Abstract
Isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) are key metabolic enzymes that convert isocitrate to α-ketoglutarate. IDH1/2 mutations define distinct subsets of cancers, including low-grade gliomas and secondary glioblastomas, chondrosarcomas, intrahepatic cholangiocarcinomas, and hematologic malignancies. Somatic point mutations in IDH1/2 confer a gain-of-function in cancer cells, resulting in the accumulation and secretion in vast excess of an oncometabolite, the D-2-hydroxyglutarate (D-2HG). Overproduction of D-2HG interferes with cellular metabolism and epigenetic regulation, contributing to oncogenesis. Indeed, high levels of D-2HG inhibit α-ketoglutarate-dependent dioxygenases, including histone and DNA demethylases, leading to histone and DNA hypermethylation and finally a block in cell differentiation. Furthermore, D-2HG is a biomarker suitable for the detection of IDH1/2 mutations at diagnosis and predictive of the clinical response. Finally, mutant-IDH1/2 enzymes inhibitors have entered clinical trials for patients with IDH1/2 mutations and represent a novel drug class for targeted therapy.
Introduction
Isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) are metabolic enzymes catalyzing the conversion of isocitrate to α-ketoglutarate (αKG), while reducing NADP to NADPH. Point mutations in IDH1/2 define distinct subsets of low-grade glioma and secondary glioblastoma (GBM), chondrosarcoma, intrahepatic cholangiocarcinomas, hematologic malignancies, as well as premalignant diseases and rare inherited metabolism disorders.Citation1–Citation9 Multiple preclinical models have provided evidence for the oncogenic potential of IDH1/2 mutations, which alter epigenetic regulation, cancer cell differentiation, and metabolism.Citation10–Citation14 Depending on the associated genomic aberrations and the cellular context, the oncogenic potential of IDH1/2 mutations ranges from an initiating event – promoting transformation – to a secondary oncogenic event conferring selective advantage to cancer cells. In vitro and in vivo preclinical studies have demonstrated that inhibition of IDH1/2-mutant enzymes decreases intracellular D-2-hydroxyglutarate (D-2HG) levels, reverses epigenetic dysregulation, and releases the differentiation block. These findings supported initiation of the ongoing clinical trials evaluating novel IDH1/2 inhibitors in IDH1/2-mutant cancers.
Normal functions of IDH enzymes
The IDH family of enzymes comprises three proteins located in the cytoplasm and peroxysomes (IDH1) and mitochondria (IDH2 and IDH3),Citation15,Citation16 which are involved in a number of cellular processes, including mitochondrial oxidative phosphorylation, glutamine metabolism, lipogenesis, glucose sensing, and regulation of cellular redox status.Citation17 IDH3 forms a heterotetrameric complex (two alpha, one beta, and one gamma subunit) catalyzing the NAD+-dependent conversion of isocitrate to αKG in the tricarboxylic acid cycle ().
Figure 1 Enzymatic activities of wild type and mutated IDH enzymes.
Abbreviations: αKG, alpha ketoglutarate; D-2HG, D-2-hydroxyglutarate; IDH, isocitrate dehydrogenase; DNA, deoxyribonucleic acid; mut, mutated; NAD, nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate; TCA cycle, tricarboxylic acid cycle.
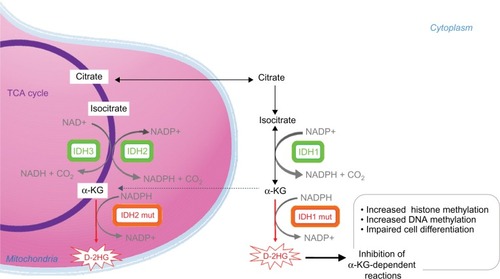
IDH1 and IDH2 are highly similar enzymes, forming homodimers and catalyzing the reversible NADP+-dependent oxidative decarboxylation of isocitrate to αKG (). NADPH is a key cellular reducing agent required for detoxification processes through reduction of glutathione and thioredoxins and activation of catalase, which are all involved in the protection against the toxicity of reactive oxygen species and oxidative DNA damage.Citation18 IDH1 is the main NADPH producer in the brain.Citation19
In specific cellular contexts such as hypoxia/pseudohypoxia and/or altered oxidative metabolism, bidirectional αKG metabolism along oxidative and reductive pathways can be activated,Citation20–Citation22 thereby allowing generation of isocitrate/citrate from αKG and glutamine. Recent evidence indicated that both IDH1 and IDH2 enzymes play fundamental roles in these alternative metabolic pathways.Citation22,Citation23 Reductive αKG/glutamine metabolism allows cancer cells to maintain pools of biosynthetic precursors and suppress mitochondrial reactive oxygen species, resulting in the sustainment of rapid rates of proliferation, even when oxidative metabolism is impaired.Citation24,Citation25
IDH mutants produce D-2HG and inhibit αKG-dependent dioxygenases
IDH1/2 mutations are heterozygous, missense mutations, leading to the substitution of the amino acids arginine 132 in IDH1 and arginine 172 or 140 in IDH2Citation2–Citation7 (). These residues play key role in substrate binding in the enzymes active site. Initial functional studies of IDH1/2-mutant cells revealed that IDH1/2 mutations decrease the ability of the mutant enzymes to convert isocitrate to αKG3 and that IDH1/2-mutant enzymes inhibit wild-type activity in a dominant-negative manner.Citation26 Further functional analysis demonstrated that IDH1/2-mutant enzymes gain neomorphic enzymatic activity, converting NADPH and αKG to NADP+ and D-2HG.Citation10,Citation27,Citation28 IDH1/2 mutant enzymes produce high levels of D-2HG in cells (50- to 100-fold higher than in normal tissues). 2HG is a chiral molecule that can exist as either a d-enantiomer or an l-enantiomer. IDH1/2 mutants exclusively produce D-2HG. Importantly, IDH1/2-mutant cells have normal αKG levels.Citation10,Citation28 In physiological condition, D-2HG intracellular concentration is low. D-2HG is not known to play any physiological metabolic role, and its production remains poorly understood.
