Abstract
Platinum(IV) coordination complexes like oxoplatin (cis,cis,trans-diammine-dichlorido-dihydroxido-platinum[IV]) show high stability and therefore can be utilized orally for outpatient care. Although oxoplatin is capable of binding directly to DNA after prolonged incubation, platinum(IV) agents are considered to be largely inert prodrugs that are converted to highly cytotoxic platinum(II) compounds by reducing substances, enzymes, or microenviron-mental conditions. Reaction of oxoplatin with 0.1 M hydrogen chloride mimicking gastric acid yields cis-diammine-tetrachlorido-platinum(IV) (DATCP[IV]), which exhibits two-fold increased activity. The presence of chlorides as ligands in the axial position results in a high reduction potential that favors transformation to platinum(II) complexes. In this study, the intracellular effect of the highly reactive tetrachlorido derivative was investigated in comparison with an equipotent dose of cisplatin. Genome-wide expression profiling of NCI-H526 small cell lung cancer cells treated with these platinum species revealed clear differences in the expression pattern of affected genes and concerned cellular pathways between DATCP(IV) and cisplatin. Application of DATCP(IV) resulted in extensive downregulation of protein and ATP synthesis, cell cycle regulation, and glycolysis, in contrast to cisplatin, which preferentially targeted glutathione conjugation, pyruvate metabolism, citric acid cycle, and the metabolism of amino acids and a range of carbohydrates. Thus, the oxoplatin metabolite DATCP(IV) constitutes a potent cytotoxic derivative that may be produced by gastric acid or acidic areas prevailing in larger solid tumors, depending on the respective pharmaceutical formulation of oxoplatin. Furthermore, DATCP(IV) exhibits intracellular effects that are clearly different from the expected reduced product cisplatin(II). In conclusion, activation of the platinum(IV) complex oxoplatin seems to involve the generation of a cytotoxic six-coordinate species, dependent on prevailing conditions, and its effects need to be considered in addition to the effects of the potential final platinum(II) product.
Introduction
Cisplatin (cis-diammine-dichlorido-platinum[II]) was established as a drug that is active against a range of malignancies, including testicular, ovarian, head and neck, bladder, esophageal, and small cell lung cancer (SCLC).Citation1,Citation2 However, tumors like colon and breast cancer show limited sensitivity, and cisplatin-induced resistance and severe side effects are frequently observed.Citation3 Second-generation platinum(II)-based drugs include carboplatin, which has similar anticancer activity but fewer side effects than cisplatin, and oxaliplatin, which exhibits cytotoxicity against cisplatin-refractory cancer types like colorectal tumors.Citation4 In an attempt to develop platinum drugs with enhanced stability that are suitable for oral application, axial ligands were introduced, yielding platinum(IV) coordination complexes with increased kinetic inertness and reduced reactivity, resulting in decreased degradation in the bloodstream, lower toxicity, and partial efficacy in cisplatin-resistant tumor cell lines.Citation5,Citation6 Thus, pharmacokinetic properties of these agents can be fine-tuned by modification of the axial substituents. Satraplatin (bisacetato-ammine-dichlorido-cyclohexylamine-platinum[IV]; JM 216), an orally applicable cisplatin analog, constitutes one of the first third-generation platinum complexes that has undergone clinical trials with limited success.Citation7
Because it is generally accepted that reduction of the platinum(IV) central atom has to occur prior to binding to target DNA, these molecules are believed to represent prodrugs.Citation8,Citation9 The following reduction produces platinum(II) species that bind to DNA and lead to the formation of intra-and/or interstrand adducts, which results in cell cycle arrest in the G2M phase and cell death.Citation9,Citation10 Cellular reducing substances such as ascorbic acid and thiol-containing species like metallothioneins and glutathione are regarded as activators of platinum(IV) prodrugs.Citation11,Citation12
A further orally applicable platinum(IV) anticancer drug that is currently under development is oxoplatin, which was synthesized for the first time by Chugaev and Khlopin in the Russian Federation in 1927 ().Citation13 Its cytotoxic activity was not demonstrated in rat tumor models until 1977.Citation14 Presnov et al compared antitumor and pharmacokinetic properties of oxoplatin with those of cisplatin. Therapeutic and maximum tolerated doses were 10-fold higher for oxoplatin than for cisplatin. Additionally, oxoplatin exhibited a prolonged therapeutic effect, antimetastatic activity, and inhibition of tumor growth similar to, or even better than, cisplatin. Oxoplatin can bind directly to DNA; however, this process is so slow that it is of minimal biological relevance.Citation15 The in vitro cytotoxicity of oxoplatin and its possible activation by reduction through reaction with hydrogen chloride (HCl) and ascorbic acid were investigated in a previous study.Citation16 Because oxoplatin may represent a prodrug of cisplatin, the effects of both platinum drugs on gene expression patterns of a sensitive cell line were compared using microarrays for genome-wide expression analysis.Citation16
Figure 1 Chemical structures of the platinum compounds used or discussed (iproplatin and ormaplatin) in the present study. The full chemical formulas are cis, cis,trans-diammine-dichlorido-dihydroxido-platinum(IV) for oxoplatin, cis-diammine-tetrachlorido-platinum(IV) for DATCP(IV), cis-dichloro-trans-dihydroxy-bis-isop ropylamine-platinum(IV) for iproplatin, and tetrachloro-(D,L-trans)-l,2-diaminocyclohexane-platinum(IV) for ormaplatin, respectively.
