Abstract
Purpose
Lead (Pb) is an environmental pollutant responsible for various organ damages including renal injury. It seems that OS and associated events are crucial mechanisms of lead-induced renal dysfunction. The current study aimed to explore the potential protective effects of glycine against renal injury caused by lead in mice.
Materials and methods
Mature male mice (n=32) were allocated into four groups. The following treatment regimens were the control (vehicle-treated); Pb-acetate (20 mg/kg/day, gavage); Pb-acetate + glycine (500 mg/kg/day, IP); and Pb-acetate + glycine (1,000 mg/kg/day, IP). Pb-acetate + glycine was administered for 14 consecutive days, Pb-acetate was given first and then glycine at least 6 hours later. On day 15, the subjects were anesthetized, and samples were collected. Serum biomarkers such as BUN and serum creatinine were monitored along with formation of reactive oxygen species, lipid peroxidation, kidney GSH level, and histopathological changes.
Results
Based on the results, BUN and serum creatinine levels significantly increased following exposure to lead. Glycine supplementation (500 and 1,000 mg/kg, IP) decreased BUN and creatinine serum levels (P<0.001). Biomarkers of OS were also reduced in renal tissue following glycine therapy in Pb-exposed mice (P<0.001). Histopathological changes were observed in mice treated with lead as tubular dilation, protein cast, vacuolization, and inflammation. In this regard, glycine inhibited histopathological alterations in kidney caused by lead exposure.
Conclusion
It was found that glycine treatment significantly mitigated Pb-induced renal injury most likely through alleviating OS and the associated deleterious outcomes on the kidney tissue.
Introduction
Humans are continuously exposed to lead (Pb), a toxic heavy metal, which is an environmental pollutant.Citation1 Accumulation of lead in various organs such as bone, liver, kidney, and reproductive system is associated with deleterious effects.Citation2–Citation5
One of the potential adverse effects of prolonged and high-level Pb exposure is renal injury and nephropathy. Pb-induced nephropathy usually occurs as a result of prolonged exposure to this heavy metal and constant high plasma Pb level of >60 μg/dL. Meanwhile, prolonged Pb exposure at lower plasma levels could also cause nephrotoxicity.Citation6,Citation7 According to animal and histopathological studies, the most affected segment of kidney in Pb-induced renal injury is the proximal tubule.Citation8 Tubular atrophy, interstitial fibrosis, hypertrophic arteriolar changes, and accumulation of inflammatory cells are common histological alterations in kidney tissues in experimental models of Pb nephrotoxicity.Citation9,Citation10
In Pb-induced nephrotoxicity, different mechanisms have been reported.Citation5 Among these mechanisms, OS and its associated events are noteworthy,Citation5,Citation11,Citation12 which include increased reactive oxygen species (ROS) levels, biological target disruption, and defects in cellular antioxidant mechanisms.Citation12,Citation13 Therefore, it seems rational to use antioxidants as therapeutic options against Pb toxicity.
Glycine, an amino acid with the simplest structure, is an intricate part of protein structure. Several pharmacological properties are attributed to glycine. Interestingly, it was found that glycine supplementation could protect biological targets against a variety of xenobiotics.Citation14–Citation17 Some studies mentioned the nephroprotective properties of this amino acid.Citation18 Regarding its cytoprotective activity, different mechanisms are suggested. Based on the findings, glycine can effectively reduce OS caused by xenobiotics.Citation14–Citation17,Citation19
The current study aimed to evaluate the potential nephroprotective effects of glycine against Pb nephrotoxicity in an animal model.
Materials and methods
Chemicals
Glycine (2-aminoacetic acid), malondialdehyde, thiobarbitu-ric acid, dithiobis-2-nitrobenzoic acid, GSH, 2′,7′-dichloro-fluorescein diacetate (DCFH-DA), and EDTA were provided by Sigma-Aldrich (St. Louis, MO, USA). Trichloroacetic acid (TCA), dithiothreitol (DTT), ferric chloride hexahydrate, 2, 4, 6-Tris (2-pyridyl)-s-triazine (TPTZ), and Tris (hydroxy-methyl) aminomethane hydrochloride were provided by Merck (Darmstadt, Germany).
