded that the success rate was significantly higher in USACDT. Although the mean infusion time and the rate of complications were lower in USACDT, statistical significance was not achieved.Citation21
Additionally, bleeding risk is higher in patients treated with thrombolytic agents than in those treated with anticoagulants alone. Recently, major bleeding reported in the CaVenT (Catheter-Directed thrombolysis for Deep Vein Thrombosis) and the Acute Venous Thrombosis (Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT)) studies were 3% and 1.7%, respectively.Citation20,Citation22 However, most procedure-related bleedings were minor and located at the access site.Citation23
The identification of new cases with combined thrombophilia and the description of their clinical management will be useful to gain knowledge on this rare but severe thrombophilia. Here, we present a case with three prothrombotic defects who developed extended DVT in left lower limb that had a favorable clinical and radiological evolution with fibrinolysis, mechanical thrombectomy and anticoagulation.
Case Report
A 17-year-old male was admitted to the emergency department for assessment of pain and enlargement of the left lower extremity for 24 hours. The patient described prolonged rest 3 days before admission to our clinic due to lumbar pain. He had no cardiovascular risk factors and referred no previous personal thrombotic events. At the age of 3, he was diagnosed of hereditary AT deficiency type I (AT activity: 47%, AT antigen: 40%) (reference range 80–120%), mild free and total PS deficiency (45% both) (reference range 70–120%) and prothrombin variant G20210A in heterozygosis, due to the broad thrombotic family history (). His father developed a spontaneous thrombosis in the right lower extremity at the age of 30, with PTS associated with venous ulcers. The father is currently on indefinite anticoagulation with VKA due to hereditary AT deficiency and maintains an appropriate international normalized ratio (INR) range (2.5–3.5). No thrombotic recurrences have been reported in 12 years of anticoagulation. His mother carries a type I PS deficiency and the PT G20210A polymorphism in heterozygosis. Neither the mother nor maternal grandfather, sister and maternal aunt carrying these two prothrombotic defects had previous thrombotic events, although the maternal aunt reported 3 miscarriages.
Figure 1 Pedigree of the studied family. Filled symbols represent thrombotic events. The age (yo: years old) of the thrombotic events is between brackets. The arrow points the proband. The prothrombotic genetic defects identified in each subject (all heterozygous) are also indicated.
Molecular characterization of AT deficiency revealed a heterozygous point mutation in intron 5 of SERPINC1 (NM_000488.4): c.1154–14G>A already described in Human Gene Mutation Database (HGMD) in other patients with type I deficiency (CS941423; rs542881762), which creates a cryptic splicing signal in the intron responsible for a variant with four in-frame additional residues that cause intracellular polymerization and traces of disulfide-linked dimers in plasma.Citation24,Citation25
Sequencing of PROS1 revealed a relatively common heterozygous mutation (NM_000313.4): c.1501T>C (p. Ser501Pro), responsible for the PS Heerlen variant (CM951058) that slightly increases the risk of thrombosis.Citation26
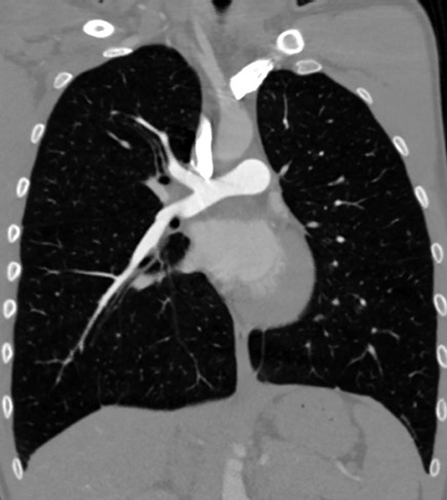
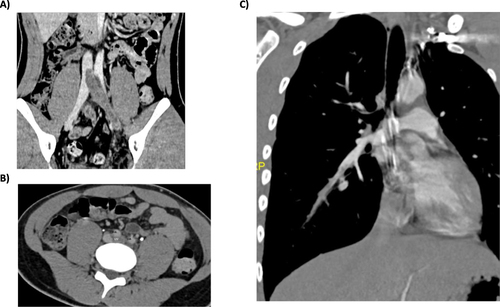
