Abstract
Background
Impulsive aggression (IA) is considered a maladaptive form of aggression that is reactive and overt and occurs outside of the acceptable social context. Many children and adolescents with attention-deficit/hyperactivity disorder (ADHD) display clinically significant aggression, with the predominant subtype being IA. However, there is currently no Food and Drug Administration-approved medication specifically to treat IA. The pathophysiology of IA is not fully understood, although it has been suggested to include the dopamine, norepinephrine, and serotonin systems.
Methods
SPN-810 (extended-release molindone) is being developed for the novel indication of IA and is currently being studied in patients treated for ADHD. Molindone is an indole derivative and a dopamine D2 receptor antagonist.
Results
The in vitro pharmacological studies described in the current manuscript demonstrate that the active substance molindone (SPN-810M) is a potent antagonist for the dopamine receptors, D2S and D2L, and the serotonin receptor, 5-HT2B, at therapeutic concentrations. The in vitro studies further demonstrate that the antagonist effect of SPN-810M is due to the parent drug and not the metabolites, and that the antagonism is not affected by the presence of norepinephrine or dopamine neurotransmitters. In addition, studies investigating the potential differential effects of the enantiomers of SPN-810M have demonstrated that the R(−) enantiomer is more potent than S(+), showing greater regulatory effect on D2S and D2L receptors.
Conclusion
Overall, the results of the in vitro SPN-810M pharmacological studies provide some insight into how SPN-810M modulates the serotonin and dopamine pathways that play a role in IA.
Introduction
Impulsive aggression (IA) is considered a maladaptive form of aggression that is reactive, overt, and occurring outside the acceptable social context.Citation1,Citation2 The presence of IA features in patients diagnosed with neuropsychiatric disorders such as attention-deficit/hyperactivity disorder (ADHD) amplifies the risk of poor outcomes even when treatment of the primary disorder is optimized.Citation3 Many children and adolescents with ADHD display clinically significant aggression, with the predominant subtype being IA.Citation3 IA becomes clinically distinguishable once ADHD-targeted therapy is maintained. This has been demonstrated in the Multimodal Treatment Study of Children with ADHD, in which 54% of the children with ADHD presented with clinically significant aggression prior to 14 months of treatment (medication with or without behavioral therapy); however, despite treatment, 44% of these children with aggression at baseline exhibited persistent IA.Citation1 Although there are guideline recommendations for adjunctive targeted therapy when IA persists with ADHD treatment,Citation1,Citation4–Citation6 the agents being used have not proven to be adequately effective and have tolerability issues; furthermore, there are limited data for clinicians due to the paucity of randomized clinical trials.Citation3 Currently, there is no US Food and Drug Administration (FDA)-approved medication specifically indicated to treat IA that may be observed in children with ADHD, and therefore there is a need for an effective medication with an improved tolerability profile.Citation3,Citation7
Although alterations in the central nervous system (CNS) biology have been implicated, the pathophysiology of IA is not fully understood.Citation1,Citation3 It has been hypothesized that IA is associated with the recruitment of the acute threat response system structures — the amygdala, hypothalamus, and periaqueductal gray.Citation8 The recruitment of these structures in order to initiate IA may reflect the functioning of the ventromedial prefrontal cortex, the dorsomedial prefrontal cortex, and the anterior insular cortex.Citation8 Abnormalities in central serotonergic, noradrenergic, and dopaminergic function may also be involved in IA.Citation9,Citation10 In addition, IA has been associated with dopamine hyperactivity as a result of deficient serotonergic functioning and regulation of the dopamine system in brain regions such as the nucleus accumbens and prefrontal cortex, which are linked to reward-related motivation.Citation11
Dopamine binds to five subtypes of G-protein-coupled receptors, categorized as D1-like (D1 and D5) or D2-like (D2, D3, and D4) receptors.Citation12,Citation13 Through alternative splicing, two isoforms of the D2 receptors are generated, D2S (short) and D2L (long), which include 29 additional amino acids in the third intracellular loop.Citation12,Citation13 The D2 receptors are known targets of antipsychotic drugs.Citation12,Citation13 Serotonergic neurons originate from the raphe nuclei and relay information using various neural pathways and serotonin receptors.Citation14 Seven serotonin receptors have been identified to date: 5-HT1 through to 5-HT7.Citation14 The role of serotonin in IA is not completely understood, although low levels of serotonin have been associated with IA.Citation11 The activation or antagonism of some of the serotonin receptors has been shown to inhibit aggressive behavior, while that of others increases this behavior.Citation14–Citation16
Although it is not yet completely known how SPN-810 (extended-release molindone) reduces IA, previous receptor binding studies have shown that molindone does indeed block the D2 receptors.Citation17 This current manuscript will discuss in vitro pharmacological studies and elucidate the effect of molindone (SPN-810M) on the dopamine and serotonin receptors that have been implicated in IA.