Animals and treatments
A total of 32 adult male BALB/c mice (25–30 g) were purchased from the Laboratory Animals Breeding Center, Shiraz University of Medical Sciences (SUMS), Shiraz, Iran, and kept under standard conditions (12L:12D, photo schedule; 18–22°C; appropriate ventilation, ≈40% humidity) and fed ad libitum with water and commercial rodent pellets (Behparvar®, Tehran, Iran). The animal procedures were done at Pharmaceutical Sciences Research Center, School of Pharmacy, SUMS. The local ethics committee of AJA University of Medical Sciences, Tehran, Iran, approved this study (#97-01-36-17657).
The animals received humane care and were handled regarding the guidelines of the ethics committee of AJA University of Medical Sciences.
The subjects were allocated into four groups of eight mice and the following treatment regimens were introduced: the control (vehicle-treated); Pb-acetate (20 mg/kg/day, gavage); Pb-acetate (20 mg/kg/day, gavage) + glycine (500 mg/kg/day, IP); and Pb-acetate (20 mg/kg/day, gavage) + glycine (1,000 mg/kg/day, IP). Pb-acetate + glycine were given for 14 consecutive days. Pb-acetate dose was selected based on previous studies that mentioned 20 mg/kg of Pb-acetate as the nephrotoxic dose.Citation21 Glycine was administered at least 6 hours after Pb-acetate administration. On day 15, samples were collected.
Sample collection
Mice were anesthetized (thiopental 80 mg/kg, IP) to drain blood samples from abdominal vena cava. Both kidneys were excised and weighed. Right kidneys were kept in 10% formalin for histopathological evaluations. Total antioxidant capacity, lipid peroxidation, ROS production, and GSH contents were determined in the left kidneys.
Histopathology and organ weight index
The kidney was weighed, using the following formula: Weight index = [Wet weight of organ (g)/Body weight (g)]×100.
Buffered formalin solution (0.4% sodium phosphate monobasic [NaH2PO4], 0.64% sodium phosphate dibasic [Na2HPO4], and 10% formaldehyde in distilled water) was used to fix the kidney samples for histopathological evaluations. The kidney samples were stained with H&E followed by paraffin embedding and cutting in 5 μm sections. Tissue histopathological alterations were evaluated by a pathologist in a blind manner using a light microscope (Olympus BX41; Olympus Optical Co. Ltd, Tokyo, Japan). Early proximal tubular vacuolization, tissue inflammation, protein cast, and tubular dilation were graded as mild (+), moderate (++), or severe (+++) in each group.
Plasma biochemistry
To obtain plasma sedimentation, blood samples were drained into tubes (coated with EDTA) and then centrifuged (10,000 g, 15 minutes, 4°C). BUN and creatinine plasma levels were determined using commercially available kits (Pars Azmun®, Tehran, Iran).Citation22 Plasma Pb level was measured by an atomic absorption method.