In the emergency department, edema in the left lower extremity as well as pain on mobilization and palpation were objectified. The blood test showed a high reactive C protein (9.27 mg/dL) (reference range <0.50 mg/dL), 19000 leukocytes (reference range 4000–11000) (86% neutrophils) and a D dimer of 84.23 µg/mL (reference range <0.50 µg/mL). The ultrasound revealed an increase in the diameter and echogenic content in the tibial, peroneal, popliteal, femoral, external iliac and common iliac veins, in relation to extensive DVT. Anticoagulation with low molecular weight heparin (LMWH, enoxaparin 1 mg/kg every 12 hours) was initiated. While the patient received heparin, AT replacement (50 IU/kg) to achieve an AT level between 80% and 120% was required to reach an optimal anticoagulation (target anti-Xa activity between 0.7–1.2 IU). For this purpose, anti-Xa activity and AT activity were measured. Abdominal computed tomography (CT) showed a DVT in the left lower limb, reaching the proximal inferior vena cava (IVC) and occupying 50% of the contralateral iliac vein at the confluence area, with moderate soft tissue edema. Also, the thoracic CT angiography findings were compatible with acute segmental PE ().
Figure 2 CT angiography findings at diagnosis. (A) Thrombosis in the left iliac vein that appears in the IVC; (B) Thrombosis in the left iliac vein without clear compression syndrome at the crossing behind the right iliac artery; (C) PE in branches of the right basal pyramid artery.
Given the high probability of PTS in the setting of extensive DVT, percutaneous treatment was initially proposed. Just before the endovascular treatment, AT supplementation (50 UI/Kg) to reach an AT activity close to 100% was administered.
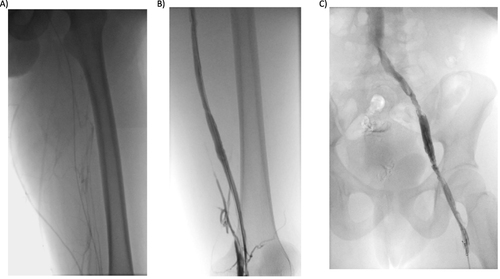
At the beginning of the procedure, an IVC filter was placed, and an ultrasound-guided puncture in the left posterior tibial vein was performed. A multi-side-hole infusion USACDT was placed within the thrombosed segment from the iliac to the distal popliteal ().
Figure 3 Phlebography from the posterior tibial vein. (A) Before rt-PA infusion: occupation of the deep tibial, popliteal and femoral venous territory, with little drainage through the superficial vein territory; B and (C) Following rt-PA infusion: permeability of the posterior tibial, popliteal, femoral, iliac and cava venous system.
To reduce the risk of bleeding, a low-dose fibrinolytic regime infusion rate of 0.6 mg per hour was used, and it was maintained for 24 hours with a total dose of 16 mg of rt-PA. Anticoagulation with unfractionated heparin was required to ensure an easier anticoagulation adjustment, depending on activated partial thromboplastin time (APTT) and fibrinogen levels.
In agreement with the local hospital protocol, during fibrinolytic infusion, fibrinogen should be checked every 4 hours while the patient receives rt-PA. If fibrinogen <200 mg/dl, the rt-PA is reduced to half the dose, while the rt-PA pump should be discontinued if fibrinogen <100 mg/dl.
The following day, the control phlebography revealed a significant improvement, with the remains of thrombus in the femoral, common femoral and popliteal veins. A pulse-pressure mechanical thrombectomy was then performed in the affected areas, achieving permeability and adequate flow without residual thrombosis. May–Thurner type venous compression was not confirmed in the final phlebography, so a stent was not required. Before finishing the procedure, the IVC filter was then removed.
