Abstract
Background
Fibrotic diseases result from an exuberant response to chronic inflammation. Myelofibrosis is the end result of inflammation in bone, caused by an inflammatory process triggered by production of abnormal myeloid cells driven by mutations affecting the JAK–STAT pathway. Inflammatory cytokine overproduction leads to increased mesenchymal cell proliferation, culminating in fibrosis. Although JAK2 inhibitors, such as the JAK1/2 inhibitor ruxolitinib and the JAK2/FLT3/CSF1R/IRAK1 inhibitor pacritinib suppress abnormal clone expansion in myelofibrosis, ruxolitinib does not appear to prevent or reverse bone-marrow fibrosis in most patients. In two Phase III clinical trials, pacritinib, however, demonstrated improvements in platelet counts and hemoglobin and reductions in transfusion burden in some patients with baseline cytopenias, suggesting it may improve bone-marrow function. Unlike ruxolitinib, pacritinib suppresses signaling through IRAK1, a key control point for inflammatory and fibrotic signaling.
Purpose
To investigate potential antifibrotic effects of pacritinib in an animal model of liver fibrosis relevant to the observed course of human disease.
Methods
Pacritinib, negative control (vehicle), and positive control (the angiotensin 2-receptor antagonist and PPARγ partial agonist telmisartan) were assessed in the murine Stelic animal model, which mimics the clinically observed progression from hepatic steatosis to nonalcoholic steatohepatitis, liver fibrosis, and hepatocellular carcinoma. Histopathological analysis used hematoxylin and eosin staining. Body and liver weight changes, nonalcoholic fatty-liver disease activity scores, and plasma cytokeratin 18 fragment levels (a biomarker of hepatic necrosis) were measured.
Results
Pacritinib-treated mice had significantly (P<0.01) reduced fibrotic areas in liver compared to vehicle control and significantly (P<0.05) lower levels of CK18. The antifibrotic effect of pacritinib was comparable to that of telmisartan, but without significant effects on fat accumulation.
Conclusion
These results, the first to demonstrate hepatic antifibrotic effects for pacritinib in an animal model of liver disease, provide preliminary support for potential clinical applications of pacritinib in fibrotic diseases other than myelofibrosis.
Introduction
Fibrosis is the end product of chronic inflammation, and has aptly been called “a wound-healing response that has gone out of control.”Citation1 Common to all fibrotic diseases are activation and proliferation of endothelial cells and fibroblasts in response to inflammation induced by Toll-like receptors that activate IRAK1 causing downstream inflammatory and profibrotic cytokines, including TGFβ. Fibroblasts subsequently differentiate into myofibroblasts and secrete increasing levels of extracellular matrix proteins including collagens.Citation2 This ultimately results in replacement of normal tissues with fibrotic tissue, along with attendant disruption in function that may be limited to single organs (eg, liver fibrosis, pulmonary fibrosis) or involve a systemic process, such as scleroderma.Citation3
Myelofibrosis (MF) is a clonal hematopoietic neoplasm originating at the level of multipotential hematopoietic stem cells characterized by an inflammatory response in the bone marrow, leading to progressive fibrosis that impairs normal marrow function, resulting in cytopenias and extramedullary hematopoiesis.Citation4 A hallmark of bone-marrow response to MF cells is vascular proliferationCitation5 and increasing reticulin and collagen fibrosis in the bone marrow associated, with marked elevation in inflammatory cytokines, including TGFβ.Citation6 A central role for JAK–STAT signaling in the pathogenesis of myeloproliferative neoplasms, such as MF, was suggested by the discovery that a gain-of-function mutation in the JAK2 gene (JAK2V617F) was found in 50%–60% of patients with MF.Citation7–Citation9 In light of the unmet need for the treatment of patients with MF, inhibitors of JAK2 have been extensively studied in the clinic.