ROS formation
In total, 200 mg of kidney samples homogenized on ice in 5 mL Tris-HCl buffer (40 mM, pH =7.4, 4°C) was mixed with 100 μL homogenized sample and 1 mL of Tris-HCl buffer (pH 7.4) plus 10 μL of DCFH-DA until reaching 10 μM as the final concentration.Citation23,Citation24 The prepared samples were incubated in the dark for 15 minutes at 37°C (Gyromax™ incubator shaker). The sample luminescence intensity was recorded with a FLUOstar Omega® multifunctional microplate reader at λexcitation =485 nm and λemission =525 nm.Citation25
Lipid peroxidation
For this purpose, TBA reactive substances were applied; the reaction mixture contained 500 μL of homogenized tissue (10% w/v in KCl, 1.15% w/v), 1 mL of TBA (0.375% w/v), and 3 mL of phosphoric acid (1% w/v, pH =2). After vigorously mixing the mixture and bain-marie heating for 45 minutes at 100°C, n-butanol (2 mL) was added following cooling of the sample.Citation14,Citation26,Citation27 Then, the samples were centrifuged at 10,000 g for 5 minutes, followed by vigorous mixing. Finally, based on the developed color, the absorbance was measured at λ =532 nm with an EPOCH® plate reader (BioTek, Highland Park, VT, USA).Citation28
Total antioxidant capacity in kidney tissue
The Ferric Reducing Antioxidant Power (FRAP) assay evaluates absorbance changes at λ =595 nm based on the blue-colored Fe2+, tripyridyltriazine, derived from the colorless oxidized Fe3+ using electron-donating antioxidant activity.Citation29 In order to prepare the FRAP reagent freshly, acetate buffer (10 vol, 300 mM/L, pH 3.6) was mixed with TPTZ (1 vol, 10 mM/L in 40 mM/L hydrochloric acid) and ferric chloride (1 vol, 20 mM/L). The FRAP reagent was prepared on the same day of assessment and kept in the dark. Tris-HCl buffer (250 mM Tris-HCl, 200 mM sucrose, and 5 mM DTT, pH 7.4, 4°C) was used for kidney tissue homogenesis on ice.Citation14,Citation26,Citation27,Citation30 Next, 50 μL of the homogenized sample (50 μL) plus deionized water (150 μL) was added to the FRAP reagent (1.5 mL). Then, samples were incubated in the dark (37°C for 5 minutes), and the absorbance rate was read at λ =595 nm with an EPOCH® plate reader (BioTek). The sample’s protein content was measured, using Bradford method, for data standardization.Citation31
Kidney GSH level
GSH levels were determined spectrophotometrically as the indicator using 5,5′-dithiobis-2-nitrobenzoic acid (DTNB).Citation32 For this purpose, to homogenize kidney samples, 200 mg of tissue was mixed with 8 mL EDTA (0.04 M, 4°C) on ice. Then, 5 mL of the provided homogenate was mixed with 4 mL of distilled water (4°C) plus 1 mL TCA (50% w/v; 4°C). The mixture was vortexed and centrifuged (10,000 g, 4°C, 15 minutes). Then, the supernatant (2 mL) was added to Tris-HCl buffer (4 mL, 40 mM, pH =8.9) plus DTNB (100 μL, 10 mM in methanol). The absorbance rate of the prepared solution was read at 412 nm with an EPOCH® plate reader (BioTek).
Data analysis
Data are presented as mean ± SD. Data were analyzed using one-way ANOVA with Tukey’s multiple comparison tests as the post hoc. The level of significance was set at P<0.05.
Results
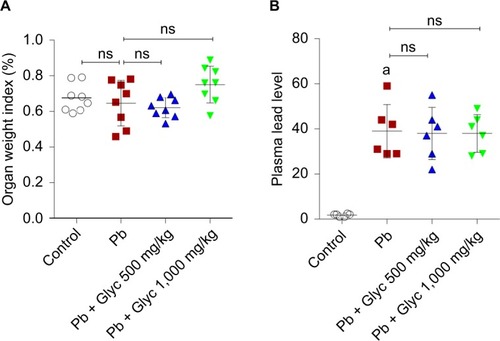
There were no significant changes in the kidney/body weight ratio in the current study, when Pb-treated and control animals were compared (). On the contrary, no significant change in the body weight of animals was detected in the current study. Plasma level in lead was significantly higher in Pb-exposed animals compared with the controls (). However, there were no significant changes in plasma Pb levels between glycine-supplemented and Pb-treated animals ().
Figure 1 Kidney weight index (A) and Pb level (B) in mice.