Materials and methods
Compounds tested
Molindone Hydrochloride (SPN-810M) (USP reference standard) (Supernus Pharmaceuticals, Inc., Rockville, MD, USA); Molindone Hydrochloride SPN-810M P1-2 metabolite and Molindone Hydrochloride (SPN-810M) P3-2 metabolite (Advinus Therapeutics Ltd., Bangalore, India); Molindone (SPN-810M) R(–) enantiomer and S(+) enantiomer (Supelco, Bellefonte, PA, USA).
Radioligand binding
For D2S and D2L radioligand binding, the methods described by Hayes et alCitation18 were followed. Briefly, D2S and D2L receptors in human recombinant Chinese hamster ovary (CHO) cells were labeled with 0.16 nM [3H]-spiperone. All cell lines described in this manuscript were purchased commercially and authorization for the ethical use of these cell lines was obtained prior to usage (additional information on all cell lines and their source is provided in Table S1). Assays (in duplicate) were conducted in incubation buffer containing 50 mM Tris-HCl, pH 7.4; 1.4 mM ascorbic acid; 0.001% BSA; and 150 mM NaCl. Non-specific binding was defined with 10 µM haloperidol. Following 120-minute incubation at 25°C, radioligand binding was quantified. Using the methods described in Bonhaus et alCitation19 with slight modifications, 5-HT2B receptors were labeled with 1.2 nM [3H]-lysergic acid diethylamide in human recombinant CHO-K1 cells. Assays were conducted in incubation buffer containing 50 mM Tris-HCl, pH 7.4; 4 mM CaCl2; and 0.1% ascorbic acid. Non-specific binding was defined with 10 µM 5-HT. Following 60-minute incubation at 37°C, radioligand binding was quantified.
[35S]GTPgS binding
A GTPγS binding assay was used to evaluate the antagonist activity of SPN-810M at the human D2S and D2L receptors expressed in transfected CHO cells. Total [35S]GTPγS (guanine 5′-O-[γ-thio]triphosphate) binding was performed in duplicate based on the method used by Senogles et al, with minor modifications.Citation20 Briefly, test compounds were preincubated with the membranes (D2S: 0.05 mg/mL; D2L: 0.1 mg/mL) and GDP (guanosine 5′-diphosphate) (D2S: 3 µM; D2L: 10 µM) in modified HEPES buffer (20 mM HEPES, pH 7.4; 100 mM NaCl; 10 mM MgC12; 1 mM EDTA; 1 mM DTT) for 20 minutes and scintillation proximity assay beads were then added for another 60 minutes at 30°C. The reaction was initiated by 0.3 nM [35S]GTPγS for an additional 30 minutes at 30°C for D2S and 15 minutes at 30°C for D2L incubation period. The bound [35S]GTPγS was detected using a scintillation counter (PerkinElmer Microbeta).
Impedance measurement and cellular dielectric spectroscopy
To evaluate the antagonist activity of SPN-810M at the human D2S receptor expressed in transfected HEK-293 cells, the CellKey (cellular dielectric spectroscopy) detection method was used to measure effects of the compound on impedance modulation. Cells were seeded onto 96-well plates coated with fibronectin at 2×105 cells/well in HBSS buffer + 20 mM HEPES (Thermo Fisher Scientific, Waltham, MA, USA) with 0.1% BSA and equilibrated for 45 minutes at 28°C. Plates were placed onto the system and measurements were made at 28°C. HBSS (basal and stimulated controls), reference antagonist (IC50 determination), or the test compounds were preincubated for 15 minutes before the addition of HBSS (basal control) or the reference agonist dopamine at 30 nM (EC80). Impedance measurements were monitored for 10 minutes. The standard reference antagonist butaclamol was tested in each experiment at several concentrations to generate a concentration-response curve from which its IC50 value was calculated. Assays were run in duplicate. The analysis of other receptors (eg, 5-HT1A) used similar assay methods.