Ruxolitinib is an oral JAK1/2-kinase inhibitor that has been approved by the US Food and Drug Administration for patients with intermediate- or high-risk MF and those with polycythemia vera who have had inadequate response to or are intolerant of hydroxyurea.Citation10 Ruxolitinib has been shown to result in potent reductions in inflammatory cytokine-expression levels in patients with MF,Citation11 and its approval in MF was based on data from studies that demonstrated only modest reductions in bone-marrow fibrosis in a fraction of treated patients (16%), with fibrosis unchanged (32%) or progressing (19%) in a higher percentage of patients.Citation12–Citation15 Therefore, effective antifibrotic strategies in MF remain an unmet need upon which translational research continues to focus.
Pacritinib is an oral inhibitor of JAK2, FLT3, CSF1R, and IRAK1Citation16–Citation18 that has demonstrated activity in clinical studies of patients with MF and other myeloproliferative neoplasms.Citation19–Citation22 In Phase II studies, pacritinib was associated with reduction in spleen volume and improvement in symptoms in patients with MFCitation20 and myeloid malignanciesCitation22 without substantial limiting myelosuppression. In the randomized Phase III PERSIST-1Citation23 and PERSIST-2Citation20 studies of pacritinib versus best available therapy in patients with MF, improved hematopoietic function (eg, increased platelet count, increased hemoglobin, reduced transfusion burden) was noted in patients with baseline cytopenias, suggesting restoration in marrow function possibly due to an effect in part on bone-marrow fibrosis.
The kinase profile of pacritinib was evaluated in a kinome-wide screening study and demonstrated – in addition to its effect on JAK2 – that it potently inhibits phosphorylation of IRAK1 at an IC50 <20 nM.Citation18 IRAK1 is a kinase situated at a critical juncture of inflammatory signaling from Toll-like receptors and cellular signaling by IL1 leading to downstream activation of both p38 MAPK and p-ERK. The biomarker profile of pacritinib was determined in a panel of human primary cell-based systems designed to model various disease states, including inflammation and fibrosis.Citation24 At physiologically relevant levels in a system modeling T-cell-dependent B-cell activation, pacritinib robustly reduced levels of the proinflammatory cytokines soluble IL6 (sIL6), TNFα, sIL17A, and sIL17F. Along with TGFβ, IL6 is crucial to promoting TH17-cell differentiation.Citation25 These cells in turn secrete IL17A and IL17F, which promote neutrophil recruitment and infiltration, leading to inflammation and fibrosis.Citation26,Citation27 IL17 also stimulates macrophages to produce proinflammatory cytokines.Citation28 In addition to its effects on biomarkers, pacritinib has been found to have an antiproliferative effect on endothelial cells and fibroblasts.Citation24 Inhibition of several key kinases involved in the elaboration of proinflammatory cytokines, particularly IL17A, and the noted antiproliferative effects provide a strong rationale for examining whether pacritinib is able to modify the fibrotic process in MF. Since clinical assessment of this hypothesis requires invasive procedures in clinical trials of extended duration, this was tested in a standard, well-characterized preclinical model of a fibrotic disease.
Liver fibrosis is a response to chronic hepatocyte injuryCitation29 that may progress to end-stage cirrhosis.Citation30,Citation31 Although it is potentially reversible,Citation32 there are currently no approved therapies that have been shown to modify the course of liver fibrosis. Numerous rodent models have been developed in which liver injury is induced by trauma, toxins, or chronic infections.Citation33–Citation36 None of these, however, recapitulate the natural history commonly seen in patients with nonalcoholic (NA) liver disease. Recently, the Stelic animal model (STAM) was introduced.Citation37 In this model, designed to replicate the clinically observed, insulin-resistance-driven progression from hepatic steatosis to NA steatohepatitis (NASH), liver fibrosis, and hepatocellular carcinoma, neonatal male mice are treated with a carefully titrated dose of streptozotocin, followed by feeding with a high-fat diet. Fatty liver (FL) is induced by 5 weeks and NASH by 6 weeks in 100% of the animals. Here, we report the effects of pacritinib in the STAM mouse model, compare these with effects of telmisartan, an angiotensin 2-receptor antagonist and PPARγ partial agonist with known antifibrotic effects in rodent models, and present hypotheses on the mechanisms potentially involved.