Abbreviations: Glyc, glycine; ns, not significant; Pb, plasma lead.
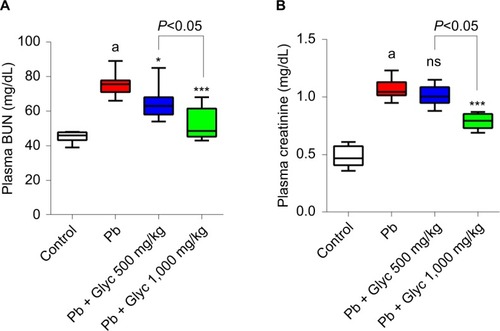
Pb exposure significantly increased plasma biomarkers in renal damage (). BUN and creatinine plasma levels were significantly higher in the Pb-treated mice compared with the controls (). In addition, glycine supplementation significantly reduced serum BUN and creatinine levels ().
Figure 2 Plasma biomarkers of renal injury such as BUN (A) and plasma creatinine (B) in lead (Pb)-treated mice.
Abbreviations: Glyc, glycine; ns, not significant compared with Pb group; Pb, plasma lead..
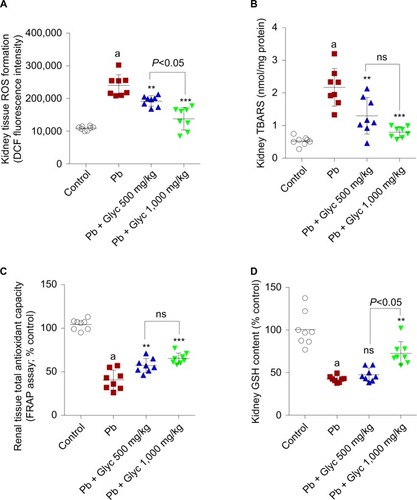
OS biomarkers were significantly higher in Pb-treated animals (). A significant level of ROS in addition to an elevated level of lipid peroxidation was observed in the Pb group compared with the controls (). The kidney GSH content and tissue antioxidant capacity were significantly reduced in the Pb group (). OS bio-markers of renal tissue were significantly reduced following glycine therapy (500 and 1,000 mg/kg, IP) in Pb-exposed mice ().
Figure 3 Biomarkers of OS in renal tissue of lead (Pb)-treated animals.
Abbreviations: DCF, dichlorofluorescein; Glyc, glycine; ns, not significant; Pb, plasma lead.
In Pb-treated mice, histopathological changes included tubular dilation, protein cast, vacuolization, and inflammation ( and ). Glycine therapy (500 and 1,000 mg/kg, IP) mitigated renal histopathological alterations in mice exposed to lead ( and ).
Figure 4 Renal histopathological alterations in the lead (Pb)-exposed mice.
Abbreviation: Pb, plasma lead.
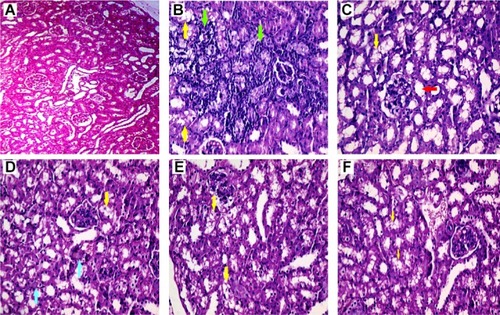
Table 1 Grades of renal histopathological changes in the lead (Pb)-treated animals
Discussion
Heavy metals are toxic environmental pollutants, and exposer to them can be detrimental.Citation33 Lead (Pb) is a toxic heavy metal, which possesses a wide range of adverse effects in humans.Citation34 Neurological abnormalities, dysregulation of the endocrine system, different types of malignancies, bone and skeletal disorders, reproductive toxicity, as well as hepatic and renal injury have been reported in association with Pb overexposure.Citation35,Citation36 Pb-induced nephrotoxicity is a complication that could lead to renal failure.Citation37,Citation38 Since there is no safe and convenient agent against renal dysfunction caused by lead, this study aimed to investigate the potential protective effects of the amino acid glycine against Pb nephrotoxicity. This study indicated that glycine supplementation (500 or 1,000 mg/kg, IP) mitigated Pb-induced renal injury, possibly by attenuating OS and its associated complications.