The patient did not refer pain or bleeding and he initiated VKA (warfarin) together with bridge therapy (LMWH at therapeutic doses). The INR and anti-Xa activity were closely measured (every 12–24 hours) until two determinations in the therapeutic range were obtained. Considering the extensive thrombosis and the triple thrombophilia, we considered an optimal INR range between 2.5 and 3.5.
Nine months later, the angio-CT revealed total resolution of the PE (). One year after the acute episode, no DVT in lower limbs was described in control ultrasounds. The patient is currently asymptomatic, and the INR remains in therapeutic range. Due to the high-risk multiple thrombophilia, the patient requires long-term anticoagulation. He referred adequate treatment adherence and no further adverse events have been reported.
Case Discussion and Review of the Literature
Up to 10% of the population is affected by one or more currently known inherited thrombophilia.Citation27 However, combined thrombophilic defects are pretty rare, representing 7.8–8.3% of the patients with thrombophilia.Citation12 Affected patients usually develop thrombosis at an early age and require long-term anticoagulation due to the high recurrence risk.Citation11,Citation12
The family shown here is an example of the clinical heterogeneity of thrombophilic factors and the consequence of their combination. The father, carrier of type I AT deficiency, according to the high risk of thrombosis of this deficiency had a thrombotic event at the age of 30 years. In contrast, the mother, his sister, his aunt, and his grandmother, despite carrying two prothrombotic defects (PS Heerlen and PT G20210A), had no thrombotic events, probably because these two defects are milder, and they did not have any cardiovascular risk factors. However, the proband, carrier of the three prothrombotic defects, had the most severe clinical phenotype according to the age of the first event (the patient was 17 years old).
Although the patient carries the highest thrombophilia risk (hereditary AT deficiency type I) in addition to 2 more mild to moderate inherited thrombophilia traits (PS deficiency and PT G20210A mutation in heterozygosis), he had not required previous thromboprophylaxis with LMWH, as he did not go through any prothrombotic settings like immobilization or high-risk thrombotic surgery. But, in the setting of prolonged rest due to lumbar pain, he developed an extended DVT in the left leg, reaching the IVC.
The experience obtained with the management of the thrombotic event in the proband may also help the treatment of new cases with combined thrombophilia. Anticoagulation is the standard of care for thromboembolic disease. It helps reduce clot formation and prevent PE.Citation22 However, chronic complications derived from VT, especially PTS, may reach up to 40% of the affected individuals and imply a worsening in quality of life.Citation28 The rapid thrombus removal may improve deep venous flow and hence decreases PTS incidence.Citation23 Endovascular therapies, including local fibrinolysis, mechanical or combined strategies improve acute symptoms and reduce chronic sequelae, leading to higher rates of vein patency and preservation of valve function compared to anticoagulation alone.Citation18,Citation19,Citation23 Thrombolysis enhances immediate clot removal, and drugs such as urokinase and rt-PA are infused into a vein or locally using a catheter guided technique. CDT or pharmacomechanical CDT are likely to be safer and more effective than systemic thrombolytic therapy and could hold promise in preventing PTS. CDT is a promising option for massive and submassive PE with hemodynamic instability due to its rapid reversal of right ventricular dysfunction in people with acute PE compared to anticoagulation alone.Citation29 However, regarding DVT in lower extremities, the improvement in PTS symptoms is controversial and individualized treatment should be made.Citation18,Citation19 When choosing endovascular therapies, several factors including age, severity and duration of symptoms, anatomical distribution of the thrombosis, response to systemic anticoagulation and bleeding risk should be considered.Citation17 Enden et al included 189 patients with iliofemoral DVT, and the use of CDT was associated with a 26% reduction of PTS risk development and 3% rate of major bleeding.Citation20 Liu et al included 38 patients with extensive DVT in lower extremities and reported a 90% complete lysis and no major bleedings. Competent femoral valves were observed in 86% of the patients. The PTS rate was 17% during a mean 20-month follow-up.Citation30
There is scarce data regarding the use of DOACs in patients with combined thrombophilia,Citation31 In this setting, we considered AVK as the best anticoagulation option.