Methods
Animal model
The STAM mouse model has been described previously.Citation37 Two-day old male C57BL/6J mice (Japan SLC, Tokyo, Japan) were given a single 200 μg subcutaneous dose of streptozotocin. At 4 weeks of age, they were fed a sterilized high-fat diet (HFD32; CLEA, Tokyo, Japan). The sterilized high-fat diet and water were provided ad libitum. Animals were housed and cared for in accordance with the Japanese Pharmacology Society Guidelines for Animal Use,Citation38 and the study was approved by the SMC Laboratories institutional animal care and use committee. They were maintained in a specific-pathogen-free facility under controlled temperature, humidity, lighting, and air exchange. Pacritinib (CTI Bio-Pharma, Seattle, WA, USA) was suspended in vehicle consisting of 0.5% weight/volume methylcellulose (4,000 cP) and 0.1% volume/volume Tween 80. Telmisartan (Boehringer Ingelheim, Ingelheim am Rhein, Germany), the positive control, was dissolved in pure water. Groups consisted of eight mice each. The treatment schedule for the vehicle (negative control), pacritinib, and telmisartan is shown in . Body weight was recorded prior to treatment and then daily. Mice were observed for signs of toxicity, moribundity, and mortality for approximately 60 minutes after each administration. Animals were killed by exsanguination through direct cardiac puncture under ether anesthesia at 9 weeks of age.
Table 1 Treatment schedule for all groups during weeks 6–9
Histopathological analysis
For hematoxylin and eosin (H&E) staining, sections were cut from paraffin blocks of liver tissue prefixed in Bouin solution and stained with Mayer’s hematoxylin (Lillie’s modification; Muto Pure Chemicals, Tokyo, Japan) and eosin (Wako Pure Chemicals Industries, Osaka, Japan) solution. NA fatty-liver disease (NAFLD) activity score was calculated according to the criteria of Kleiner et al.Citation39
Plasma cytokeratin 18 fragment levels
Plasma CK18 (M30) level was quantified using the Mouse Cytokeratin 18-M30 enzyme-linked immunosorbent-assay kit (Cusabio, College Park, MD, USA) as per label instructions. Briefly, plasma (1:500 in sample diluent) was added to the wells and incubated at 37°C for 2 hours. After removal of liquid, biotin antibody was added and wells incubated at 37°C for 1 hour, then washed with buffer three times and incubated with 100 μL of HRP–avidin for 1 hour. Enzymatic activity was detected using tetramethylbenzidine substrate and absorbance measured at 450 nm after a 30-minute incubation.
Statistical tests
Statistical analyses were performed using Bonferroni multiple-comparison tests on GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Results are expressed as the means ± SD.
Results
Deaths
Deaths prior to day 21 in the treatment groups were none in the vehicle group, one in the telmisartan group (day 10) and three in the pacritinib group (days 5, 7, and 10). For animals in the pacritinib group, severe weight loss was noted prior to death, but pathology results did not reveal any unusual findings (eg, vascular occlusion or cerebral bleeding). Based on these results, the 200 mg/kg pacritinib dose was deemed intolerable and mice treated with 150 mg/kg thereafter (). No further deaths occurred.
Weight changes
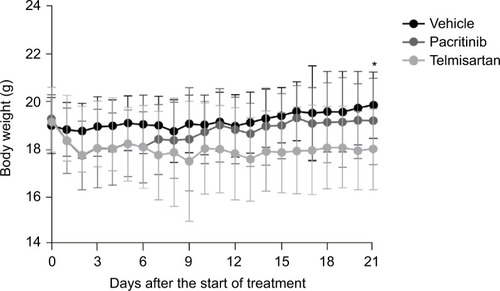
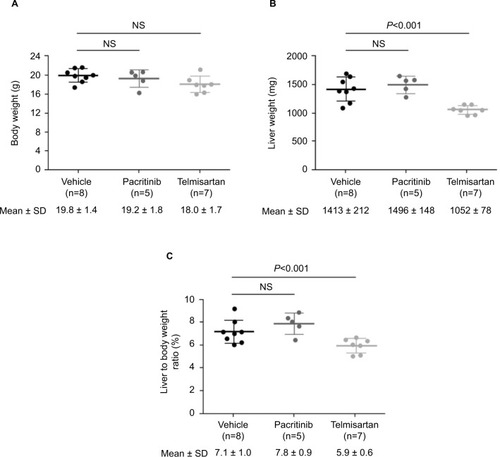
There was no significant difference in mean body weight between the vehicle and pacritinib groups on any day. The mean body weight of the positive control (telmisartan) group was significantly lower than that of the vehicle group on day 21, as assessed by the two-way analysis of variance (ANOVA) Bonferroni test (). On the necropsy day (day 0 of week 9), there were no significant differences in mean body weight among any of the treatment groups, as assessed by the one-way ANOVA Bonferroni test (). The telmisartan group had significantly decreased mean liver weight (P<0.001) and mean liver:body weight ratio (P<0.05) compared with the vehicle group (, and ), and there were no significant differences between the pacritinib and vehicle groups.