Different mechanisms have been proposed for the adverse effects of Pb toward biological systems. Among these mechanisms, OS and its associated complications have a fundamental role.Citation39 It has been well documented that Pb exposure is associated with significant ROS formation, defects in cellular antioxidant mechanisms, and disruption of biological targets such as cell membrane and different intracellular proteins.Citation40 Pb-associated OS is also important in nephrotoxicity mechanism.Citation40,Citation41 Some investigations mentioned the importance of antioxidant therapy against Pb-induced renal injury.Citation39 The obtained results emphasize on the importance of OS and its related consequences in the pathogenesis of Pb nephrotoxicity. In consensus with previous studies, we found that Pb exposure is associated with significant ROS formation, lipid peroxidation, kidney tissue GSH depletion, and defects in the renal antioxidant capacity. On the contrary, glycine supplementation (500 or 1,000 mg/kg, IP) damped off OS consequences in the kidney caused by lead exposure. These findings could indicate that the antioxidative activities of glycine might be important to its nephroprotective mechanisms.
Glycine is an amino acid with a simple structure, incorporated in protein structure. Glycine has different physiological and pharmacological features, and its anti-inflammatory, antioxidant, and osmoregulatory activities are reported in various experimental models.Citation42,Citation43 The impact of glycine on OS and its consequences are considered as its main cytoprotective activity.Citation15,Citation19 This amino acid is also a component of the GSH as a vital cellular antioxidant defense system. In the current investigation, higher levels of GSH were detected in the glycine-treated groups (), which might be an indication for higher rate of GSH synthesis in glycine-treated animals. We found that glycine treatment significantly reduced Pb-induced OS in the kidney tissue. On the contrary, no significant increase in plasma Pb level was detected after glycine treatment (). This might indicate that glycine is able to chelate Pb or enhance its excretion from the body ().
Several studies showed the positive effects of glycine on mitochondria.Citation14,Citation17,Citation20 One study showed that glycine treatment enhanced mitochondrial ATP content and prevented mitochondrial permeabilization and swelling.Citation14 Glycine treatment also modulates mitochondria-mediated cell death and apoptosis.Citation17,Citation44 Mitochondria is an important source of ROS.Citation45 Hence, a possible mechanism for the antioxidative properties of glycine might be mediated through affecting mitochondria-facilitated ROS formation. The precise effect of glycine on cellular mitochondria and its role in renal dysfunction caused by Pb exposure need further studies in order to be understood clearly.
Other organs rather than kidney might also be affected by Pb (eg, reproductive system and the liver). Therefore, evaluating the mechanism of Pb toxicity and the protective effects of chemicals such as glycine in these organs warranted further investigations.
Glycine is an endogenous and safe chemical. Hence, this amino acid might be clinically applicable against a variety of complications such as Pb-induced renal injury. To understand the findings of the current study in clinical setting, further studies are warranted.
Conclusion
Based on the results, glycine therapy could significantly reduce renal injury in mice exposed to Pb and the probable mechanism is the alleviation of OS.
