Abstract
Benzodiazepines are the most widely prescribed class of psychoactive drugs in current therapeutic use, despite the important unwanted side effects that they produce, such as sedation, myorelaxation, ataxia, amnesia, and ethanol and barbiturate potentiation and tolerance. They exert their therapeutic effects via binding to the benzodiazepine binding site of gamma-aminobutyric acid (GABA) type A receptors, and allosterically modulating the chloride flux through the ion channel complex. First isolated from plants used as tranquilizers in folkloric medicine, some natural flavonoids have been shown to possess selective affinity for the benzodiazepine binding site with a broad spectrum of central nervous system effects. Since the initial search for alternative benzodiazepine ligands amongst the flavonoids, a list of successful synthetic derivatives has been generated with enhanced activities. This review provides an update on research developments that have established the activity of natural and synthetic flavonoids on GABA type A receptors. Flavonoids are prominent drugs in the treatment of mental disorders, and can also be used as tools to study modulatory sites at GABA type A receptors and to develop GABA type A selective agents further.
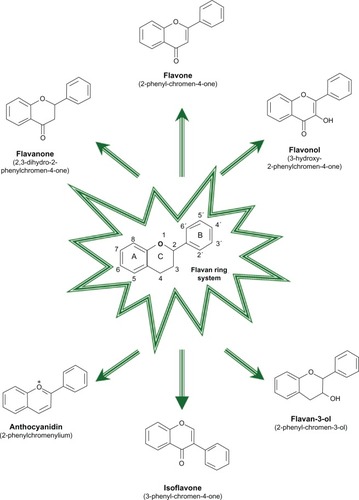
Flavonoids
Flavonoids may have existed in nature for almost one billion years, and over 9000 chemically unique flavonoids have been identified in plant sources. These compounds are low molecular weight substances, found in all vascular plants, and are phenylbenzopyrones () with an assortment of basic structures.
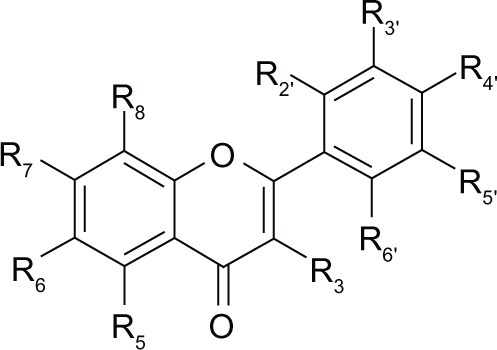
Flavonoids occur as aglycones, glycosides, and methylated derivatives. In plants, flavonoid aglycones occur in a variety of structural forms. For convenience, the rings are labeled A, B, and C (). The individual carbon atoms are based on a numbering system which uses ordinary numerals for A and C, and “primed” numerals for the B ring. The different ways of closing this ring associated with the different oxidation degrees of ring A define the various classes of flavonoids. Most flavonoids occur in natural association with sugar in conjugated form and, within any one class, may be characterized as, eg, monoglycosidic or diglycosidic. The glycosidic linkage is normally located at position 3 or 7 and the carbohydrate unit can be L-rhamnose, D-glucose, glucorhamnose, galactose, or arabinose.Citation1 The chemical diversity, size, three-dimensional shape, and physical and biochemical properties of flavonoids allow them to interact with targets in different subcellular locations to influence biological activity in plants, animals, and microbes.Citation2
Apart from being important dietary components, many therapeutic benefits of flavonoids are known in animal systems. Flavonoids have antioxidant, antiproliferative, antitumor, anti-inflammatory, and proapoptotic activities, and some molecular targets have been identified.Citation3–Citation7 The health-promoting effects of flavonoids may relate to interactions with key enzymes, signaling cascades involving cytokines and transcription factors, or antioxidant systems.Citation8
Because flavonoids can be found ubiquitously in plants, they are major constituents of a variety of fruit and vegetables, beverages, such as tea and wine, and seeds such as cocoa beans and grape seeds. Flavonoids undergo extensive biotransformation and conjugation that occur during their absorption from the gastrointestinal tract, in the liver, and finally in cells.Citation9–Citation11 Dietary flavonoids are substrates for phase I and phase II enzymes in the small intestine and liver. They are deglycosylated and metabolized into glucuronides, sulfates, and O-methylated derivatives.Citation12