Table 1 IDH mutations estimates in solid tumors and hematologic malignancies
IDH mutations are associated with epigenetic reprogramming, altering cancer cell differentiation
D-2HG and αKG are highly similar molecules, differing only by the presence of a C2 hydroxyl group in D-2HG instead of the C2 carbonyl of αKG. D-2HG can occupy the same binding pocket as α-KG and acts as a weak competitive inhibitor of αKG-dependent dioxygenases. αKG and Fe2+ are used as cofactors of the activity of >60 αKG-dependent dioxygenases,Citation12,Citation29 which are involved in a wide range of cellular processes such as hypoxia, angiogenesis, maturation of collagens of the extracellular matrix, and regulation of epigenetics. In vitro ectopic expression of IDH1/2 mutants produces high D-2HG levels that inhibit αKG-dependent dioxygenases, including histone demethylases Jumonji 2 (JMJD2) and Jmj C domain-containing histone demethylase-1 (JHDM1), and DNA demethylases ten-eleven translocation 2 (TET2) proteins, resulting in the impairment of key steps in histone and DNA demethylation. The epigenetic deregulation induced by IDH1/2 mutant enzymes translates into histone and DNA hypermethylation in a passage-dependent manner.Citation10–Citation13,Citation30–Citation35
Indeed, methylation profile of several human malignancies showed that IDH1/2-mutant tumors display a typical CpG island methylator phenotype characterized by high degree of DNA hypermethylation in CpG-rich domains. Hypermethylation is the dominant feature of IDH1/2-mutant acute myeloid leukemias (AMLs), and these mutants display similar DNA methylation profiles. Interestingly, TET2-mutant cells display an overlapping hypermethylation signature with IDH1/2-mutants cells.Citation11,Citation13 Gene expression profile of large cohorts of gliomas and AML has shown that IDH1/2-mutant tumors display a distinct gene expression profile enriched for genes expressed in progenitor cells.Citation11,Citation13,Citation14,Citation36–Citation38 Importantly, such wide epigenetic modifications were associated with altered expression of genes involved in cellular differentiation, thereby resulting in a block to cellular differentiation, which can be reversed by pharmacological inhibition of the mutant enzymes.Citation11,Citation13,Citation14,Citation39–Citation41 Consequently, as observed in TET2 mutants, increased expression of stem cell markers and impaired differentiation are seen in cells expressing IDH1/2 mutant enzymes.Citation11,Citation13,Citation42 Hypermethylation can also compromise the binding of methylation-sensitive insulator proteins, which may result in the loss of insulation between topological DNA domains and aberrant gene activation, as recently demonstrated in IDH1-mutant gliomasphere models.Citation43
Importantly, there is a correlation between intracellular concentrations of D-2HG and the epigenetic effects in IDH1/2-mutant tumors. Indeed, as D-2HG is a weak competitor of αKG,Citation12 the phenotype of immature cell is only observed when a high level of accumulation of D-2HG is reached.Citation44 Besides IDH1/2-mutant cancers, abnormal accumulation of D-2HG has been observed in D-2HG aciduria (D-2HGA), a rare inherited metabolic disorder characterized by extremely variable clinical presentations ranging from fatal neonatal encephalopathy and cardiomyopathy to asymptomatic cases. Mutations in D2HGDH and IDH2 are the molecular basis of this metabolic disorder, with a tendency to a more severe clinical phenotype in IDH2-mutant patients.Citation45 Interestingly, although D-2HG levels are excessively high in patients with D-2HGA, no cancers have been reported so far in this population, which suggests that D-2HG accumulation is not sufficient alone to induce cancer. Indeed, while expression of mutant IDH1/2 enzymes was associated with increased progenitor cell marker expression and impaired cell differentiation, such epigenetics effects were not sufficient alone to drive oncogenesis. This suggests that other molecular alterations including FLT3 or NRAS are required to promote full transformation of IDH1/2-mutant clones in AML models.Citation46,Citation47
Finally, other metabolites are known to inhibit αKG-dependent dioxygenases. For example, excess of succinate is observed in case of mutations affecting genes coding for succinate dehydrogenase. Similar to IDH1/2 mutations, such alterations have been linked with DNA hypermethylation, decreased expression of key genes involved in neuroendocrine differentiation, and a block in cellular differentiation.Citation48
IDH mutations are associated with metabolic reprogramming
Few investigations have questioned metabolic changes associated with IDH1/2 mutations. Recent metabolic flux analyses have shown that IDH1 mutations render tumor cells more dependent on mitochondrial oxidative tricarboxylic acid metabolism and compromise the conversion of glutamine to citrate and fatty acids under hypoxia as compared to IDH1-wild type cells.Citation49 Interestingly, such compromised metabolic reprogramming resulted in decreased cell growth of IDH1-mutant cells upon hypoxia. Of note, D-2HG inhibits both ATP synthase and mTOR signaling in glioma cells, resulting in growth arrest and cell death under conditions of glucose limitation.Citation50 Large-scale metabolic profiling of IDH1-mutant patient-derived glioma models showed that IDH1-mutant glioma cells display extreme vulnerability to depletion of the coenzyme nicotinamide adenine dinucleotide (NAD+),Citation51 an essential metabolite involved in several fundamental cellular processes such as energetic metabolism, regulation of transcription, DNA repair, cell cycle progression, and apoptosis. Overall, IDH1/2 mutations are likely associated with wide metabolic reprogramming. Further deciphering of alterations in cellular metabolism associated with IDH1/2 mutations should highlight novel opportunities for therapeutic intervention and drug development.