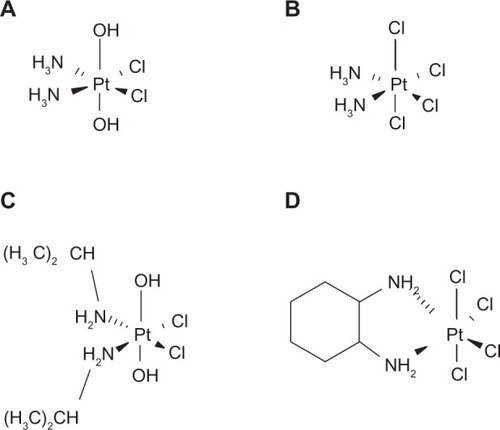
The antiproliferative activity of cisplatin was not affected by previous incubation with 0.1 M HCl; however, these highly acidic conditions resulted in two-fold enhanced cytotoxicity for oxoplatin due to its conversion to cis-diammine-tetrachlorido-platinum(IV) (DATCP[IV]) (). Similar platinum(IV) complexes, namely iproplatin and ormaplatin (also termed tetraplatin) (), had been investigated in clinical trials that were abandoned because of high toxicity of ormaplatin and low activity of iproplatin.Citation17,Citation18 Both agents are prodrugs that are converted to platinum(II) species with increased activity via reduction that takes place rapidly for ormaplatin and slowly for iproplatin.Citation19 A breakthrough was achieved with bis(carboxylato)-platinum(IV) analogs, showing reduction potentials situated between drugs with either chloride or hydroxide axial ligands.Citation20 Two bis(carboxylato)-platinum(IV) compounds, namely satraplatin and LA-12 (bis[acetato]-adamantylamine-[ammine]-dichlorido-platinum[IV]), have been investigated in clinical trials.Citation21 Satraplatin has two acetate moieties and needs to be hydrolyzed and subsequently reduced in order to exert an anticancer effect.
The presence of axial chloride or acetate ligands results in a slightly higher lipophilicity compared with the platinum(II) analog, whereas hydroxide substituents lead to significantly lower lipophilicity.Citation22,Citation23 According to these data, the tetrachlorido metabolite of oxoplatin, DATCP(IV), is expected to be reduced immediately in the cytoplasm and its actual cytotoxic effects to be caused by the main resulting reduction product cisplatin. In order to test this assumption, the effects of cisplatin and DATCP(IV) on global gene expression of the platinum-sensitive SCLC cell line NCI-H526 were investigated by microarrays in the present study.
Materials and methods
Chemicals and cell line
Unless otherwise noted, all chemicals and solutions were obtained from Sigma-Aldrich (St Louis, MO). Oxoplatin and DATCP(IV) were synthesized according to standard procedures by Chiracon (Luckenwalde, Germany) and kindly provided by IPSS (Berlin, Germany). The NCI-H526 cell line was obtained from the American Tissue Culture Collection (ATCC, Manassas, VA). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Seromed, Berlin, Germany), 4 mM glutamine, and antibiotics.