Flavonoid absorption from the intestine occurs by several different pathways. Flavonoid aglycones can be easily absorbed into the intestinal cells because their lipophilicity facilitates their passage across the mucosal phospholipid bilayer of cells. Lactase phlorizin hydrolase has a crucial role in the absorption of flavonoids bearing β-glycoside linkages.Citation13 On the other hand, flavonoid monoglycosides can be transported by the sodium glucose transporter-1Citation14,Citation15 on the brush border membrane of intestinal cells. Most flavonoid glycosides entering enterocytes are deglycosylated by β-glucosidases, namely, broad-specificity cytosolic β-glucosidase.Citation16 The flavonoids appear to be subjected to glucuronidation, sulfation, and methylation in the intestinal epithelial cells before entering circulation.Citation17 The flavonoid conjugates then gain access into hepatocytes where they are further methylated, glucuronidated, or sulfated.Citation18 These flavonoid conjugates are excreted into the urine and also into bile fluid, thereby returning to the intestinal lumen.Citation19 Subsequently, they may be reabsorbed again, mainly in the large intestine. Further metabolism occurs in the colon, where enzymes of the gut microflora induce the breakdown of flavonoids to phenolic acids which may undergo absorption and be further metabolized in the liver.Citation11,Citation20 The present review focuses on advances in our knowledge pertaining to the action of flavonoids on the central nervous system, more precisely their effect on gamma amino butyric acid (GABA) type A receptors.
GABA type A receptor
Neuropharmacology is based simply on the fundamental balance between chemical excitation and inhibition. These processes are indispensable in the networks of neurons, and all neurons have receptors for inhibitory and excitatory neurotransmitters. The GABA system is one of the mechanisms that takes care of chemical inhibition in the brain, and has been widely used for pharmacological modulation of brain function. Most brain neurons express GABA type A receptors, which are considered to be the most important for pharmacological modulation. These receptors are heteropentameric GABA-gated chloride channels belonging to the Cys-loop ligand-gated ion channel superfamily that also includes the nicotinic acetylcholine receptors, glycine receptors, and 5-HT3 receptors.Citation21,Citation22 The subunits of all these receptors share a common ancestral structure. In addition to the rapid actions of GABA via GABA type A receptors, GABA also modulates neural activity, albeit on a slower time scale, via activation of GABA type A receptors belonging to the G protein-coupled receptor superfamily. GABA type A receptor subunits are encoded by 19 different genes that have been grouped into eight subclasses based on sequence homology (α1–6, β1–3, γ1–3, δ, ε, θ, π, ρ1–3). Alternative splicing contributes to additional receptor diversity.Citation23,Citation24 Knowing the number of subunits and potential combinations, the quantity of possible receptor subtypes could be enormous. However, only 11 structurally and functionally distinct receptor subtypes have been conclusively identified and are reasonably abundant in at least parts of the brain. They represent combinations of 2α and 2β subunits, together with a single γ2 or δ subunit. A further 15 receptor subtypes exist with high probability and a more limited distribution.Citation25 These numbers do not account for additional heterogeneity based on two different types of α or β subunits in one receptor complex,Citation26 or due to alternative splicing of subunits. GABA type A receptors with different subunit compositions exhibit different pharmacology and channel gating properties, are differentially expressed during development and in the adult brain, accumulate at different neuronal cell surfaces, and are subject to differential regulation by extracellular cues.Citation27
GABA type A receptors can be allosterically modulated by benzodiazepines, barbiturates, steroids, anesthetics, anticonvulsants, and many other drugs, the number of which is constantly increasing.Citation28,Citation29 These compounds do not interact directly with the GABA binding site, but exert their actions by binding to allosteric sites at GABA type A receptors that influence the binding properties of other binding sites present on these receptors and so modulate GABA-induced chloride ion influx ().