Spectrum of cancer types with IDH mutations
First identified in colorectal cancer, IDH1/2 mutations affecting IDH1 (R132) or IDH2 (R140, R172) are found across a broad spectrum of cancer types ().
Brain tumors
IDH1 mutations were described in 2008, in exome-sequencing studies of GBM (WHO grade IV astrocytoma).Citation2 Subsequent studies have shown that IDH1/2 mutations occur in a mutually exclusive manner in ~80% of WHO grade II/III oligodendrogliomas, astrocytomas, and oligoastrocytomas and secondary GBM (ie, GBM that had progressed from lower grade gliomas).Citation3,Citation52 Conversely, in primary GBM, IDH1/2 mutations are found in only 6% of patients, suggesting distinct mechanisms of tumorigenesis between GBM and lower grade gliomas.Citation3,Citation53 IDH1 R132H mutation represents 80% of all IDH mutations.Citation52 Rarely, other mutations are found affecting either IDH1 at Arg132 (including R132S, R132C, R132G, and R132L substitutions) or IDH2 at Arg172 (R172K most frequently; ). IDH1 R132H mutation can be diagnosed by immunohistochemistry or sequencing, while other mutations can be identified only by sequencing.Citation54
IDH-mutant gliomas represent a distinct subset of gliomas, with specific clinical and molecular characteristics.Citation36–Citation38,Citation55 In nearly all IDH1/2-mutant gliomas, the IDH1/2 mutation precedes other molecular alterations, suggesting that IDH1/2 mutation is an early causative event in the pathogenesis of this brain tumor subset.Citation37,Citation38,Citation55–Citation59 In line with this hypothesis, IDH1/2 mutation is the only molecular alteration that is almost constantly conserved at recurrence, and analysis of IDH1/2-mutant tumors showed that IDH1/2-mutant proteins are almost ubiquitously expressed in tumor cells.Citation56–Citation58,Citation60 Accordingly, the IDH1/2 mutation is often referred to as a “trunk” initiating event in the clonal evolutionary tree of IDH1/2-mutant gliomas. Secondary genetic alterations occurring during the evolution of IDH1/2-mutant gliomas are often referred as “lineage-defining events”, as TP53 and ATRX mutations characterize tumors of astrocytic lineage, whereas hTERT promoter mutation and 1p/19q codeletion are associated with oligodendroglial tumors. Thereafter, as the disease progresses toward more aggressive tumors, tumor cells often acquire “tertiary” oncogenic alterations, frequently involving cell cycle regulation and growth control pathways, resulting in more malignant behavior.Citation37,Citation38,Citation55,Citation57
Importantly, IDH1/2 mutations have been associated with prognostic and predictive values as biomarkers in gliomas, and assessment of the IDH1/2 status is being implemented in routine clinical practice for patients with primary brain tumors.Citation61 Natural history of IDH1/2-mutant glioma differs from the one of IDH1/2-wild type tumors. Recent studies have demonstrated that IDH1/2 mutations are associated with younger age, better prognosis, and better response to treatment. As an illustration, recent randomized Phase III trials have demonstrated that patients with IDH1/2-mutant gliomas had better overall survival after treatments with radiation therapy and chemotherapy.Citation62–Citation64 Among IDH1/2-mutant gliomas, patients with oligodendroglial tumors harboring 1p19q codeletion have the more favorable prognosis. The mechanisms underlying the relative chemo- and radiosensitive phenotypes associated with IDH1/2 mutations are not fully understood. Epigenetic silencing of the methyl-guanine methyl transferase (MGMT) – which encodes a DNA repair protein that counteracts the cytotoxic effect of alkylating agents – promoter gene by methylation is frequently observed in IDH1/2-mutant tumors and is associated with a partial inability of the tumor to repair the alkylating agent-induced DNA damage.