Phosphoinositide hydrolysis assays and homogeneous time-resolved fluorescence (HTRF)
HEK-293 cells and CHO cells expressing 5-HT2A and 5-HT2B, respectively, were used in the IP-one HTRF® assay kit. For each assay, the test compound was incubated with the cells (5×105/mL) in stimulation buffer of IP1 Tb kit and 5-HT (5-HT2A: 100 nM; 5-HT2B: 30 nM) for 30 minutes at 37°C. The assays were done in duplicate. The analysis of other receptors (eg, D1, D3, D5, 5-HT2C, 5-HT6, and 5-HT7) used similar assay methods. Measurements were performed at the excitation wavelength of 337 nm, and the emission wavelength of 620 nm (donor) and 665 nm (acceptor). The HTRF ratio was calculated by dividing the acceptor signal (665 nm) by the donor signal (620 nm), which normalizes the signal and corrects the results for effects due to the optical characteristics of the media and assay components.
Results
Radioligand binding and functional activity of SPN-810M on the dopamine and serotonin receptors
SPN-810M was tested for potential agonist or antagonist effect on neurotransmitter receptors, specifically toward the D2S, D2L, and 5-HT2B receptors. The radioligand binding assays demonstrated that SPN-810M inhibited binding in response to an agonist for the D2S, D2L, and 5-HT2B receptors ().
Figure 1 Radioligand binding assays of SPN-810M for (A) D2S, (B) D2L, and (C) 5-HT2B. The IC50 for D2S, D2L, and 5-HT2B was 0.0363 µM, 0.0505 µM, and 0.0808 µM, respectively.
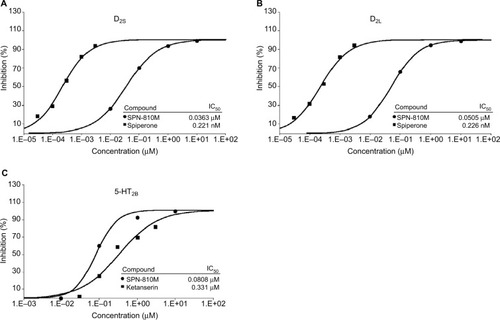
Using a concentration range of 3 nM–10 µM, SPN-810M demonstrated an antagonist effect on D2S in the impedance-based cellular functional assay performed on human recombinant HEK-293 cells. The concentration of SPN-810M at which percent inhibition of control agonist response is reduced by half (IC50) was identified as 0.14 µM. Furthermore, in the [35S]GTPγS binding assay, SPN-810M at concentrations ranging from 1 nM to 10 µM showed a similar IC50 of 0.0844 µM in response to control agonist for D2S (). Similarly, SPN-810M (1 nM–10 µM) was found to act as an antagonist for the D2L receptor with an IC50 of 0.11 µM in the [35S]GTPγS binding assay (). At the SPN-810M concentrations tested (3 nM–10 µM), the inhibitory effect on D1, D3, and D5 receptors was not as potent as that noted for D2S and D2L, with IC50 values ranging from 3.2 µM to 8.3 µM. In addition, there was no significant inhibition of the alpha-adrenergic receptors.
Figure 2 Effect of SPN-810M on (A) D2S, (B) D2L, and (C) 5-HT2B. IC50 was determined using GTPγS assay for D2S and D2L, and HTRF for 5-HT2B. The IC50 for D2S, D2L, and 5-HT2B was 0.0844 µM, 0.11 µM, and 0.41 µM, respectively.
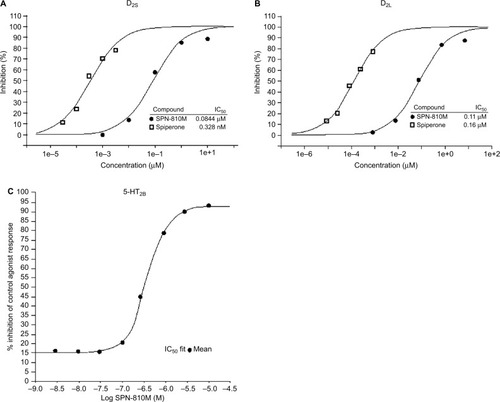
The phosphoinositide hydrolysis functional assay using concentrations of SPN-810M ranging from 3 nM to 10 µM in human recombinant CHO cells identified SPN-810M as an antagonist for serotonin 5-HT2B with the IC50 as 0.41 µM (). The functional assay also identified the IC50 of 5-HT2A, which was 14 µM, demonstrating that SPN-810M was more potent in acting as an antagonist for 5-HT2B vs 5-HT2A.