Treatment of iliofemoral DVT can be challenging. The use of anticoagulation alone can be insufficient for restoring venous competence. Symptomatic proximal DVT seems to be an optimal scenario for catheter-based endovascular techniques to clear the thrombus and prevent PTS development.
To sum up, patients with DVT in lower extremities should be carefully evaluated on a case-by-case basis. Those with acute symptomatic iliofemoral DVT, low bleeding risk and long-life expectancy are expected to be optimal candidates to combine anticoagulation and endovascular treatment.
Conclusions
Combined thrombophilia is rare and increases thrombotic risk.
Endovascular treatment can be a feasible option in proximal symptomatic DVT in lower extremities.
Ethics and Consent
The study participants have given written informed consent to participate and for publication of the data. Parental consent was obtained for the case of a minor. Institutional approval was not required for publication.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
The authors are grateful to Dr. Beatriz Valero for supportive clinical care.
References
- Phillippe HM, Hornsby LB, Tredway S, Armstrong EM, Bellone JM. Inherited thrombophilia. J Pharm Pract. 2014;27(3):227–233. doi:10.1177/0897190014530390
- Khan F, Tristlcher T, Khan SR, Rodger MA. Venous thromboembolism. Lancet. 2021;398(10294):64–77. doi:10.1016/S0140-6736(20)32658-1
- Lindhoff-Last E, Luxembourg B. Evidence-based indications for thrombophilia screening. VASA. 2008;37(1):19–30. doi:10.1024/0301-1526.37.1.19
- Takagi Y, Kato I, Ando Y, et al. Antithrombin- resistant prothrombin Yukuhashi mutation also causes thrombomodulin resistance in fibrinogen clotting but not in protein C activation. Thromb Res. 2014;134(4):914–917. doi:10.1016/j.thromres.2014.07.040
- Ammollo CT, Semeraro F, Colucci M, Simioni P. Factor IX Padua enhances the fibrinolytic resistance of plasma clots. Thromb Haemost. 2014;111(2):226–232. doi:10.1160/TH13-06-0489
- Armstrong EM, Bellone JM, Hornsby LB, Treadway S, Phillippe HM. Acquired thrombophilia. J Pharm Pract. 2014;27(3):234–242. doi:10.1177/0897190014530424
- Marco-Rico A, Marco-Vera P. Antithrombin deficiency and thrombosis: a wide clinical scenario in a single institution. J Blood Med. 2023;14(1):499–506. doi:10.2147/JBM.S416355
- Croles FN, Borjas-Howard J, Nasserinejad K, Leebeek FW, Meijer K. Risk of venous thrombosis in antithrombin deficiency: a systematic review and Bayesian meta-analysis. Semin Thromb Hemost. 2018;44(4):315–326. doi:10.1055/s-0038-1625983
- Corral J, de la Morena-Barrio ME, Vicente V. The genetics of antithrombin. Thromb Res. 2018;169:23–29. doi:10.1016/j.thromres.2018.07.008
- Connors JM. Thrombophilia testing and venous thrombosis. NEJM 2017; 377(12):1177–1187. doi:10.1056/NEJMra1700365
- Losonczy H, Nagy A, Toth O, et al. Prevalence and clinical significance of single and combined inherited thrombophilias. Blood. 2005;106(11):1632. doi:10.1182/blood.V106.11.1632.1632
- Pabinger I, Vossen CY, Lang J, et al. Mortality and inherited thrombophilia: results from the European prospective cohort on thrombophilia. J Thromb Haemost. 2012;10(2):217–222. doi:10.1111/j.1538-7836.2011.04573.x
- Marco-Rico A, Marco-Vera P. Thrombotic antiphospholipid síndrome and direct oral anticoagulants: unmet needs and review of the literature. Semin Thromb Hemost. 2023;49(7):736–743. doi:10.1055/s-0043-1767728