Hematologic malignancies
IDH1 (R132) or IDH2 (R140 and R172) mutations are found in myeloid malignancies, that is, myelodysplastic syndromes (MDS), AML, and myeloproliferative neoplasms, and also in angioimmunoblastic T-cell lymphoma (AITL).Citation4,Citation5,Citation65,Citation66 In myeloid malignancies, they are considered as an initiating event in 19% of patients with IDH1 mutations and 34% of patients with IDH2 mutations.Citation67 However, IDH1 mutations are likely to be implicated in early stages of de novo AML as others are, that is, NPM1, DNMT3A, TET2, and ASXL1.Citation68
In de novo AML, IDH1/2 mutations are associated with older age, normal karyotype, and NPM1 mutations. IDH1/2 and TET2 mutations are mutually exclusive.Citation69 IDH1 mutations are found in 6%–16% of de novo AML and are associated with a poorer outcome in patients treated with intensive chemotherapy,Citation70 even in patients with favorable prognosis as per European LeukemiaNet (ELN) classification.Citation71–Citation73
IDH2 mutations are found in 8%–19% of de novo AML. IDH2 R140Q mutation is the most frequent (75%–80%) whereas IDH2 R172K mutation is found in 20% of the cases. The prognostic impact of these mutations differs strongly. Depending on the mutational spectrum, IDH2 R140Q mutations confer favorable or no impact on overall survival.Citation4,Citation65,Citation67,Citation74,Citation75 Conversely, patients with IDH2 R172K mutation have a worse prognosis, with lower complete remission rate, higher relapse rate, and lower overall survival.Citation76,Citation77
IDH1/2 mutations are found in 4% to 12% of MDS cases, with a higher incidence (up to 23%) in high-risk MDS. IDH1/2 mutations are associated with an older age, DNMT3A, ASXL1, SRSF2 mutations, and higher rate of transformation to AML.Citation65,Citation78–Citation80
In myeloproliferative neoplasms, the incidence of IDH1/2 mutations range between 2% and 4%, rising up to 31% after transformation to AML. They are associated with older age and SRSF2 mutations. In patients with myelofibrosis, IDH1/2 mutations confer worse prognosis.Citation81
Besides myeloid neoplasms, IDH2 mutations (mostly R172K) are found in 20%–45% of patients with AITL although its prognostic value is not yet known.Citation66
Other malignancies associated with IDH mutations
IDH1/2 mutations have been detected in enchondromas and chondrosarcomas but rarely found in other mesenchymal tumors such as osteosarcomas. IDH1 mutations are the most frequent (40%–52%), and IDH2 mutations are present in 6%–11% of the cases.Citation7,Citation8
IDH1/2 mutations occur in up to 25% of intrahepatic cholangiocarcinomas. Again, IDH1 mutations are the most frequent (11%–24%) and IDH2 mutations are seen in 2%–6% of the cases.Citation6,Citation39,Citation82
Finally, sporadic cases of IDH1/2 mutations have been reported in other types of solid cancers: thyroid cancer, melanoma, prostate carcinoma, lung cancer, breast adenocarcinoma, colorectal cancer, esophageal cancer, and bladder cancer.Citation15,Citation16,Citation83
D-2HG as a predictive biomarker in IDH-mutant cancers
D-2HG released in the serum and/or urine by cancer cells harboring IDH1/2 mutations is a biomarker for IDH1/2 mutations, presumably reflecting the neomorphic enzymatic activity of the mutant enzymes. D-2HG levels are of interest for both the diagnosis and monitoring of patients with IDH1/2-mutant malignancies.Citation84–Citation89
At diagnosis, D-2HG is a strong predictive biomarker for the presence of IDH1/2 mutations in AML. High total 2HG concentration was highly predictive of the presence of an IDH1/2 mutation, although separation of the d- and l-enantiomers distinguished IDH1/2-mutant and -wild-type AML with greater specificity.Citation86,Citation87,Citation90
Increased serum and/or urine D-2HG levels predict IDH1/2 mutation in AML and intrahepatic cholangiocarcinoma and may be used as predictive biomarker for tumor response/recurrence. Prospective evaluation of D-2HG levels during the treatment of newly diagnosed AML treated with standard chemotherapy revealed that both D-2HG level and mutated-IDH1/2 allele burden decreased with response to treatment. Failure to normalize D-2HG levels is associated with treatment failure, whereas elevated D-2HG levels at complete remission are associated with poorer outcome, suggesting that D-2HG is a biomarker predictive of clinical response to intensive chemotherapy in AML patients with IDH1/2 mutations.Citation86,Citation87 On the opposite, D-2HG levels are in the normal range in patients with IDH-mutant gliomas,Citation91 suggesting that the brain–blood barrier prevents D-2HG from entering the circulation. Nevertheless, recent studies suggested that the urinary levels of D-2HG may increase the sensitivity and specificity for IDH1/2 mutation detection in glioma patients.Citation92,Citation93 Although the clinical value of noninvasive detection and monitoring of D-2HG levels has been well established in AML, the feasibility in glioma remains unclear. Most promising strategies are based on advanced imaging approaches, currently under investigation to determine their utility in clinical practice. Indeed, magnetic resonance spectroscopy (MRS) may detect and measure in vivo 2HG levels in patients harboring IDH1/2-mutant tumors.Citation94–Citation98 Recent pilot studies evaluating clinical applications of MRS in IDH1/2-mutant gliomas have shown that 2HG levels correlated with tumor volume and cellularity, and that cytotoxic therapy resulted in a decrease in 2HG levels, suggesting that MRS could assist as noninvasive tool for diagnosis and treatment follow-up.Citation96,Citation97
Targeting of IDH-mutant tumors
The discovery of IDH1/2 mutations has resulted in a number of novel therapeutic approaches (), which either restore normal IDH1/2 function or block production or downstream effects of D-2HG.