Human metabolites of SPN-810M
The major human metabolites of SPN-810M, P1-2 and P3-2, were evaluated for antagonistic effects on the dopamine and serotonin receptors. The binding assays identified no binding affinity for P1-2 on the tested receptors. Although the metabolite P3-2 had a slight antagonist effect on 5-HT2B at concentrations greater than 10 µM, the phosphoinositide hydrolysis functional assay demonstrated an antagonist effect only at 100 µM. Additional binding assays revealed that the SPN-810M metabolites P1-2 and P3-2 did not bind to other neurotransmitter receptors, such as norepinephrine, GABA, glutamate, histamine, or acetylcholine receptors.
Potential effect of dopamine and norepinephrine on the in vitro pharmacology profile of SPN-810M
Additional assays were carried out to determine the effect of 0.1 µM, 1 µM, and 10 µM dopamine or norepinephrine on SPN-810M antagonism of D2S, D2L, and 5-HT2B. D2S antagonistic activity of SPN-810M was unaffected by the presence of 0.1 µM dopamine or norepinephrine (). The antagonist effect of SPN-810M on the D2L receptor was also unaffected by the presence of norepinephrine at concentrations tested (0.1 µM, 1 µM, and 10 µM), with an IC50 ranging from 0.05 µM to 0.11 µM (). Likewise, the antagonist effect of SPN-810M on 5-HT2B was not affected in the presence of dopamine or norepinephrine (IC50 ranging from 0.19 µM to 0.45 µM; ). No agonist effect of SPN-810M on 5-HT2A, 5-HT2B, 5-HT2C, 5-HT6, 5-HT7, D1, D2S, D2L, D3, or D5 was observed in the presence of dopamine or norepinephrine.
Table 1 IC50 of SPN-810M on neurotransmitter receptors D2S, D2L, and 5-HT2B in the presence of neurotransmitters dopamine and norepinephrineTable Footnotea
SPN-810M enantiomers: R(−) enantiomer vs S(+) enantiomer
Results from the [35S]GTPγS binding assays demonstrate that antagonist potency of the R(−) enantiomer of SPN-810M is ~100-fold and 300-fold higher for D2S and D2L, respectively, than the S(+) enantiomer, based on the IC50 (). Further experiments demonstrate that the R(−) enantiomer also has a greater inhibitory effect than the S(+) enantiomer for the 5-HT2B receptor (IC50=0.2 µM vs 1.8 µM, respectively; ).
Table 2 IC50 of SPN-810M enantiomers for neurotransmitter receptors D2S, D2L, and 5-HT2B
Discussion
The results of the in vitro pharmacology studies demonstrated that at concentrations within the clinically relevant range for the intended therapeutic dose (36 mg/day) of SPN-810 (extended-release molindone), SPN-810M is a potent antagonist for the D2S and D2L dopamine receptors and the serotonin receptor 5-HT2B; it is not as potent for the 5-HT2A receptor. The findings also indicate that R(−) is a more potent enantiomer of SPN-810M than S(+), showing greater effect on D2S and D2L receptors, and that the parent drug, SPN-810M, and not the major human metabolites (P1-2 and P3-2), has the antagonist effect on the target dopamine or serotonin receptors. In addition, the P1-2 and P3-2 metabolites did not demonstrate any inhibitory effect on several other neurotransmitter receptors. Finally, the data show that the presence of dopamine and norepinephrine neurotransmitters does not interfere with the antagonistic effect of SPN-810M on D2S, D2L, and 5-HT2B, suggesting that known concomitant psychostimulants used to treat ADHD would not affect the pharmacological activity of SPN-810M in clinical settings.
SPN-810 (extended-release molindone) has been shown to improve IA as investigated in patients treated for ADHD.Citation7,Citation21,Citation22 The results of these in vitro pharmacology studies provided insight regarding the potential mechanism, suggesting it does so mainly by modulating dopamine and also serotonin.