Histological analysis
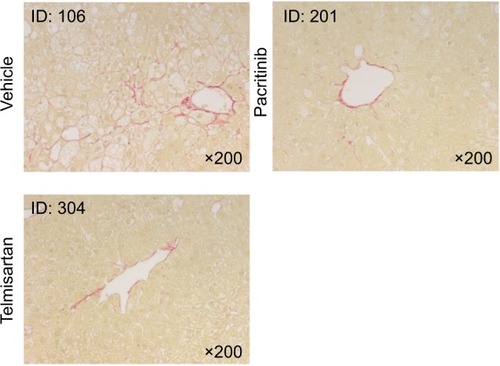
Representative micrography of the H&E-stained liver sections is shown in . Liver sections from the vehicle group exhibited severe micro- and macrovesicular fat deposition, hepatocellular ballooning, and inflammatory cell infiltration. The telmisartan group showed a significant (P<0.01) reduction in NAFLD-activity score compared with the vehicle group (, ). Although a trend toward lower steatosis and hepatocyte-ballooning scores were noted in the pacritinib group relative to the vehicle group, the difference in NAFLD score between the two groups was not significant. Representative micrography of Sirius red-stained sections of liver is shown in . Liver sections from the vehicle group exhibited collagen deposition in the pericentral region of liver lobules. Both the pacritinib and the telmisartan groups showed significant (P<0.01 for pacritinib, P<0.001 for telmisartan) decreases in the mean percentage of fibrosis area compared with the vehicle control group (0.74%±0.17%, 0.73%±0.12%, and 1.08%±0.16%, respectively).
Figure 3 Representative micrography of H&E-stained liver sections on day of death.
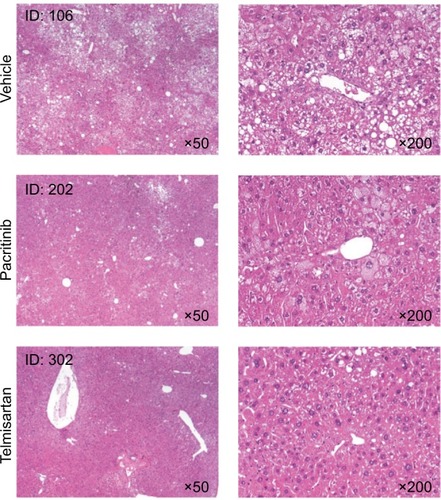
Figure 4 NAFLD-activity scores for all groups on day of death.