All compounds were prepared as stock solutions of 2 mg/mL in DMSO and aliquots stored at −20°C.
Cell proliferation assay
Cells were harvested, counted, and distributed into the wells of flat-bottomed 96-well microtiter plates at a density of 1 × 104 cells/well in 100 µL medium. A total of 100 µL of appropriate dilutions of test compounds were added to each well, and the plates were incubated under tissue culture conditions for 4 days. Stock solutions of the compounds were diluted more than 100-fold for use in assays. Solvent control wells were included in all tests. Dose-response curves were obtained by assessment of cell growth at twofold drug dilutions in triplicate and used for calculation of the IC50 values. Cell proliferation was quantified using a modified tetrazolium dye assay (MTT; EZ4U, Biomedica, Vienna, Austria).
Genome-wide gene expression analysis
Lysates of 30 × 106 cells (extraction buffer: 4 M guanidine isothiocyanate, 0.5% sodium N-lauroyl sarcosinate, 10 mM EDTA, 5 mM sodium citrate, 100 µM β-mercaptoethanol; 30 minutes, 4°C) were added to cesium trifluoroacetate and centrifuged (46,000 rpm, 15°C, 20 hours). Supernatant containing DNA was removed and RNA precipitated with ice-cold 96% ethanol. Pellets were washed and, following removal of ethanol, resuspended in sterile water. RNA content was measured photometrically.
Gene expression analysis was performed using the Applied Biosystems Human Genome Survey Microarray V2.0 (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Therefore, 2–5 µg mRNA (20–50 µg total RNA) was reversely transcribed (RT) to first-strand cDNA (MyCycler thermocycler, BioRad, Hercules, CA). The RT mixture was labeled on ice and purified according to the manufacturer’s instructions for the Applied Biosystems 1700 RT Labeling Kit. Hybridization of cDNA and microarray analysis (Applied Biosystems 1700) was carried out following the manufacturer’s chemiluminescence detection kit protocol. Data for each cell line (n = 2) were filtered, normalized, and log2-transformed, before further processing was carried out using Microsoft Excel software/SAM (false discovery rate of 10%; Statistical Analysis of Microarray, Stanford University, Stanford, CA). ABI 1700 gene identities can be accessed via the Panther classification system (www.pantherdb.org). ID mapping, pathway assignment, and over-representation analysis of cellular pathways were performed using the Reactome version 35 database (www.reactome.org).
Results
NCI-H526 SCLC cells were treated with either 4.1 µM cisplatin or 1.35 µM DATCP(IV) for 3 days, which resulted in cell cycle arrest but a cell viability of over 93% (data not shown). Under these conditions, decreases in mitochondrial activity were detectable on day 4 in chemosensitivity assays in the previous study.Citation16 Cells were harvested and counted and lysates prepared for genome-wide expression analysis. The 40 genes found to be either overexpressed or downregulated to the largest extent in treated NCI-H526 cell in response to cisplatin or DATCP(IV), respectively, in comparison with untreated medium controls, are listed in . These data demonstrate that the majority of genes are clearly differentially affected by the two compounds. The folate receptor 1 (FOLR1) is the gene that is upregulated by both platinum drugs. Similarly, analysis of the 40 most downregulated genes revealed no concordance.
Table 1 Alterations of gene expression in platinum drug-treated NCI-H526 cells. The 40 genes exhibiting highest down- or upregulated expression in treated NCI-H526 small cell lung cancer cells in response to cisplatin or DATCP(IV), respectively, compared with untreated cells (fold Δ: n-fold change in gene expression treated/untreated cells)
Because these gene expression differences in NCI-H526 cells may be quantitative rather than qualitative, all genes that were downregulated or upregulated more than four-fold, respectively, in response to one of the two platinum complexes were checked for over-representation in pathway analysis employing the Reactome database. In the case of cisplatin, over-represented pathways involving downregulated genes included glutathione conjugation, pyruvate metabolism, citric acid cycle, and cellular signal transduction, as well as metabolism of a range of carbohydrates and amino acids (). Corresponding upregulated pathways comprised metabolic regulation, energy metabolism, and distinct signaling cascades, including mediators like glucagon, phospholipase C (PLC), calmodulin (CaM), adenylate cyclase, and cAMP-responsive element binding protein (CREB). For DATCP(IV), downregulation of genes participating in protein synthesis and turnover, replication, transcription, respiration, cell cycle regulation, p53-dependent and -independent damage response, glycolysis/gluconeogenesis, and others were found (). Upregulated transcripts included those involved in metabolism of xenobiotics, metal ion transport, H-RAS activation, and cell junction organization. According to the Reactome database, with the single exception of the “metabolism of carbohydrates” (REACT_474/1383/1520) partially overlapping pathways, none of the processes mostly affected by either cisplatin and DATCP(IV) was the same, which indicates important differences in their mechanisms of cytotoxicity.