SPN-810M: a potent antagonist for D2S, D2L, and 5-HT2B
Molindone has been found to be a potent antagonist for D2 receptors.Citation17 Gene expression profiling has demonstrated that molindone’s effect on lipid metabolism is markedly weaker than that of the antipsychotics clozapine and risperidone.Citation17,Citation23 Molindone does not act like antipsychotics such as haloperidol, as it has been shown to elicit little to no parkinsonism or extrapyramidal symptoms and has a low propensity for weight gain.Citation24,Citation25 The results of the current radioligand binding assays and functional activity studies extend these findings and identify SPN-810M as an antagonist for D2S, D2L, and 5-HT2B. SPN-810M also demonstrated an inhibitory effect on the D1, D3, D5, and 5-HT2A receptors, but to a much lesser degree. The majority of atypical antipsychotics have a high affinity for 5-HT2A,Citation26 although amisulpride is an example of a highly effective atypical antipsychotic that does not occupy any 5-HT2A receptors.Citation27 In any case, the lack of 5-HT2A potency suggests that SPN-810M does not act like known atypical antipsychotic drugs (with a high affinity for 5-HT2A).Citation26 Overall, these in vitro results suggest that SPN-810M at clinically relevant doses is pharmacologically distinct from both typical and atypical antipsychotic drugs, with the exception of amisulpride.
Previous studies have shown that single enantiomers and their racemates can be equipotent in in vitro and in vivo pharmacological testsCitation28 or that each enantiomer can have a different potency, as seen with the dopamine D1-like receptor partial agonist SKF 83959 enantiomer R(+) and the S(−) enantiomers, MCL 202 and MCL 201, respectively.Citation29 Results from the present study investigating the differential effects of the enantiomers of SPN-810M show that the racemic molecule and the R(−) enantiomer did not demonstrate significant differences in their in vitro action. However, the R(−) form showed ~100, 300, and eight times more potency as an antagonist for D2S, D2L, and 5-HT2B receptors, respectively, than the S(+) enantiomer. The fact that the potency was similar for the R(−) enantiomer on D2S and D2L at 0.048 µM and 0.021 µM, respectively, suggests that the SPN-810M R(−) enantiomer could play a role in the D2 autoreceptor of the dopaminergic system. In particular, SPN-810M may affect both the presynaptic D2S autoreceptors and the postsynaptic D2L receptors.Citation12,Citation13
Active metabolites of certain drugs, such as triptans, can induce CNS side effects by binding to 5-HT or other receptors.Citation30 The major human metabolites (P1-2 and P3-2) of SPN-810M did not display antagonistic effects on the dopamine or serotonin receptors in vitro. Furthermore, the metabolites did not demonstrate an inhibitory effect on several other neurotransmitter receptors tested, such as norepinephrine, GABA, glutamate, histamine, or acetylcholine receptors. This finding is in agreement with a study conducted by Balsara et al, which concluded that a molindone metabolite does not exert neuroleptic activity.Citation31 The lack of an effect due to the SPN-810M metabolites suggests that SPN-810M is not likely to have metabolite-related CNS side effects, improving the tolerability in a clinical setting.
The effect of psychostimulants on SPN-810M antagonism of D2S, D2L, and 5-HT2B
Psychostimulants are considered first-line pharmacotherapy for the treatment of ADHD and refractory aggression, and they usually improve ADHD symptoms as well as aggressive behaviors by increasing synaptic dopamine levels in the brain.Citation32–Citation35 Other pharmacotherapies for ADHD include norepinephrine reuptake inhibitors.Citation4 However, IA symptoms that are present prior to treatment persist in ~44% of children with ADHD, despite ADHD therapy.Citation1,Citation36 Studies suggest that IA may respond to adjunctive treatments administered with optimized first-line pharmacotherapy for ADHD.Citation1,Citation37 As such, the additional assays described above were carried out to determine the effect of dopamine or norepinephrine on D2S, D2L, and 5-HT2B in the presence of SPN-810M. Specifically, we wanted to evaluate whether these neurotransmitters 1) increased or decreased the antagonistic effect of SPN-810M, and/or 2) caused a reversal in function of SPN-810M from an antagonist to an agonist. The results from the in vitro pharmacology studies demonstrate that the antagonist effects of SPN-810M on the D2S, D2L, and 5-HT2B receptors were not altered by the presence of dopamine or norepinephrine, as very little change in potency was observed. Furthermore, the presence of dopamine or norepinephrine did not lead to SPN-810M reversing from an antagonistic to an agonistic effect.