Figure 2 Schematic model of the GABA type A receptors.
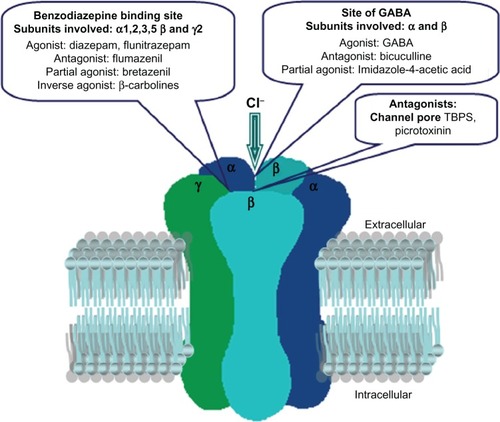
Benzodiazepines are anticonvulsive, sedative-hypnotic, and anxiolytic compounds in clinical use. They produce allosteric changes that enhance the action of GABA on GABA type A receptors, increasing the GABA-induced frequency of opening of the chloride channels and the apparent affinity of the receptor for GABA. Benzodiazepines represented a major advance in psychopharmacology in the 1960s and have been some of the most widely prescribed drugs. They are sedating and, more importantly, produce physical dependence, such that significant withdrawal symptoms are observed on treatment cessation. In addition, benzodiazepines can be drugs of abuse. This inspired the development of subtype-selective agonists, which might retain the beneficial effects of an antianxiety and anticonvulsant profile, while no longer being sedative, ataxic, or having dependence liability.Citation30
The location of the benzodiazepine binding site at the α and γ subunit interface indicates that the pharmacology of the benzodiazepine receptor subtypes is mainly determined by the α and γ isoforms forming this site, whereas β subunits, although needed to construct a channel, do not greatly affect the sensitivity of the GABA type A receptors to benzodiazepine ligands. The traditional benzodiazepine agonists (such as diazepam) are active at GABA type A receptors containing a γ subunit, a β subunit, and one of the α subunits, ie, α 1, 2, 3, or 5.Citation31 The benzodiazepine-sensitive GABA type A receptors can be further subdivided, in that receptors containing the α1 subunit have a higher sensitivity to a subpopulation of benzodiazepine ligands, such as quazepam or zolpidem (an imidazopyridine).Citation28,Citation31,Citation32 Furthermore, receptors containing the α2 or α3 subunit have an intermediate affinity for zolpidem, whereas those containing α5 have very low affinity for this drug.
Receptors containing the α4 or α6 subunits, together with β and γ2, do not bind the traditional benzodiazepine agonists, including zolpidem, but demonstrate high affinity for some ligands, such as flumazenil and Ro15-4513, or bretazenil.Citation28 Both the potency and efficacy for benzodiazepine ligands depend on the nature of the α subunit.
The benzodiazepine binding site ligands so far identified do not distinguish well between the α2 and α3 or between the α4 and α6 subunits. However, all four subunits can produce functional channels in vitro when coexpressed with other subunits, and their differential distribution in the brain suggests that they modulate different behavioral circuitry.