Table 2 Ongoing clinical trials evaluating IDH inhibitors
Hypomethylating agents
Hypomethylating agents (HMAs) may be of interest in the context of CpG island methylator phenotype induced by IDH1/2 mutations. Azacitidine and decitabine are DNA methyltransferase (DNMT) inhibitors that demonstrated significant clinical benefit not only in high-risk MDS but also in AML.Citation99–Citation101 The outcome of IDH1/2-mutant AML patients treated with HMAs has been retrospectively analyzed, although results are difficult to interpret due to the small number of patients (n=27). These series suggested the lack of association between IDH1/2 mutations and efficacy of HMAsCitation102,Citation103 or showed a better response to DNMT inhibitors among patients with IDH1/2-mutant AML.Citation104
In gliomas, recent preclinical studies have reported that treatment with HMAs reduces DNA methylation of promoter loci of genes involved in glial differentiation. Treatment with HMAs resulted in reduction in cell proliferation and tumor regression in patient-derived IDH1-mutant glioma xenograft models.Citation105,Citation106 These approaches are currently evaluated in early phase trials (NCT02223052 and NCT02332889).
IDH mutant enzymes inhibitors
Preclinical in vitro and in vivo studies have validated the proof of concept that targeted inhibition of IDH1/2 mutants resulted in normalization in a dose-dependent manner of 2-HG, reversal of histone and DNA hypermethylation, and release of cellular differentiation block.Citation39,Citation41,Citation107,Citation108 AGI-5198 and AGI-6780 are selective inhibitors of mutant IDH1 and IDH2 enzymes, respectively. They normalized 2HG, reversed histone and DNA hypermethylation, and induced differentiation of not only TF-1 erythroleukemia cells but also primary human AML cells harboring IDH1/2 mutations.Citation39,Citation41 AGI-5198 in a dose-dependent manner reduced 2HG and in turn was associated with tumor growth inhibition in vitro and in vivo. In IDH1-mutant glioma models, AGI-5198 induced expression of genes associated with astrocytic and oligodendrocytic differentiation and reduced repressive histone trimethylation marks at these gene promoters.Citation40 Together, these studies indicate that differentiation therapy may be achievable in cancers with IDH1/2 mutations, thereby supporting the initiation of clinical trials (). AG-120 and AG-221 are first-in-class, oral, potent, reversible, selective inhibitors of the IDH1 and IDH2 mutant enzymes, respectively. Separate first-in-human, Phase I, dose-escalation studies of AG-120 and AG-221 are underway in patients with IDH1/2-mutated hematologic malignancies (NCT02074839 and NCT01915498). Similar Phase I dose escalation studies of AG-120 in patients with IDH1-mutated gliomas and other solid tumors (NCT02073994) and of AG-221 in patients with IDH2-mutated gliomas, other solid tumors, and AITL (NCT02273739) are now open. The primary objective of these studies is to establish the safety and tolerability profile of AG-120 and AG-221, while secondary objectives are to characterize the pharmacokinetics, pharmacodynamics, and clinical efficacy. Preliminary unpublished clinical data from the ongoing Phase I trials for AG-120 and AG-221 indicates favorable safety profile, reduction of D-2HG levels, and finally encouraging response rate.Citation109–Citation111 These results support initiation of randomized Phase III and combination studies in AML ().
Immunotherapy
Recent studies have investigated vaccination-based immunotherapy to target IDH1 mutations. In principle, IDH1/2 mutants are ideal tumor-specific neoantigens due to their uniform occurrence at specific codons and ubiquitous expression throughout all tumor cells. Accordingly, recent preclinical studies showed that vaccination with IDH1 R132H-specific peptide elicited an MHC class II-specific antitumor response against IDH1 R132H-expressing tumor cells and reduced the growth of intracranial tumors.Citation112,Citation113 These preliminary results suggest that mutant IDH1-targeted immunotherapies can elicit potent antitumor immune responses. Clinical trials are ongoing to evaluate such strategies.
BCL-2 inhibition
Recent preclinical works in patient-derived models of IDH1/2-mutant AML have identified synthetic lethal interaction between the antiapoptotic gene BCL-2 and mutant-IDH1/2, showing that IDH1/2-mutant AML cells are more sensitive than their wild-type counterparts to the BCL-2 inhibitor ABT-199.Citation114 ABT-199 was further tested in AML patients in a Phase II trial that has enrolled 32 patients.Citation115 Interestingly, of the five patients who achieved complete remission with or without incomplete marrow recovery, three had IDH1/2 mutations. These preliminary data suggest that BCL-2 inhibition may be of interest in patients with IDH1/2-mutant AML.
Conclusion
The discovery of IDH1/2 mutations highlights the unique role of the “oncometabolite” D-2HG in oncogenesis. The druggable gain-of-function of the mutant enzymes has led to the generation of a new class of drugs. Relevant preclinical models and results of early Phase I trials in adults with hematologic malignancies demonstrate that targeting IDH1/2 mutant is a valid strategy. This is a new model of differentiation therapy that warrants combination strategies.
Disclosure
The authors report no conflicts of interest in this work.