Theorized mechanism of action
Our in vitro assays provide some insight into the potential mechanisms of SPN-810M. The results of the in vitro pharmacology studies indicate that SPN-810M is a potent antagonist for D2S, D2L, and 5-HT2B. In children (aged 6–12 years) diagnosed with ADHD and presenting with IA, SPN-810 (extended-release molindone) use at doses up to 36 mg/day resulted in significant improvements in aggressive behavior based on the Retrospective-Modified Overt Aggression Scale (R-MOAS).Citation21,Citation22 The exact mechanism of action by which SPN-810 improved aggressive behavior is not completely understood. We hypothesize that by acting as a 5-HT2B receptor antagonist, SPN-810M can influence the mesoaccumbens dopamine pathway by reducing the outflow of dopamine.Citation38 Although we found that SPN-810M is an antagonist for 5-HT2B, it was a more potent antagonist for the D2S and D2L receptors, suggesting that SPN-810M may affect the presynaptic D2S autoreceptors to adequately regulate the synthesis and release of dopamine into the synaptic cleft and block the postsynaptic D2L receptors to reduce phasic DA signaling in regions of the brain that are likely to be associated with aggression.Citation12,Citation13,Citation39 At therapeutic doses, antagonistic effects of SPN-810M for D2S and D2L, as well as for 5-HT2B, may work in concert to synergistically contribute to the clinical effects. Indeed, further in vivo neuropharmacological studies including fMRI studies may enable better understanding of the mechanism of action of this drug, with the goal of improving care for patients with IA.
Acknowledgments
This study was supported by Supernus Pharmaceuticals, Inc. The authors would like to thank Eurofins Cerep Panlabs, France, for their assistance in the assay techniques; and IMPRINT Science, New York, for editorial support of this manuscript, which was funded by Supernus Pharmaceuticals, Inc.
Supplementary material
Table S1 Source and reference information for all utilized cell lines
Disclosure
CY and GG are employees of Supernus Pharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
References
- JensenPSYoungstromEASteinerHConsensus report on impulsive aggression as a symptom across diagnostic categories in child psychiatry: implications for medication studiesJ Am Acad Child Adolesc Psychiatry200746330932217314717
- ConnorDFOn the challenge of maladaptive and impulsive aggression in the clinical treatment settingJ Child Adolesc Psychopharmacol20162612326835875
- SaylorKEAmannBHImpulsive aggression as a comorbidity of attention-deficit/hyperactivity disorder in children and adolescentsJ Child Adolesc Psychopharmacol2016261192526744906
- PliszkaSRCrismonMLHughesCWThe Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorderJ Am Acad Child Adolesc Psychiatry200645664265716721314
- Scotto RosatoNCorrellCUPappadopulosETreatment of maladaptive aggression in youth: CERT guidelines II. Treatments and ongoing managementPediatrics20121296e1577158622641763
- PappadopulosEMacintyreII JCCrismonMLTreatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part IIJ Am Acad Child Adolesc Psychiatry200342214516112544174
- AdewoleTBrittainSTJohnsonJKLiransoTFindlingRLLong-term efficacy and safety of extended-release molindone (SPN-810) to manage impulsive aggression (IA) in children with attention deficit hyperactivity disorder (ADHD)Poster presented at: American Academy of Child and Adolescent PsychiatryOctober 24–29, 2016New York, NY
- BlairRJThe neurobiology of impulsive aggressionJ Child Adolesc Psychopharmacol20162614926465707
- KraemerGWClarkeASSocial attachment, brain function, and aggressionAnn N Y Acad Sci19967941211358853599
- KavoussiRArmsteadPCoccaroEThe neurobiology of impulsive aggressionPsychiatr Clin North Am19972023954039196921
- SeoDPatrickCJKennealyPJRole of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disordersAggress Violent Behav200813538339519802333
- LindgrenNUsielloAGoinyMDistinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sitesProc Natl Acad Sci U S A200310074305430912651945
- UsielloABaikJHRougé-PontFDistinct functions of the two isoforms of dopamine D2 receptorsNature2000408680919920311089973
- NarvaesRMartins de AlmeidaRMAggressive behavior and three neurotransmitters: dopamine, GABA, and serotonin—A review of the last 10 yearsPsychology & Neuroscience201474601607