The functional relevance of the receptor subtypes was revealed using a combined molecular genetic and pharmacological approach.Citation33,Citation34 Mouse lines were generated in which each of the benzodiazepine-sensitive GABA type A receptors (containing α1, α2, α3, or α5 subunits) was rendered insensitive to diazepam by a point mutation in the benzodiazepine binding site (eg, α1 H101R).Citation35 A comparison of drug-induced behavioral responses in the mutated and wild-type mice then allowed identification of diazepam effects that were missing or reduced in the mutant mice. It was demonstrated that α1βγ2 receptors mediate the sedative, antegrade amnestic, and some anticonvulsant actions of diazepam.Citation35,Citation36 The anxiolytic activity of diazepam is mediated mostly by GABA type A receptors composed of α2βγ2 subunitsCitation37 and also by α3 GABA type A receptors.Citation38 This allowed sedation and anxiolysis to be separated in molecular terms, mediated by different pathways that were characterized by the presence of α1- and α2-GABA type A receptors, respectively. The α2βγ2 receptors are also implicated in some of the muscle relaxant activities of diazepam.Citation37 Receptors containing the α3 subunit seem to mediate the antiabsence effects of clonazepam. The α5βγ2 receptors seem to influence learning and memory.Citation39
The action of benzodiazepines can be blocked by flumazenil. However, flumazenil-insensitive positive modulation of GABA type A receptors has been described in receptors lacking a γ subunit by benzodiazepines at µM concentrations. It was found that recombinant α1β1 GABA type A receptors from the rat brain were sensitive to potentiation by benzodiazepine binding site ligands, with both diazepam and flumazenil acting as positive modulators.Citation40 It was also found that classical benzodiazepines produce biphasic potentiation at rat recombinant α1β2γ2 GABA type A receptors via two distinct mechanisms.Citation41 This biphasic potentiation is believed to be mediated via two sites, referred to as high-affinity and low-affinity benzodiazepine binding sites. Both sites are present on receptors composed of α1β2γ2 GABA type A subunits, and low affinity potentiation can be selectively observed at receptor combinations lacking a γ subunit, such as α1β2 GABA type A receptors. Furthermore, low affinity potentiation at both receptor combinations is insensitive to flumazenil.
Natural flavonoids as GABA type A receptor ligands
Nature provides science and society with a virtually unlimited supply of structurally diverse and biologically active molecules. While some are directly useful in commercial applications, others are valuable for studying and understanding biological phenomena at the molecular level. Flavonoids are only a modest example.
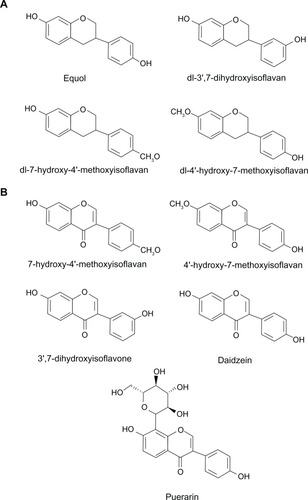
The first report of flavonoids as central nervous system ligands was described by Roche researchers. In their study, they were looking for the presence of “diazepam-like” endogenous ligands in bovine urine, in which they isolated a few isoflavan derivatives: (S-7,4′-dihydroxyisoflavan (equol); dl-3′,7-dihydroxyisoflavan; dl-4′-hydroxy-7-methoxyisoflavan; dl-7-hydroxy-4′-methoxyisoflavan; 7-hydroxy-4′-methoxyisoflavone (formononetin); and 4′-hydroxy-7-methoxyisoflavone; 3′,7-dihydroxyisoflavone) () with low affinity for the benzodiazepine binding site.Citation42 Some years later, using a radioreceptor-guided purification protocol, we were able to isolate one of these isoflavan ligands (equol) from bovine rumen contents, an important natural source of “diazepam-like” compounds.Citation43 These isoflavans were most probably derived from plant sources in the bovine diet.