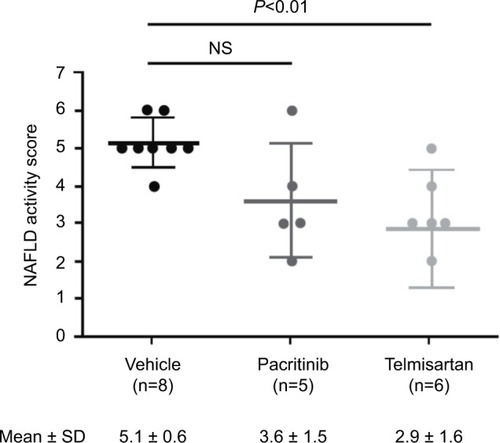
Table 2 Nonalcoholic fatty-liver disease-activity scoreCitation39
Plasma CK18 levels
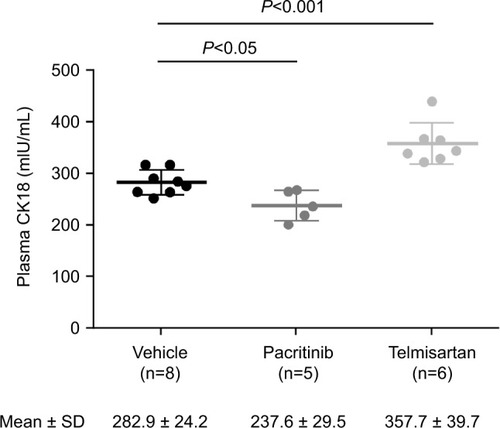
On the day of death, mean plasma CK18 M30 levels were 282.9±24.2, 237.6±29.5, and 357.7±39.7 mIU/mL for vehicle, pacritinib, and telmisartan, respectively (). Relative to vehicle (negative control) CK18 M30 levels were significantly lower (P<0.05) in the pacritinib group and significantly higher (P<0.001) in the telmisartan group.
Discussion
Pharmacological inhibition of JAK2 has previously been reported to attenuate liver fibrosis in rodent models, although the mechanism has not been fully elucidated.Citation40 The observation that pacritinib reduces levels of sIL6, sIL17A, and sIL17F in a system of peripheral blood mononuclear cells, fibroblasts, and endothelial cells that models T-cell-dependent B-cell activationCitation24 suggests a number of potential intervention points in addition to JAK2 signaling through which it could exert an anti-inflammatory and/or antifibrotic effect ().
Figure 7 Inflammation pathways and potential pacritinib intervention points in fibrosis.
Abbreviations: Pac, pacritinib; PBMCs, peripheral blood mononuclear cells; ECs, endothelial cells.
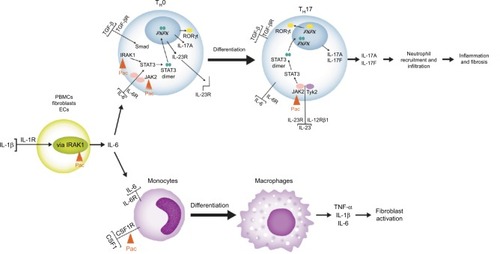
IRAK1 is a serine/threonine kinase that plays a key role in the IL1–IL6 axisCitation41 and has a major role in steatosis associated liver cirrhosis and progression to hepatocellular carcinoma.Citation42 Knockdown of IRAK1 in human hepatic stellate cells (HSCs) results in reductions in the release of inflammatory cytokines associated with local inflammation and promotion of fibrosis.Citation43 IRAK1 is critical to signaling by Toll-like receptors activated by fatty acids and other lipid derivatives, and appears to be central to lipid-mediated inflammation.Citation44,Citation45 In mouse models of acute and chronic inflammation, IRAK1 deletion dampens inflammatory responses by disfavoring naïve T-cell differentiation into TH17 cells, thereby decreasing levels of IL17, the proinflammatory cytokine that plays a pivotal role in HSC activation, which gives rise tô90% of myofibroblasts in liver-fibrosis models.Citation46–Citation48 Therefore, inhibition of IRAK1 by pacritinib may underlie the observed reduction in IL6 levels, causing a consequent depletion of TH17 cells, IL17A, and IL17F.