- Weitz J, Eikelboom JW, Samama MM. New antithrombotic drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. CHEST. 2012;141(Suppl 2):e120S–e141S. doi:10.1378/chest.11-2294
- Castillo-Pérez M, Jerjes-Sánchez C, Rodríguez D, Paredes-Vázquez JG, Panneflek J, Vázquez-Guajardo M. Clinical outcomes of very elderly patients treated with ultrasound-assisted catheter-directed thrombolysis for pulmonary embolism: a systematic review. J Thromb Thrombolysis. 2021;52(1):260–271. doi:10.1007/s11239-021-02409-3
- Giri J, Sista AK, Weinberg I, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation. 2019;140(20):e774–e801. doi:10.1161/CIR.0000000000000707
- Sagris M, Tzoumas A, Kokkinidis DG, Korosoglou G, Lichtenberg M, Tzavellas G. Invasive and pharmacological treatment of deep vein thrombosis: a scoping review. Curr Pharm Des. 2022;28(10):778–786. doi:10.2174/1381612828666220418084339
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi:10.1016/j.chest.2015.11.026
- Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. doi:10.1002/14651858.CD002783.pub4
- Enden T, Haig Y, Klow NE, et al.; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomized controlled trial. Lancet. 2012;379(9810):31–38. doi:10.1016/S0140-6736(11)61753-4
- Farrokhi M, Khurshid M, Mohammadi S, et al. Comparison of ultrasound-accelerated versus conventional catheter-directed thrombolysis for deep vein thrombosis: a systematic review and meta-analysis. Vascular. 2022;30(2):365–374. doi:10.1177/17085381211010532
- Weinberg I, Vedantham S, Salter A, et al. ATTRACT Trial Investigators. Relationships between the use of pharmacomechanical catheter-directed thrombolysis, sonographic findings and clinical outcomes in patients with acute proximal DVT: results from the ATTRACT Multicenter Randomized Trial. Vasc Med. 2019;24(5):442–451. doi:10.1177/1358863X19862043
- Wang CN, Deng HR. Percutaneous endovenous intervention plus anticoagulation versus anticoagulation alone for treating patients with proximal deep vein thrombosis: a meta-analysis and systematic review. Ann Vasc Surg. 2018;49:39–48. doi:10.1016/j.avsg.2017.09.027
- Dela Morena Barrio ME, López-Gálvez R, Martínez-Martínez I, et al. Defects of splicing in antithrombin deficiency. Res Pract Thromb Haemost. 2017;1(2):216–222. doi:10.1002/rth2.12025
- Human Gene Mutation Database (HGMD); 2023. Available from: www.hgmd.cf.ac.uk. Accessed August 24, 2023.
- Larsen OH, Kjaergaard AD, Hvas AM, Nissen PH. Genetic variants in the protein S (PROS1) gene and protein S deficiency in a Danish population. TH Open. 2021;5(4):479–488. doi:10.1055/s-0041-1736636
- Dautaj A, Krasi G, Bushati V, et al. Hereditary thrombophilia. Acta Biomed. 2019;90(10–S):44–46. doi:10.23750/abm.v90i10-S.8758
- Harter K, Levine M, Henderson SO. Anticoagulation drug therapy: a review. West J Emerg Med. 2015;16(1):11–17. doi:10.5811/westjem.2014.12.22933
- Dilektasli AG, Demirdogen Cetinoglu E, Acet NA, et al. Catheter-directed therapy in acute pulmonary embolism with right ventricular dysfunction: a promising modality to provide early hemodynamic recovery. Med Sci Monit. 2016;22:1265–1273. doi:10.12659/MSM.897617
- Liu G, Zhao Z, Cui C, et al. Endovascular management of extensive lower extremity acute deep vein thrombosis with AngioJet rheolytic thrombectomy plus catheter-directed thrombolysis from contralateral femoral access. Phlebology. 2019;34(4):257–265. doi:10.1177/0268355518790407
- Khidder L, Gendron N, Mauge L. Inherited thrombophilia in the era of direct oral anticoagulants. Int J Mol Sci. 2022;23(3):1821. doi:10.3390/ijms23031821