Table 2 Over-representation pathway analysis of genes more than four-fold down- or upregulated in NCI-H526 small cell lung cancer cells treated with cisplatin. Gene expression was assessed using Applied Biosystems Human Genome Survey Microarray V2.0, and data were analyzed using the Reactome database
Table 3 Over-representation pathway analysis of genes more than four-fold down- or upregulated in NCI-H526 small cell lung cancer cells treated with DATCP(IV). Gene expression was assessed using Applied Biosystems Human Genome Survey Microarray V2.0, and data were analyzed using the Reactome database
Discussion
The development of orally applicable platinum-based anticancer drugs is currently being intensively pursued in order to avoid intravenous administration, allowing for outpatient care.Citation1,Citation2 Among the first oral platinum coordination complexes established are picoplatin(II) and satraplatin(IV), which have shown promise in preclinical and clinical trials but have so far failed to gain approval.Citation4 Platinum(IV) compounds are considered prodrugs that are converted to their cytotoxically active platinum(II) forms primarily at the target site.Citation23 Oxoplatin is converted to platinum(II) species by intracellular-reducing agents such as ascorbic acid and glutathione. Furthermore, exposure to 0.1 M HCl, representing gastric acidity, resulted in two-fold increased antiproliferative activity.Citation16 Reduction/activation of oxoplatin at a low pH is an advantage for targeted release in the acidic microenvironment of solid tumors.
Although 40 years have passed since the discovery of the anticancer activity of cisplatin, the mechanism of action of platinum complexes is still unclear.Citation24 The question of whether platinum(IV) compounds have intrinsic activity or whether they serve as prodrugs that are reduced to platinum(II) molecules before reaching their DNA target remains to be resolved. Platinum(IV)–ammine complexes containing the chelating ligand 1,2-diaminocyclohexane combined with a variety of coordinating anions were found to react with 9-methylxanthine, 9-methylhypoxanthine, and guanosine-5′-monophosphate, providing evidence that not all platinum(IV) compounds represent prodrugs.Citation25 Oxoplatin is capable of forming DNA adducts in a rather slow process.Citation15 Oxoplatin was furthermore found to accumulate in tumor tissue, and metabolization resulted in the formation of several species, amongst them cisplatin, pointing to the role of oxoplatin as a prodrug of cisplatin; however, this hypothesis has not been validated so far. Oxoplatin reacted with 0.1 M HCl as well as DATCP(IV) yielded identical infrared spectra and cytotoxic effects.Citation16 The reduction potential of the platinum-based drugs depends mainly on the axial ligands, with chloride substituents reduced most easily, hydroxide groups most stable, and carboxylate ligands lying between the two extremes.Citation23,Citation26
According to the ATCC, the NCI-H526 cell line, originating from a bone metastasis of an SCLC patient prior to therapy, expresses neuron-specific enolase, brain enzyme of creatine kinase, and p53 mRNA. Comparison of the gene expression patterns of control and treated NCI-H526 cells revealed significant differences in the expression pattern of target genes for cisplatin and DATCP(IV). Cisplatin-downregulated transcripts are involved in glutathione conjugation, pyruvate metabolism and citric acid cycle, cell signal transduction and metabolism of a range of carbohydrates and amino acids, and upregulation of pathways employed in metabolic regulation, energy metabolism, and cell signaling in NCI-H526 cells, which point to a restricted and selective cellular response. In contrast, DATCP(IV) suppresses expression of a host of genes participating in many aspects of cellular processes like protein synthesis and turnover, replication, transcription, respiration, cell cycle regulation, p53-dependent and -independent damage response, and glycolysis/gluconeogenesis. Upregulated transcription of genes involved in the metabolism of xenobiotics and metal ion transport seems to be important in drug resistance. The finding of only one single overlapping pathway, namely “metabolism of carbohydrates and glycolysis/gluconeogenesis”, corroborates the fundamental differences in the mechanisms of cytotoxicity induced by the two platinum compounds and contradicts the exclusive role of DATCP(IV) as a prodrug of cisplatin.