Author contributions
MS, RH, MMO, AAS, and NA conceived and planned the experiments. RH, MMO, and AAS performed the measurements. MS, NA, and RH contributed to sample preparation and interpretation of the results. All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Abbreviations
| BALB | = | Bagg Albino |
| BUN | = | blood urea nitrogen |
| FRAP | = | ferric reducing antioxidant power |
| GSH | = | glutathione |
| IP | = | intraperitoneal |
| OS | = | oxidative stress |
| TBA | = | thiobarbituric acid assay |
Acknowledgments
Authors acknowledge their gratitude to the Pharmaceutical Sciences Research Center (no. 17657) of Shiraz University of Medical Sciences for technical support. The authors also wish to thank Mr H Argasi at the Research Consultation Center of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- CrinnionWJThe CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physiciansAltern Med Rev201015210110920806995
- MasonLHHarpJPHanDYPb neurotoxicity: neuropsychological effects of lead toxicityBiomed Res Int201420141018
- Navas-AcienAGuallarESilbergeldEKRothenbergSJLead exposure and cardiovascular disease--a systematic reviewEnviron Health Perspect2007115347248217431501
- RastogiSKRenal effects of environmental and occupational lead exposureIndian J Occup Environ Med200812310320040966
- JiaQHaXYangZHuiLYangXOxidative stress: a possible mechanism for lead-induced apoptosis and nephrotoxicityToxicol Mech Methods201222970571022894711
- BernardBPBeckerCEEnvironmental lead exposure and the kidneyJ Toxicol Clin Toxicol1988261–21343290509
- EkongEBJaarBGWeaverVMLead-related nephrotoxicity: a review of the epidemiologic evidenceKidney Int200670122074208417063179
- SongXBLiuGLiuFAutophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cellsCell Death Dis201786e286328594408
- BertkeSJLehmanEJWurzelbacherSJHeinMJMortality of lead smelter workers: a follow-up study with exposure assessmentAm J Ind Med2016591197998627350012
- InglisJAHendersonDAEmmersonBTThe pathology and pathogenesis of chronic lead nephropathy occurring in QueenslandJ Pathol197812426576363988
- GoyerRAMechanisms of lead and cadmium nephrotoxicityToxicol Lett1989461–31531622650022
- SudjarwoSAEraikoKSudjarwoGWKoerniasariProtective effects of piperine on lead acetate induced-nephrotoxicity in ratsIran J Basic Med Sci20172011122729299200
- WangLWangHHuMCaoJChenDLiuZOxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to leadArch Toxicol200983541742719347332
- HeidariRGhanbarinejadVMohammadiHMitochondria protection as a mechanism underlying the hepatoprotective effects of glycine in cholestatic miceBiomed Pharmacother2018971086109529136945
- HeidariRJamshidzadehAANiknahadHThe hepatoprotection provided by taurine and glycine against antineoplastic drugs induced liver injury in an ex vivo model of normothermic recirculating isolated perfused rat liverTrends Pharm Sci2016215976
- HeidariRMohammadiHAhmadiAAProtective effect of glycine and tri-methyl glycine (betaine) against heavy metals-induced oxidative stress in liver-derived post-nuclear supernatant (PNS)Trends Pharm Sci201842113124
- PalPBPalSDasJSilPCModulation of mercury-induced mitochondria-dependent apoptosis by glycine in hepatocytesAmino Acids20124251669168321373768
- YinMZhongZConnorHDProtective effect of glycine on renal injury induced by ischemia-reperfusion in vivoAm J Physiol Renal Physiol20022823F417F42311832421
- SenthilkumarRViswanathanPNaliniNEffect of glycine on oxidative stress in rats with alcohol induced liver injuryPharmazie2004591556014964423
- ShethHHafezTGlantzounisGKSeifalianAMFullerBDavidsonBRGlycine maintains mitochondrial activity and bile composition following warm liver ischemia-reperfusion injuryJ Gastroenterol Hepatol201126119420021175814
- MoneimAEADkhilMASjjohmA-QThe protective effect of flax-seed oil on lead acetate-induced renal toxicity in ratsJ Hazard Mater201119425025521872391