Pacritinib may also inhibit TH17-cell differentiation through effects on the transcription factor RORC (RORγt in mice), induction of which depends on full activation of STAT3 in processes dependent upon IRAK1 and JAK2, both of which pacritinib inhibits. Following differentiation, JAK2 associated with the IL23/IL12Rβ1 receptor plays a role in increased IL17A transcription, thus representing another possible intervention point for pacritinib. Finally, pacritinib also inhibits CSF1R kinase, thereby disfavoring the differentiation of monocytes to macrophages,Citation49 which promote myofibroblast survival and contribute to the development of liver fibrosis.Citation50,Citation51 Notably, controlling macrophage differentiation as an antifibrotic strategy in MF via a different pathway (using recombinant human pentraxin 2) is the subject of ongoing clinical investigation.Citation52
The present study investigated whether pacritinib, acting through one or more of these mechanisms, could exert antifibrotic effects in a mouse model that recapitulated the clinical progression commonly seen in human liver disease. In the STAM mouse model, pacritinib had no significant effect on body weight, liver weight, liver:body weight ratio, or NAFLD score relative to vehicle. As such, it did not significantly affect fat accumulation, the inflammatory trigger for liver fibrosis. Nonetheless, it significantly reduced fibrotic area, suggesting inhibition of the inflammatory and subsequent fibrotic response to steatosis. In the same assay, telmisartan, an angiotensin 2 receptor antagonist and PPARγ partial agonist that has demonstrated antifibroticCitation53 and hepatoprotectiveCitation54 activity in rodent models, most likely through downregulation of TGFβ and suppression of HSC activation,Citation55,Citation56 was used as a positive control. In contrast to pacritinib, telmisartan had significant effects on liver weight, liver:body weight ratio, and NAFLD score, in addition to fibrosis area. These results are consistent with a clinical study that reported significantly improved NAFLD and fibrosis scores for telmisartan plus lifestyle modifications relative to lifestyle modifications alone in human patients with NASH.Citation57 Differential effects of pacritinib and telmisartan in the STAM model likely reflect the additional mechanism of action, PPARγ partial agonism, associated with telmisartan. This has effects on hepatic fatty oxidation, hepatic lipogenesis, and peripheral as well as hepatic insulin sensitivity.Citation58 Finally, the present study examined levels of circulating CK18 fragment in all three groups of animals. Plasma CK18 fragment levels represent a biomarker of the extent of hepatocyte apoptosis, with increased levels predicting clinically observed liver fibrosis,Citation59 NASH occurrence, and NASH severity.Citation60 CK18 levels were significantly reduced relative to vehicle control in animals treated with pacritinib, a finding in line with the significantly reduced extent of liver fibrosis observed by histopathology in this group.
The present pilot translational study has several limitations. A relatively small number of animals was tested, and biomarkers that could link the observed activity of pacritinib to the proposed mechanisms of action were not examined. Further studies are needed to elucidate the pharmacological basis for the effects of pacritinib in liver fibrosis. Bearing these caveats in mind, this is the first study to demonstrate hepatic antifibrotic effects for pacritinib in a nonclinical model of liver disease.
The results of this study lend support to longitudinal assessment of the effect of pacritinib on marrow fibrosis in patients with MF enrolled in upcoming clinical trials, and moreover provide preliminary support to pilot clinical development in liver cirrhosis, along with other fibrotic conditions, such as pulmonary fibrosis and scleroderma.
Acknowledgments
Medical writing and editorial assistance was funded by CTI BioPharma and provided under the direction of the authors by Nexus Global Group Science.
Disclosure
SAF and JWS are employed by and have equity ownership in CTI BioPharma. TH and YS are employed by SMC Laboratories. JM has received research funding paid to his institution from Incyte, Roche, Promedior, CTI BioPharma, KaloBios, and Novartis, and has consulted for Incyte and Novartis.