All platinum(IV) complexes that have reached clinical trials thus far have yielded a platinum(II) central atom, reductively formed in vivo by endogenous molecules such as glutathione and ascorbate.Citation5 Unexpectedly, the extracellular reduction of ormaplatin was primarily accomplished by protein sulfhydryl groups but not by glutathione, predominantly leading to the expected cis-dichlorido-(D,L-trans)-1, 2-diamineplatinum(II) among other products, due to substitution of chloride ligands.Citation27 In clinical trials of ormaplatin, approximately 60% of the platinum in blood was bound to proteins (50% irreversibly) at the end of infusion, and the drug exhibited severe and unpredictable neurotoxicity.Citation17,Citation28–Citation31 Similarly, the reaction of iproplatin with glutathione yielded cis-di(isopropylamine) chlorido-glutathionato-platinum(II) and not the expected cis-dichlorido-species. Therefore, binding of one of the available coordination sites of this platinum(II) product to glutathione precludes the formation of bifunctional adducts with DNA.Citation29,Citation32 Reaction with cysteine-rich cellular proteins and zinc-finger transcription factors as well as disruption of protein-DNA complexes may represent alternative targets for this iproplatin metabolite.Citation33,Citation34 The intracellular fate of DATCP(IV) has not been clarified so far, but analogically to the platinum complexes ormaplatin and iproplatin as well as in accordance with the present study, DATCP(IV) seems to bind to a host of cellular proteins, which results in shutdown of their transcription, rather than conversion to free platinum(II) compounds and DNA damage preceding impairment of transcription.
Conclusion
Here we demonstrate that the effects of DATCP(IV) on global gene expression of an SCLC cell line differ fundamentally from those of cisplatin. It is concluded that the metabolite itself, or intracellular reaction products thereof, impair a host of important proteins, resulting in the shutdown of a whole panel of genes involved in pathways effecting metabolism of cellular constituents and energy production. Thus, our data suggest that this compound may act as an anticancer drug originally and not by serving only as a prodrug of cisplatin, as was previously deduced from its chemical properties, such as the reduction potentials of tetrachlorido-platinum(IV) complexes.Citation25
Acknowledgments
This study was supported by a fund from the Jubiläumsfonds (National Bank of Austria, Grant No. 13345). We thank Dr Zoser B Salama of IPSS, Berlin, Germany, for kindly providing the chemicals oxoplatin and DATCP(IV) as well as for helpful discussion.
Disclosure
The authors report no conflict of interest associated with this work.
References
- KostovaIPlatinum complexes as anticancer agentsRecent Patents Anti-Cancer Drug Discov200611122
- KellandLThe resurgence of platinum-based cancer chemotherapyNature Rev Cancer20077857358417625587
- WangDLippardSJCellular processing of platinum anticancer drugsNature Rev Drug Discov20054430732015789122
- OlszewskiUHamiltonGA better platinum-based anticancer drug yet to come?Anticancer Agents Med Chem201010429330120187870
- HallMDMellorHRCallaghanRHambleyTWBasis for design and development of platinum(IV) anticancer complexesJ Med Chemistry2007501534033411
- HallMDAlderdenRAZhangMThe fate of platinum(II) and platinum(IV) anti-cancer agents in cancer cells and tumoursJ Struct Biol2006155384416630726
- BhargavaAVaishampayanUNSatraplatin: leading the new generation of oral platinum agentsExpert Opin Investig Drugs2009181117871797
- HambleyTWBattleARDeaconGBModifying the properties of platinum(IV) complexes in order to increase biological effectivenessJ Inorg Biochem1999771–231210626347