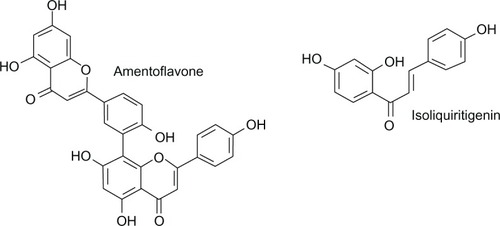
The next valuable antecedent in this story of flavonoids active in the central nervous system was the discovery of the biflavonoid, amentoflavone () as a high affinity ligand for the benzodiazepine binding site. This natural compound (Karmelitter Geist®) was isolated from an extract of several commercially available medicinal plants and used to treat nervous disorders.Citation44
Amentoflavone is one of the most potent flavonoids in displacing benzodiazepine binding to rat brain membranes, but is inactive in vivo. It binds, in vitro, in a mixed-type competitive and noncompetitive manner to brain receptors, with an affinity comparable with that of diazepam. Studies on subtype specificity showed that amentoflavone had little or no affinity for α4-containing or α6-containing receptors.Citation45 This biflavonoid can be extracted from Ginkgo biloba but removed from herbal preparations such as EGb 761.Citation46 Using a functional assay employing recombinant GABA type A receptors expressed in oocytes, amentoflavone has been shown to be a weak negative allosteric modulator of GABA action, acting independently of classical flumazenil-sensitive benzodiazepine-modulatory sites.Citation46 It was also reported that amentoflavone influences a variety of G protein-coupled receptors for serotonin, dopamine, and opioids at nM concentrations, while having no effect on the binding of muscimol, a GABA type A agonist to GABA type A receptors.Citation47
Since then and contemporary with the work described above, research in our laboratories was devoted to the identification of natural ligands for the benzodiazepine binding site in plants. In particular, we were looking for benzodiazepines or benzodiazepine-like compounds, and the discovery of the properties of amentoflavoneCitation44 drove our investigation to similar ligands in plants known to contain flavonoids and we also extended this study to other plants traditionally used as tranquillizers.Citation48–Citation51
In vivo and in vitro studies with the principal flavonoid and flavonol derivatives () have clarified their pharmacological actions on the binding of benzodiazepines. The first monoflavonoid described as a specific ligand for the benzodiazepine binding site was chrysin (5,7-dihydroxyflavone).Citation52 This compound, isolated from Passiflora caerulea L, is a selective and competitive inhibitor of [3H]flunitrazepam binding to the benzodiazepine binding site. Chrysin is almost equipotent to diazepam as an anxiolytic, but does not exhibit sedative or myorelaxant effects.Citation53 It was postulated that this natural monoflavonoid is a partial agonist of the central benzodiazepine binding site. Additionally, both intraperitoneal and oral administration of chrysin in mice produced a significant hyperalgesic effect in the tail-immersion test that involved GABA type A receptors, as does flumazenil, a specific antagonist for the benzodiazepine binding site; bicuculline, a GABA type A receptor antagonist and picrotoxin, a chloride channel blocker, could antagonize the hyperalgesia of chrysin.Citation54
Table 1 Flavone and flavonol derivatives
The dried flower heads of Matricaria recutita L are used in folk medicine to prepare a spasmolytic and sedative tea. The fractionation of the aqueous extract of this plant led to the detection of several fractions with significant affinity for the benzodiazepine binding site and to the isolation and identification of apigenin (5,7,4′-trihydroxyflavone). Apigenin competitively binds to the benzodiazepine binding site of the GABA type A receptor, has clear anxiolytic activity in mice when administered intraperitoneally, without showing evidence of sedation or muscle relaxant effects at doses similar to those used for classical benzodiazepines and no anticonvulsant action.Citation55 However, other studies, performed in rats, found that apigenin fitted the profile of an inverse benzodiazepine agonist and was sedative and mildly proconvulsant, but not anxiolytic.Citation56,Citation57 Meanwhile, others reported that apigenin fitted the profile of a benzodiazepine antagonist.Citation58 The discrepancies between the in vitro and in vivo results obtained for apigenin may be due to its in vivo metabolism or the use of different species, ie, rats or mice, because mice have higher baseline levels of anxiety. Thus, apigenin appears to have diverse effects at the benzodiazepine binding site and the nature of these effects is unclear. Overall, it seems that the effects of apigenin on GABA type A receptors are complex and involve both flumazenil-sensitive and flumazenil-insensitive components, and that other receptors could be involved in the behavioral effects of this drug.Citation59,Citation60