References
- WynnTACommon and unique mechanisms regulate fibrosis in various fibroproliferative diseasesJ Clin Invest2007117352452917332879
- AkhmetshinaAPalumboKDeesCActivation of canonical Wnt signalling is required for TGF-β-mediated fibrosisNat Commun2012373522415826
- GabrielliAAvvedimentoEVKriegTSclerodermaN Engl J Med2009360191989200319420368
- ZahrAASalamaMECarreauNBone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategiesHaematologica2016101666067127252511
- ThieleJRompcikVWagnerSFischerRVascular architecture and collagen type IV in primary myelofibrosis and polycythaemia vera: an immunomorphometric study on trephine biopsies of the bone marrowBr J Haematol19928022272341550781
- AgarwalAMorroneKBartensteinMZhaoZJVermaAGoelSBone marrow fibrosis in primary myelofibrosis: pathogenic mechanisms and the role of TGF-βStem Cell Investig201635
- BaxterEJScottLMCampbellPJAcquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disordersLancet200536594641054106115781101
- KralovicsRPassamontiFBuserASA gain-of-function mutation of JAK2 in myeloproliferative disordersN Engl J Med2005352171779179015858187
- LevineRLWadleighMCoolsJActivating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosisCancer Cell20057438739715837627
- Jakafi [package insert]Wilmington (DE)Incyte Corporation2016
- VerstovsekSKantarjianHMesaRASafety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosisN Engl J Med2010363121117112720843246
- HarrisonCKiladjianJJAl-AliHKJAK inhibition with ruxolitinib versus best available therapy for myelofibrosisN Engl J Med2012366978779822375970
- VerstovsekSMesaRAGotlibJA double-blind, placebo-controlled trial of ruxolitinib for myelofibrosisN Engl J Med2012366979980722375971
- HarrisonCNVannucchiAMKiladjianJJLong-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosisLeukemia20163081701170727211272
- VerstovsekSMesaRAGotlibJLong-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trialJ Hematol Oncol20171015528228106
- HartSGohKCNovotny-DiermayrVSB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignanciesLeukemia201125111751175921691275
- HatzimichaelETsolasEBriasoulisEProfile of pacritinib and its potential in the treatment of hematologic disordersJ Blood Med2014514315225170285
- SingerJWAl-FayoumiSMaHKomrokjiRSMesaRVerstovsekSComprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitorJ Exp Pharmacol20168111927574472
- KomrokjiRSSeymourJFRobertsAWResults of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosisBlood2015125172649265525762180
- MascarenhasJHoffmanRTalpazMResults of the PERSIST-2 phase 3 study of pacritinib (Pac) versus best available therapy (BAT), including ruxolitinib (Rux), in patients (pts) with myelofibrosis (MF) and platelet counts<100,000/μLBlood201612822LBA5
- MesaRAEgyedMSzokeASuvorovAPerkinsAMayerJPacritinib (PAC) vs best available therapy (BAT) in myelofibrosis (MF): 60-week follow-up of the phase III PERSIST-1 trialJ Clin Oncol20163415 Suppl7065
- VerstovsekSOdenikeOSingerJWGranstonTAl-FayoumiSDeegHJPhase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignanciesJ Hematol Oncol2016913727931243
- MesaRAVannucchiAMMeadAPacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trialLancet Haematol201745e225e23628336242
- Al-FayoumiSWatsonRO’MahonyASingerJWComparative biomarker profiles of pacritinib, momelotinib, pexidartinib, and ruxolitinib using BioMAP Diversity Plus PanelEur J Cancer201669Suppl 1S136
- HunterCAJonesSAIL-6 as a keystone cytokine in health and diseaseNat Immunol201516544845725898198
- WynnTARamalingamTRMechanisms of fibrosis: therapeutic translation for fibrotic diseaseNat Med20121871028104022772564
- RousselLHouleFChanCIL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammationJ Immunol201018484531453720228195
- JovanovicDVDi BattistaJAMartel-PelletierJIL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophagesJ Immunol19981607351335219531313
- BatallerRBrennerDALiver fibrosisJ Clin Invest2005115220921815690074
- EkstedtMFranzenLEMathiesenULLong-term follow-up of patients with NAFLD and elevated liver enzymesHepatology200644486587317006923
- YounossiZMKoenigABAbdelatifDFazelYHenryLWymerMGlobal epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomesHepatology2016641738426707365
- EllisELMannDAClinical evidence for the regression of liver fibrosisJ Hepatol20125651171118022245903