- FoltinováVŠvihálková ŠindlerováLHorváthVMechanisms of effects of platinum (II) and (IV) complexes. Comparison of cisplatin and oxaliplatin with satraplatin and LA-12, new PT(IV)-based drugsScripta Medica Facultatis Medicae Universitatis Brunensis Masarykianae200881105116
- NakaiTAndoMOkamotoYModulation of oxidative DNA damage and DNA-crosslink formation induced by cis-diammine-tetrachloro-platinum(IV) in the presence of endogenous reductantsJ Inorg Biochem201110511521134595
- CuboLHambleyTWSanz MiguelPJThe preparation and characterization of trans-platinum(IV) complexes with unusually high cytotoxicityDalton Trans20114034434720936210
- CarrJLTingleMDMcKeageMJSatraplatin activation by haemoglobin, cytochrome C and liver microsomes in vitroCancer Chemother Pharmacol20065748349016172904
- KonovalovaALPresnovMAZheligovskaiaNNTreshchalinaEMAntitumor effect of complex compounds of tetravalent platinumDoklady Akademii Nauk SSSR19772341223226880861
- PresnovMAKonovalovaALKozlovAMThe antitumor activity of oxoplatinumNeoplasma198532173834039040
- NovákováOVránaOKiselevaVIBrabecVDNA interactions of antitumor platinum(IV) complexesEur J Biochem19952286166247737155
- OlszewskiUAchFUlspergerEIn vitro evaluation of oxoplatin: an oral platinum(IV) anticancer agentMet Based Drugs200934891619587824
- SchilderRJLaCretaFPPerezRPPhase I and pharmacokinetic study of ormaplatin (tetraplatin, NSC 363812) administered on a day 1 and day 8 scheduleCancer Res19945437097178306332
- TraskCSilverstoneAAshCMA randomized trial of carboplatin versus iproplatin in untreated advanced ovarian cancerJ Clin Oncol199197113111372045855
- ChoiSFilottoCBisanzoMReduction and anticancer activity of platinum(IV) complexesInorg Chem19983725002504
- PlattsJAErmondiGCaronGMolecular and statistical modeling of reduction peak potential and lipophilicity of platinum(IV) complexesJ Biol Inorg Chem201116336137221080205
- MontañaAMBatallaCThe rational design of anticancer platinum complexes: the importance of the structure-activity relationshipCurr Med Chem200916182235226019519389
- GalanskiMRecent developments in the field of anticancer platinum complexesRecent Pat Anticancer Drug Discov20061228529518221042
- HarperBWKrause-HeuerAMGrantMPAdvances in platinum chemotherapeuticsChemistry2010167064707720533453
- GibsonDThe mechanism of action of platinum anticancer agents: what do we really know about it?Dalton Trans200948106811068920023895
- TalmanEGKidaniYMohrmannLReedijkJCan Pt(IV)-amine complexes act as ‘prodrugs’?Inorg Chim Acta1998283251255
- ReithoferMRBytzekAKValiahdiSMTuning of lipophilicity and cytotoxic potency by structural variation of anticancer platinum(IV) complexesJ Inorg Biochem2011105465121134601
- ChaneySGGibbonsGRWyrickSDPodhaskyPAn unexpected biotransformation pathway for tetrachloro-(d,l-trans)-1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in the L1210 cell lineCancer Res1991519699731988140
- PendyalaLWalshJRHuqMMUptake and metabolism of iproplatin in murine L1210 cellsCancer Chemother Pharmacol19892515182590997
- PendyalaLArakaliAVSansonePDNA binding of iproplatin and its divalent metabolite cis-dichloro-bis-isopropylamine platinum (II)Cancer Chemother Pharmacol1990272482502265462
- McKeageMJBoxallFEJonesMHarrapKRLack of neurotoxicity of oral bisacetatoamminedichlorocyclohexylamine-platinum(IV) in comparison to cisplatin and tetraplatin in the ratCancer Res1994546296318306321
- O’RourkeTJWeissGRNewPPhase I clinical trial of ormaplatin (tetraplatin, NSC 363812)Anticancer Drugs1994555205267858283
- VolckovaEWeaverEBoseRNInsight into the reactive form of the anticancer agent iproplatinEur J Med Chem2008431081108417707553
- EastmanAGlutathione-mediated activation of anticancer platinum(IV) complexesBiochem Pharmacol19873623417741783689445
- KidoYKhokharARSiddikZHGlutathione-mediated modulation of tetraplatin activity against sensitive and resistant tumor cellsBiochemical Pharmacol19944716351642