The flavonoids, kaempferol (3,5,7,4′-tetrahydroxyflavone) and cirsiliol (5,3′,4′-trihydroxy-6,7-dimethoxyflavone), isolated from Tilia tomentosa Moench and Salvia guaranitica, respectively, exhibited a very low affinity for the benzodiazepine binding site and were devoid of anxiolytic actions by the intraperitoneal route. However, cirsiliol produced sedation in mice as measured by the holeboard test and had thiopental-potentiating effects.Citation61–Citation63 Recently, a comparative study of the anxiolytic activity of the flavonols, kaempferol, quercetin, (3,5,7,3′,4′-pentahydroxyflavone), and myricetin (3,5,7,3′,4′,5′-hexahydroxyflavone) in the elevated plus-maze after oral and intraperitoneal administration in mice showed that only kaempferol and quercetin were active after oral administration.Citation64 The anxiolytic activity of kaempferol was also partially antagonized by concomitant administration of flumazenil.Citation65 No anxiolytic effects were observed when kaempferol and quercetin were given via the intraperitoneal route. It was hypothesized that flavonoids could act as prodrugs which are transformed into their active hydroxyphenylacetic acid metabolites by intestinal microflora.Citation64
Wogonin (5,7-dihydroxy-8-methoxyflavone), isolated from Scutellaria baicalensis Georgi, a Chinese medicinal herb, has been reported to have anxiolytic and anticonvulsant activity in orally treated mice, that could be blocked by coadministration of flumazenil,Citation66,Citation67 with no sedative or myorelaxant effects. Another flavonoid isolated from this herb, oroxylin A (5,7-dihydroxy-6-methoxyflavone), inhibits [3H] flunitrazepam binding and, orally administered in mice, acts as a neutralizing allosteric modulator blocking the anxiolytic, myorelaxant, and motor incoordination effects but not the sedative and anticonvulsant effects elicited by diazepam, a full benzodiazepine agonist.Citation68
Dinatin, a synonym of hispidulin (4′,5,7-trihydroxy-6-methoxyflavone) and skrofulein, a synonym of cirsimaritin (4′,5-dihydroxy-6,7-dimethoxyflavone), two flavones isolated from the medicinal plant from Artemisia herba-alba Asso, were found to be antagonists or weak partial agonists of the benzodiazepine binding site, and inhibited the binding of [3H] diazepam to rat brain membranes in vitro by a mixed competitive and noncompetitive mechanism.Citation69 Hispidulin, the 6-methoxy derivative of apigenin, was further isolated, together with apigenin from Salvia officinalis (sage) using benzodiazepine binding site assay-guided fractionation.Citation70 This flavone has been demonstrated to have anticonvulsant activity in a model of epilepsy in seizure-prone Mongolian gerbils and to cross the blood-brain barrier.Citation71 Unlike apigenin, hispidulin has been shown to act as a positive allosteric modulator of α1,3,5,6β2γ2S GABA type A receptor subtypes.Citation71
Daidzein (4′,7-dihydroxyisoflavone) and its 8-C glycoside, puerarin (7,4′-dihydroxy-8-C-glucosylisoflavone) (), two isoflavones isolated from Puerariae radix, a Chinese traditional herb used to treat drunkenness and alcoholic addiction, were found to inhibit the binding of [3H] flunitrazepam to rat brain membranes.Citation72
Leptospermum scoparium Forst contains the lipophilic flavonoids, 5,7-dimethoxyflavone, 5,7-dimethoxy-6-methylflavone, 5-hydroxy-7-methoxy-6-methylflavone, and 5-hydroxy-7-methoxy-6,8-dimethylflavone, which interact specifically with the benzodiazepine binding site. A dry extract of the tincture prepared from this plant and containing these flavones induced sedative and anxiolytic effects in rats.Citation73