References
- SjöblomTJonesSWoodLDThe consensus coding sequences of human breast and colorectal cancersScience2006314579726827416959974
- ParsonsDWJonesSZhangXAn integrated genomic analysis of human glioblastoma multiformeScience200832158971807181218772396
- YanHParsonsDWJinGIDH1 and IDH2 mutations in gliomasN Engl J Med2009360876577319228619
- MardisERDingLDoolingDJRecurring mutations found by sequencing an acute myeloid leukemia genomeN Engl J Med2009361111058106619657110
- KosmiderOGelsi-BoyerVSlamaLMutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasmsLeukemia20102451094109620376084
- BorgerDRTanabeKKFanKCFrequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotypingOncologist2012171727922180306
- AmaryMFDamatoSHalaiDOllier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2Nat Genet201143121262126522057236
- AmaryMFBacsiKMaggianiFIDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumoursJ Pathol2011224333434321598255
- KranendijkMStruysEASalomonsGSVan der KnaapMSJakobsCProgress in understanding 2-hydroxyglutaric aciduriasJ Inherit Metab Dis201235457158722391998
- DangLWhiteDWGrossSCancer-associated IDH1 mutations produce 2-hydroxyglutarateNature2009462727473974419935646
- FigueroaMEAbdel-WahabOLuCLeukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiationCancer Cell201018655356721130701
- XuWYangHLiuYOncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenasesCancer Cell2011191173021251613
- LuCWardPSKapoorGSIDH mutation impairs histone demethylation and results in a block to cell differentiationNature2012483739047447822343901
- TurcanSRohleDGoenkaAIDH1 mutation is sufficient to establish the glioma hypermethylator phenotypeNature2012483739047948322343889
- YenKEBittingerMASuSMFantinVRCancer-associated IDH mutations: biomarker and therapeutic opportunitiesOncogene201029496409641720972461
- CairnsRAMakTWOncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunitiesCancer Discov20133773074123796461
- ReitmanZJYanHIsocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolismJ Natl Cancer Inst20101021393294120513808
- LeeSMKohHJParkDCSongBJHuhTLParkJWCytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cellsFree Radic Biol Med200232111185119612031902
- BleekerFEAtaiNALambaSThe prognostic IDH1(R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastomaActa Neuropathol2010119448749420127344
- DeberardinisRJSayedNDitsworthDThompsonCBBrick by brick: metabolism and tumor cell growthCurr Opin Genet Dev2008181546118387799
- MullenARWheatonWWJinESReductive carboxylation supports growth in tumour cells with defective mitochondriaNature2011481738138538822101431
- MullenARHuZShiXOxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defectsCell Rep2014751679169024857658
- JiangLShestovAASwainPReductive carboxylation supports redox homeostasis during anchorage-independent growthNature2016532759825525827049945
- IcardPPoulainLLincetHUnderstanding the central role of citrate in the metabolism of cancer cellsBiochim Biophys Acta20121825111111622101401
- HillerKMetalloCMProfiling metabolic networks to study cancer metabolismCurr Opin Biotechnol2013241606823206561
- ZhaoSLinYXuWGlioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alphaScience2009324592426126519359588
- WardPSPatelJWiseDRThe common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarateCancer Cell201017322523420171147
- GrossSCairnsRAMindenMDCancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutationsJ Exp Med2010207233934420142433
- LoenarzCSchofieldCJExpanding chemical biology of 2-oxoglutarate oxygenasesNat Chem Biol20084315215618277970
- NgSSKavanaghKLMcDonoughMACrystal structures of histone demethylase JMJD2A reveal basis for substrate specificityNature20074487149879117589501
- ChowdhuryRYeohKKTianYMThe oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylasesEMBO Rep201112546346921460794
- TsukadaYFangJErdjument-BromageHHistone demethylation by a family of JmjC domain-containing proteinsNature2006439707881181616362057
- KohliRMZhangYTET enzymes, TDG and the dynamics of DNA demethylationNature2013502747247247924153300
- PastorWAAravindLRaoATETonic shift: biological roles of TET proteins in DNA demethylation and transcriptionNat Rev Mol Cell Biol201314634135623698584
- KoivunenPLeeSDuncanCGTransformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activationNature2012483739048448822343896
- NoushmehrHWeisenbergerDJDiefesKIdentification of a CpG island methylator phenotype that defines a distinct subgroup of gliomaCancer Cell201017551052220399149
- BratDJVerhaakRGAldapeKDComprehensive, integrative genomic analysis of diffuse lower-grade gliomasN Engl J Med2015372262481249826061751
- CeccarelliMBarthelFPMaltaTMMolecular profiling reveals biologically discrete subsets and pathways of progression in diffuse gliomaCell2016164355056326824661
- WangFTravinsJDeLaBarreBTargeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiationScience2013340613262262623558173
- RohleDPopovici-MullerJPalaskasNAn inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cellsScience2013340613262663023558169
- KernytskyAWangFHansenEIDH2 mutation-induced histone and DNA hypermethylation is progressively reversed by small-molecule inhibitionBlood2015125229630325398940
- LosmanJALooperREKoivunenP(R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversibleScience201333961271621162523393090
- FlavahanWADrierYLiauBBInsulator dysfunction and oncogene activation in IDH mutant gliomasNature2016529758411011426700815
- LuCVennetiSAkalinAInduction of sarcomas by mutant IDH2Genes Dev201327181986199824065766