- SakaueMAgoYSowaCModulation by 5-HT2A receptors of aggressive behavior in isolated miceJpn J Pharmacol2002891899212083749
- MarekGJCarpenterLLMcDougleCJPriceLHSynergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disordersNeuropsychopharmacology200328240241212589395
- OwenRRJrColeJOMolindone hydrochloride: a review of laboratory and clinical findingsJ Clin Psychopharmacol1989;942682762671060
- HayesGBidenTJSelbieLAShineJStructural subtypes of the dopamine D2 receptor are functionally distinct: expression of the cloned D2A and D2B subtypes in a heterologous cell lineMol Endocrinol1992669209261323056
- BonhausDWBachCDesouzaAThe pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptorsBr J Pharmacol199511546226287582481
- SenoglesSESpiegelAMPadrellEIyengarRCaronMGSpecificity of receptor-G protein interactions. Discrimination of Gi subtypes by the D2 dopamine receptor in a reconstituted systemJ Biol Chem19902658450745142137824
- BrittainSTStocksJDJohnsonJKLiransoTFindlingRLExtended-release molindone (SPN-810) as adjunctive therapy in the management of impulsive aggression in children with attention deficit hyperactivity disorder (ADHD) receiving optimized stimulant monotherapy and behavioral therapyPoster presented at: American Academy of Child and Adolescent PsychiatryOctober 26–31, 2015San Antonio, TX
- BrittainSTStocksJDJohnsonJKLiransoTFindlingRLAdjunctive extended-release molindone (SPN-810) to manage impulsive aggression in children with attention deficit hyperactivity disorder (ADHD) receiving optimized stimulant monotherapy and behavioral therapyPoster presented at: American Academy of NeurologyApril 15–21, 2016Vancouver, BC
- PolymeropoulosMHLicameleLVolpiSCommon effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophreniaSchizophr Res20091081–313414219150222
- SikichLFrazierJAMcClellanJDouble-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) studyAm J Psychiatry2008165111420143118794207
- SeemanPTallericoTAntipsychotic drugs which elicit little or no Parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptorsMol Psychiatry1998321231349577836
- IchikawaJIshiiHBonaccorsoSFowlerWLO’LaughlinIAMelt-zerHY5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine releaseJ Neurochem20017651521153111238736
- SeemanPAtypical antipsychotics: mechanism of actionCan J Psychiatry2002471273811873706
- AmanMGVinksAARemmerieBPlasma pharmacokinetic characteristics of risperidone and their relationship to saliva concentrations in children with psychiatric or neurodevelopmental disordersClin Ther20072971476148617825699
- NeumeyerJLKulaNSBergmanJBaldessariniRJReceptor affinities of dopamine D1 receptor-selective novel phenylbenzazepinesEur J Pharmacol20034742–313714012921854
- DodickDWMartinVTriptans and CNS side-effects: pharmacokinetic and metabolic mechanismsCephalalgia200424641742415154851
- BalsaraJJGadaVPNandalNVChandorkarAGPsychopharmacological investigation of the monoamine oxidase inhibitory activity of molindone, a dihydroindolone neurolepticJ Pharm Pharmacol19843696086136149285
- BladerJCPliszkaSRJensenPSSchoolerNRKafantarisVStimulant-responsive and stimulant-refractory aggressive behavior among children with ADHDPediatrics20101264e79680620837589
- GurnaniTIvanovINewcornJHPharmacotherapy of aggression in child and adolescent psychiatric disordersJ Child Adolesc Psychopharmacol2016261657326881859
- YanofskiJThe dopamine dilemma: using stimulants and antipsychotics concurrentlyPsychiatry2010761823
- ElbeDBarrAMHonerWGProcyshynRMManaging ADHD and disruptive behaviour disorders with combination psychostimulant and antipsychotic treatmentJ Psychiatry Neurosci2014393E323324758945
- MTA Cooperative GroupA 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorderArch Gen Psychiatry199956121073108610591283
- AmanMGBinderCTurgayARisperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavior disorders, and subaverage IQJ Child Adolesc Psychopharmacol200414224325415319021
- AuclairALCathalaASarrazinFThe central serotonin 2B receptor: a new pharmacological target to modulate the mesoaccumbens dopaminergic pathway activityJ Neurochem201011451323133220534001
- De MeiCRamosMIitakaCBorrelliEGetting specialized: presynaptic and postsynaptic dopamine D2 receptorsCurr Opin Pharmacol200991535819138563