Another apigenin derivative was further reported as a benzodiazepine binding site ligand. 6-Methylapigenin (4′,5,7-dihydroxy-6-methylflavone), the 6-methyl derivative of apigenin, was isolated from the roots and rhizomes of Valeriana wallichii, a known sedative medicinal plant, and inhibited [3H]flunitrazepam binding at 0.5 µM in a manner suggesting it may be a positive modulator of GABA type A receptors.Citation74 6-Methylapigenin induced anxiolytic effects in mice treated by the intraperitoneal route and was able to potentiate the sleep-enhancing properties of hesperidin (hesperetin 7-rhamnoglucoside), a flavanone glycoside also isolated from V. wallichii and Valeriana officinalis.Citation75
Epigallocatechin gallate (EGCG, (−)-cis-3,3′,4′,5,5′,7-hexahydroxy-flavane-3-gallate) (), a flavanol ester and a constituent of green tea, was demonstrated to exert dose-dependent anxiolytic, sedative-hypnotic, and amnesic activities after acute intraperitoneal administration in mice, that could be mediated, at least in part, by GABA type A receptors.Citation76,Citation77 In vitro, this flavonoid could also inhibit activation by GABA and enhance the modulatory action of diazepam on activation by GABA of recombinant human α1β2γ2L GABA type A receptors expressed in Xenopus laevis oocytes.Citation78
Some natural and synthetic flavanones have been investigated in benzodiazepine binding studies. Examples are eriodictyol (5,7,3′,4′-tetrahydroxyflavanone), hesperetin (5,7,3′-trihydroxy-4′-methoxyflavanone), and flavanone itself (), among others. All of them were found to be inactive or only weakly active in vitro.Citation73,Citation79,Citation80 However, it was recently reported that dihydromyricetin (3,5,7,3′,4′,5′-hexahydroxyflavanone) () is a positive modulator of GABA type A receptors at benzodiazepine binding sites that competitively inhibited [3H]flunitrazepam binding with moderate affinity. Dihydromyricetin is a flavonoid component of Hovenia dulcis, an herbal medicine listed among the premier antihangover plants in China that ameliorates alcohol-induced liver injuries and relieves hangover. This flavanone, administered intraperitoneally in rats, is highly effective in counteracting acute alcohol intoxication, alcohol exposure/withdrawal-induced GABA type A receptor plasticity and alcohol withdrawal syndrome symptoms, as well as reducing excessive alcohol consumption.Citation81
Luteolin (5,7,3′,4′-tetrahydroxyflavone) is a flavonoid aglycon found in a wide variety of plants. It has been reported to displace [3H]flunitrazepam from the benzodiazepine binding site in vitro, with low affinity, and it has demonstrated anxiolytic-like effects administered orally in mice. Despite the need to analyze the interaction of luteolin with the benzodiazepine binding site further, these results suggested that by itself this interaction does not seem to explain the results observed in vivo fully, thus prompting renewed interest in the analysis of possible interactions with other receptors.Citation82,Citation83
Baicalein (5,6,7-trihydroxyflavone) and baicalin (baicalein 7-O-D-glucuronide), together with wogonin and oroxylin A, are the major bioactive components in S. baicalensis. Baicalin was reported to induce an anxiolytic-like effect devoid of sedation and myorelaxation in mice when administered orally, acting via the benzodiazepine binding site. It showed significant preference for α2-containing and α3-containing subtypes compared with α1-containing and α5-containing subtypes in whole-cell patch clamp studies.Citation84 Baicalein and baicalin also showed neuroprotective and anticonvulsant effects in rats injected intraperitoneally and the anticonvulsant effect of baicalein was inhibited by flumazenil.Citation85 Other authors reported that baicalein showed anxiolytic and sedative effects when administered via the intracerebroventricular route in mice, and that this central effect were blocked by pentylenetetrazole but not by flumazenil. Therefore, they concluded that the in vivo actions of baicalein could be mediated by GABAergic nonbenzodiazepine binding sites.Citation86 Another naturally occurring flavonoid isolated from S. baicalensis, ie, K36 (5,7,2′-trihydroxy-6,8-dimethoxyflavone), exhibited the highest affinity for the benzodiazepine binding site, comparable with that of the synthetic anxiolytic, diazepam (Ki 6.05 nM). In electrophysiological experiments, K36 potentiated currents mediated by the recombinant rat α1β2γ2 GABA type A receptor expressed in X. laevis oocytes and this enhancement was demonstrated to act via the benzodiazepine binding site. Oral administration of K36 produced significant benzodiazepine binding site-mediated anxiolysis in the mouse elevated plus-maze, which was abolished upon coadministration of flumazenil. Sedation, myorelaxation, and motor incoordination were not observed. Structure-activity relationships utilizing synthetic flavonoids on the flavone backbone supported that 2′-hydroxyl-substitution is a critical moiety on flavonoids with regard to benzodiazepine binding site affinity.Citation87