- YanguasSCCogliatiBWillebrordsJExperimental models of liver fibrosisArch Toxicol20169051025104826047667
- IredaleJPModels of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organJ Clin Invest2007117353954817332881
- LiedtkeCLueddeTSauerbruchTExperimental liver fibrosis research: update on animal models, legal issues and translational aspectsFibrogenesis Tissue Repair2013611924274743
- WynnTAFibrotic disease and the T(H)1/T(H)2 paradigmNat Rev Immunol20044858359415286725
- FujiiMShibazakiYWakamatsuKA murine model for nonalcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinomaMed Mol Morphol201346314115223430399
- OhnoYAnimal experiments recommended by the Japanese Pharmacological Society (evaluation and decrease of pain): introduction and new guidelines for animal experiments for the Japanese Pharmacological SocietyNihon Yakurigaku Zasshi2007129159 Japanese17220569
- KleinerDEBruntEMvan NattaMDesign and validation of a histological scoring system for nonalcoholic fatty liver diseaseHepatology20054161313132115915461
- GranzowMSchierwagenRKleinSAngiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosisHepatology201460133434824619965
- WeberAWasiliewPKrachtMInterleukin-1 (IL-1) pathwaySci Signal20103105cm120086235
- YeZHGaoLWenDYHeYPangYYChenGDiagnostic and prognostic roles of IRAK1 in hepatocellular carcinoma tissues: an analysis of immunohistochemistry and RNA-sequencing data from the cancer genome atlasOnco Targets Ther2017101711172328356759
- ChenYZengZShenXWuZDongYChengJCMicroRNA-146a-5p negatively regulates pro-Inflammatory cytokine secretion and cell activation in lipopolysaccharide stimulated human hepatic stellate cells through inhibition of Toll-like receptor 4 signaling pathwaysInt J Mol Sci2016177E107627399683
- AhmadRShihabPKThomasRIncreased expression of the interleukin-1 receptor-associated kinase (IRAK)-1 is associated with adipose tissue inflammatory state in obesityDiabetol Metab Syndr201577126312071
- LeeJYHwangDHThe modulation of inflammatory gene expression by lipids: mediation through Toll-like receptorsMol Cells200621217418516682810
- MaitraUDavisSReillyCMLiLDifferential regulation of Foxp3 and IL-17 expression in CD4 T helper cells by IRAK-1J Immunol200918295763576919380824
- MederackeIHsuCCTroegerJSFate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiologyNat Commun20134282324264436
- TanZQianXJiangRIL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activationJ Immunol201319141835184423842754
- PixleyFJStanleyERCSF-1 regulation of the wandering macrophage: complexity in actionTrends Cell Biol2004141162863815519852
- PradereJPKluweJde MinicisSHepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in miceHepatology20135841461147323553591
- WynnTAVannellaKMMacrophages in tissue repair, regeneration, and fibrosisImmunity201644345046226982353
- VerstovsekSMesaRAFoltzLMPRM-151 in myelofibrosis: durable efficacy and safety at 72 weeksBlood20151262356
- NakagamiHKiomy OsakoMNakagamiFPrevention and regression of non-alcoholic steatohepatitis (NASH) in a rat model by metabosartan, telmisartanInt J Mol Med201026447748120818485
- TamakiYNakadeYYamauchiTAngiotensin II type 1 receptor antagonist prevents hepatic carcinoma in rats with nonalcoholic steatohepatitisJ Gastroenterol201348449150322886508
- HaliciZBilenHAlbayrakFDoes telmisartan prevent hepatic fibrosis in rats with alloxan-induced diabetes?Eur J Pharmacol20096141–314615219406119
- JinHYamamotoNUchidaKTeraiSSakaidaITelmisartan prevents hepatic fibrosis and enzyme-altered lesions in liver cirrhosis rat induced by a choline-deficient L-amino acid-defined dietBiochem Biophys Res Commun2007364480180717963695
- AlamSKabirJMustafaGGuptaUHasanSKAlamAKEffect of telmisartan on histological activity and fibrosis of non-alcoholic steatohepatitis: a 1-year randomized control trialSaudi J Gastroenterol2016221697626831610
- BensonSCPershadsinghHAHoCIIdentification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activityHypertension2004435993100215007034
- MandeliaCCollyerEMansoorSPlasma cytokeratin-18 level as a novel biomarker for liver fibrosis in children with nonalcoholic fatty liver diseaseJ Pediatr Gastroenterol Nutr201663218118726835904
- FeldsteinAEWieckowskaALopezARLiuYCZeinNNMcCulloughAJCytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation studyHepatology20095041072107819585618