- HeidariRJafariFKhodaeiFShirazi YeganehBNiknahadHMechanism of valproic acid-induced Fanconi syndrome involves mitochondrial dysfunction and oxidative stress in rat kidneyNephrology201823435136128141910
- HeidariRJamshidzadehAGhanbarinejadVOmmatiMMNiknahadHTaurine supplementation abates cirrhosis-associated locomotor dysfunctionClin Exp Hepatol201842728229904723
- HeidariRGhanbarinejadVOmmatiMMJamshidzadehAANiknahadHRegulation of mitochondrial function and energy metabolism: a primary mechanism of cytoprotection provided by carnosineTrends Pharm Sci2018414150
- HeidariRTaheriVRahimiHRShirazi YeganehBNiknahadHNajibiASulfasalazine-induced renal injury in rats and the protective role of thiolreductantsRen Fail201638113714126479898
- HeidariRNiknahadHSadeghiABetaine treatment protects liver through regulating mitochondrial function and counteracting oxidative stress in acute and chronic animal models of hepatic injuryBiomed Pharmacother2018103758629635131
- HeidariRGhanbarinejadVMohammadiHDithiothreitol supplementation mitigates hepatic and renal injury in bile duct ligated mice: potential application in the treatment of cholestasis-associated complicationsBiomed Pharmacother2018991022103229307496
- HeidariRBabaeiHRoshangarLEghbalMAEffects of enzyme induction and/or glutathione depletion on methimazole-induced hepato-toxicity in mice and the protective role of N-acetylcysteineAdv Pharm Bull2014412124409405
- KatalinicVModunDMusicIBobanMGender differences in antioxidant capacity of rat tissues determined by 2, 2′-azinobis (3-eth-ylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assaysComp Biochem Physiol C Toxicol Pharmacol20051401475215792622
- JamshidzadehAHeidariRAbasvaliMTaurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemiaBiomed Pharmacother20178651452028024286
- BradfordMMA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnal Biochem1976721–2248254942051
- SedlakJLindsayRHEstimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagentAnal Biochem19682511922054973948
- DuruibeJOOgwuegbuMOCEgwurugwuJNHeavy metal pollution and human biotoxic effectsInt J Phys Sci200725112118
- FloraGGuptaDTiwariAToxicity of lead: a review with recent updatesInterdiscip Toxicol201252475823118587
- OmmatiMMJamshidzadehAAHeidariRCarnosine and histidine supplementation blunt lead-induced reproductive toxicity through antioxidative and mitochondria-dependent mechanismsBiol Trace Elem Res1871151162
- TelismanSColakBPizentAJurasovićJCvitkovićPReproductive toxicity of low-level lead exposure in menEnviron Res2007105225626617632096
- RastogiSKRenal effects of environmental and occupational lead exposureIndian J Occup Environ Med200812310310620040966
- SommarJNSvenssonMKBjörBMEnd-stage renal disease and low level exposure to lead, cadmium and mercury; a population-based, prospective nested case-referent study in SwedenEnviron Health2013121923343055
- PatrickLLead toxicity. Part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicityAltern Med Rev200611211416813461
- LiuCMMaJQSunYZQuercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by leadEnviron Toxicol Pharmacol201030326427121787659
- MatovićVBuhaAÐukić-ĆosićDBulatZInsight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneysFood Chem Toxicol20157813014025681546
- Díaz-FloresMCruzMDuran-ReyesGOral supplementation with glycine reduces oxidative stress in patients with metabolic syndrome, improving their systolic blood pressureCan J Physiol Pharmacol2013911085586024144057
- ZhongZWheelerMDLiXL-glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agentCurr Opin Clin Nutr Metab Care20036222924012589194
- JacobTAscherEHingoraniAKallakuriSGlycine prevents the induction of apoptosis attributed to mesenteric ischemia/reperfusion injury in a rat modelSurgery2003134345746614555933
- BrookesPSYoonYRobothamJLAndersMWSheuS-SCalcium, ATP, and ROS: a mitochondrial love-hate triangleAm J Physiol Cell Physiol20042874C817C83315355853