- KranendijkMStruysEAvan SchaftingenEIDH2 mutations in patients with D-2-hydroxyglutaric aciduriaScience2010330600233620847235
- ChenCLiuYLuCCancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibitionGenes Dev201327181974198524065765
- KatsLMReschkeMTaulliRProto-oncogenic role of mutant IDH2 in leukemia initiation and maintenanceCell Stem Cell201414332934124440599
- LetouzéEMartinelliCLoriotCSDH mutations establish a hypermethylator phenotype in paragangliomaCancer Cell201323673975223707781
- GrassianARParkerSJDavidsonSMIDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolismCancer Res201474123317333124755473
- FuXChinRMVergnesL2-Hydroxyglutarate inhibits ATP synthase and mTOR signalingCell Metab201522350851526190651
- TateishiKWakimotoHIafrateAJExtreme vulnerability of IDH1 mutant cancers to NAD+ depletionCancer Cell201528677378426678339
- HartmannCMeyerJBalssJType and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomasActa Neuropathol2009118446947419554337
- BrennanCWVerhaakRGMcKennaAThe somatic genomic landscape of glioblastomaCell2013155246247724120142
- CapperDWeissertSBalssJCharacterization of R132H mutation-specific IDH1 antibody binding in brain tumorsBrain Pathol201020124525419903171
- BaiHHarmancıASErson-OmayEZIntegrated genomic characterization of IDH1-mutant glioma malignant progressionNat Genet2016481596626618343
- WatanabeTNobusawaSKleihuesPOhgakiHIDH1 mutations are early events in the development of astrocytomas and oligodendrogliomasAm J Pathol200917441149115319246647
- WakimotoHTanakaSCurryWTTargetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomasClin Cancer Res201420112898290924714777
- JohnsonBEMazorTHongCMutational analysis reveals the origin and therapy-driven evolution of recurrent gliomaScience2014343616718919324336570
- SuzukiHAokiKChibaKMutational landscape and clonal architecture in grade II and III gliomasNat Genet201547545846825848751
- LassUNümannAvon EckardsteinKClonal analysis in recurrent astrocytic, oligoastrocytic and oligodendroglial tumors implicates IDH1-mutation as common tumor initiating eventPLoS One201277e4129822844452
- StuppRBradaMvan den BentMJTonnJCPentheroudakisGGroupEGWHigh-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-upAnn Oncol201425suppl 3iii93iii10124782454
- van den BentMJBrandesAATaphoornMJAdjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951J Clin Oncol201331334435023071237
- CairncrossGWangMShawEPhase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402J Clin Oncol201331333734323071247
- CairncrossJGWangMJenkinsRBBenefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDHJ Clin Oncol201432878379024516018
- ImAPSehgalARCarrollMPDNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategiesLeukemia20142891774178324699305
- CairnsRAIqbalJLemonnierFIDH2 mutations are frequent in angioimmunoblastic T-cell lymphomaBlood201211981901190322215888
- MolenaarRJThotaSNagataYClinical and biological implications of ancestral and non-ancestral IDH1 and IDH2 mutations in myeloid neoplasmsLeukemia201529112134214225836588
- WelchJSLeyTJLinkDCThe origin and evolution of mutations in acute myeloid leukemiaCell2012150226427822817890
- SolaryEBernardOATefferiAFuksFVainchenkerWThe Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseasesLeukemia201428348549624220273
- FengJHGuoXPChenYYWangZJChengYPTangYMPrognostic significance of IDH1 mutations in acute myeloid leukemia: a meta-analysisAm J Blood Res20122425426423226625
- DöhnerHEsteyEHAmadoriSDiagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNetBlood2010115345347419880497
- MarcucciGMaharryKWuYZIDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a cancer and leukemia group B studyJ Clin Oncol201028142348235520368543
- PeterlinPRennevilleABen AbdelaliRImpact of additional genetic alterations on the outcome of patients with NPM1-mutated cytogenetically normal acute myeloid leukemiaHaematologica20151005e196e19925552703
- PatelJPGönenMFigueroaMEPrognostic relevance of integrated genetic profiling in acute myeloid leukemiaN Engl J Med2012366121079108922417203
- Abdel-WahabOPatelJLevineRLClinical implications of novel mutations in epigenetic modifiers in AMLHematol Oncol Clin North Am20112561119113322093580
- BoisselNNibourelORennevilleAHuchettePDombretHPreud-hommeCDifferential prognosis impact of IDH2 mutations in cytogenetically normal acute myeloid leukemiaBlood2011117133696369721454467
- GreenCLEvansCMZhaoLThe prognostic significance of IDH2 mutations in AML depends on the location of the mutationBlood2011118240941221596855
- LinCCHouHAChouWCIDH mutations are closely associated with mutations of DNMT3A, ASXL1 and SRSF2 in patients with myelodysplastic syndromes and are stable during disease evolutionAm J Hematol201489213714424115220
- PatnaikMMHansonCAHodnefieldJMDifferential prognostic effect of IDH1 versus IDH2 mutations in myelodysplastic syndromes: a Mayo Clinic study of 277 patientsLeukemia201226110110522033490
- TholFWeissingerEMKrauterJIDH1 mutations in patients with myelodysplastic syndromes are associated with an unfavorable prognosisHaematologica201095101668167420494930
- LashoTLJimmaTFinkeCMSRSF2 mutations in primary myelofibrosis: significant clustering with IDH mutations and independent association with inferior overall and leukemia-free survivalBlood2012120204168417122968464
- SahaSKParachoniakCAGhantaKSMutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancerNature2014513751611011425043045
- CeramiEGaoJDogrusozUThe cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics dataCancer Discov20122540140422588877
- RakhejaDKonoplevSMedeirosLJChenWIDH mutations in acute myeloid leukemiaHum Pathol201243101541155122917530
- FathiATSadrzadehHBorgerDRProspective serial evaluation of 2-hydroxyglutarate, during treatment of newly diagnosed acute myeloid leukemia, to assess disease activity and therapeutic responseBlood2012120234649465223074281
- DiNardoCDPropertKJLorenAWSerum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemiaBlood2013121244917492423641016
- JaninMMylonasESaadaVSerum 2-hydroxyglutarate production in IDH1- and IDH2-mutated de novo acute myeloid leukemia: a study by the Acute Leukemia French Association GroupJ Clin Oncol201432429730524344214
- BorgerDRGoyalLYauTCirculating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinomaClin Cancer Res20142071884189024478380
- WillekensCMicolJ-BPoinsignonVSerum 2-Hydroxyglutarate level can predict IDH2 mutation in myeloid sarcomaBlood20151262338353835
- DuranMKamerlingJPBakkerHDvan GennipAHWadmanSKL-2-Hydroxyglutaric aciduria: an inborn error of metabolism?J Inherit Metab Dis1980341091126787330
- CapperDSimonMLanghansCD2-Hydroxyglutarate concentration in serum from patients with gliomas does not correlate with IDH1/2 mutation status or tumor sizeInt J Cancer2012131376676821913188
- LombardiGCoronaGBelluLDiagnostic value of plasma and urinary 2-hydroxyglutarate to identify patients with isocitrate dehydrogenase-mutated gliomaOncologist201520556256725862748
- FathiATNahedBVWanderSAElevation of urinary 2-hydroxyglutarate in IDH-mutant gliomaOncologist201621221421926834160
- ChoiCGanjiSKDeBerardinisRJ2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomasNat Med201218462462922281806
- AndronesiOCKimGSGerstnerEDetection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopySci Transl Med20124116116ra4
- AndronesiOCLoebelFBognerWTreatment response assessment in IDH-mutant glioma patients by non-invasive 3D functional spectroscopic mapping of 2-hydroxyglutarateClin Cancer Res20152271632164126534967
- de la FuenteMIYoungRJRubelJIntegration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant gliomaNeuro Oncol201618228329026691210
- EmirUELarkinSJde PenningtonNNoninvasive quantification of 2-hydroxyglutarate in human gliomas with IDH1 and IDH2 mutationsCancer Res2016761434926669865
- FenauxPMuftiGJHellström-LindbergEAzacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemiaJ Clin Oncol201028456256920026804
- KantarjianHMThomasXGDmoszynskaAMulticenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemiaJ Clin Oncol201230212670267722689805
- DombretHSeymourJFButrymAInternational phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blastsBlood2015126329129925987659
- DiNardoCDPatelKPGarcia-ManeroGLack of association of IDH1, IDH2 and DNMT3A mutations with outcome in older patients with acute myeloid leukemia treated with hypomethylating agentsLeuk Lymphoma20145581925192924138309
- BentonCBRavandiFAndreeffMCase series of patients with acute myeloid leukemia receiving hypomethylation therapy and retrospectively found to have IDH1 or IDH2 mutationsLeuk Lymphoma20145561431143424033106
- EmadiAFaramandRCarter-CooperBPresence of isocitrate dehydrogenase mutations may predict clinical response to hypomethylating agents in patients with acute myeloid leukemiaAm J Hematol2015905E77E7925651001
- BorodovskyASalmasiVTurcanS5-azacytidine reduces methylation, promotes differentiation and induces tumor regression in a patient-derived IDH1 mutant glioma xenograftOncotarget20134101737174724077805
- TurcanSFabiusAWBorodovskyAEfficient induction of differentiation and growth inhibition in IDH1 mutant glioma cells by the DNMT Inhibitor DecitabineOncotarget20134101729173624077826
- HansenEQuivoronCStraleyKAG-120, an oral, selective, first-in-class, potent inhibitor of mutant IDH1, reduces intracellular 2HG and induces cellular differentiation in TF-1 R132H cells and primary human IDH1 mutant AML patient samples treated Ex VivoBlood20141242137343734
- QuivoronCDavidMStraleyKAG-221, an oral, selective, first-in-class, potent IDH2-R140Q mutant inhibitor, induces differentiation in a Xenotransplant ModelBlood20141242137353735
- SteinEMAltmanJKCollinsRAG-221, an oral, selective, first-in-class, potent inhibitor of the IDH2 mutant metabolic enzyme, induces durable remissions in a phase I study in patients with IDH2 mutation positive advanced hematologic malignanciesBlood201412421115115
- FanBLeKManyakELongitudinal pharmacokinetic/pharmacodynamic profile of AG-120, a potent inhibitor of the IDH1 mutant protein, in a phase 1 study of IDH1-mutant advanced hematologic malignanciesBlood20151262313101310
- DiNardoCde BottonSPollyeaDAMolecular profiling and relationship with clinical response in patients with IDH1 mutation-positive hematologic malignancies receiving AG-120, a first-in-class potent inhibitor of mutant IDH1, in addition to data from the completed dose escalation portion of the phase 1 studyBlood20151262313061306
- SchumacherTBunseLPuschSA vaccine targeting mutant IDH1 induces antitumour immunityNature2014512751432432725043048
- PellegattaSVallettaLCorbettaCEffective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial gliomaActa Neuropathol Commun20153425849072
- ChanSMThomasDCorces-ZimmermanMRIsocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemiaNat Med201521217818425599133
- KonoplevaMPollyeaDAPotluriJA phase 2 study of ABT-199 (GDC-0199) in patients with acute myelogenous leukemia (AML)Blood201412421118118