Chalcones are unique in the flavonoid family in lacking a heterocyclic C ring and exhibit the basic structure with two benzene rings linked through an α, β-unsaturated carbonyl group. Isoliquiritigenin (2′,4′,4′-trihydroxychalcone) () is a chalcone compound found in Glycyrrhiza uralensis (licorice), Allium ascalonicum, Sinofranchetia chinensis, Dalbergia odorifera, and Glycine max L. This chalcone showed anxiolytic effects in the elevated plus-maze test administered intraperitoneally in mice and rats. It also significantly potentiated pentobarbital-induced sleep in a dose-dependent manner and this effect was fully inhibited by flumazenil. The binding affinity of isoliquiritigenin was 0.453 µM and it potentiated GABA-evoked currents on isolated dorsal raphe neurons. Thus, these results suggest that this natural chalcone produces hypnotic effects by positive allosteric modulation of the benzodiazepine binding site of the GABA type A receptor.Citation88,Citation89
Synthetic derivatives: importance of 6-substitutions on the flavonoid nucleus
The results obtained with natural flavonoids encouraged us to attempt to increase their potency as benzodiazepine binding site ligands by introducing electronegative substituents into their molecules, because this feature was shown to be essential for the activity of the classical benzodiazepines.Citation90
Based on structure-activity relationship studies, incorporation of electronegative groups into the C6 and C3′ on the flavone backbone was found to yield significant increases in the binding affinities for the benzodiazepine binding site.Citation79,Citation80 It was also shown that 2′-hydroxyl was also a critical moiety on flavonoids with regard to benzodiazepine binding.Citation87 These have guided the generation of several synthetic flavonoids with high benzodiazepine binding affinity and in vivo activity, and further quantitative structure-activity relationship studies resulted in the development of several pharmacophore models.
The first report of an active derivative of flavone was a brominated compound, 6-bromoflavone. This derivative showed a high affinity for the benzodiazepine binding site and resulted in a competitive ligand for these receptors. Its pharmacological profile, when administered intraperitoneally in mice, was quite similar to that observed for diazepam.Citation91 The data to date strongly suggest that 6-bromoflavone is a full agonist of the central benzodiazepine binding site. 6-Bromo-3′-nitroflavone exhibited a different affinity for benzodiazepine binding site subtypes containing the α1 and α2/3 receptors. The plus-maze test revealed that it is anxiolytic at intraperitoneal doses of 10–300 µg/kg in mice.Citation92,Citation93 In contrast, 6-chloro-3′-nitroflavone and 6-methyl-3′-bromoflavone had no anxiolytic effects, and their pharmacological profile manifested antagonistic actions at the benzodiazepine binding site.Citation94,Citation95 In turn, 6,3′-dibromoflavone and 6-nitro-3′-bromoflavone had anxiolytic effects when administered intraperitoneally in mice, with partial agonistic behavior ().Citation96
Table 2 Synthetic flavone derivatives
The most active anxiolytic flavone derivative prepared in our laboratory was 6,3′-dinitroflavone. A 1–30 µg/kg intraperitoneal injection of this compound in mice produced anxiolytic-like effects. It is at least 30 times more potent by weight than diazepam and 3000 times more potent than flavone. It also has a very favorable separation index, being the separation index the ratio between the minimal sedative dose and the maximal anxiolytic one. Its magnitude qualifies the pharmacological selectivity of the compound.Citation97,Citation98 Inhibition of [3H]flunitrazepam binding to recombinant GABA type A receptors in transiently transfected HEK293 indicated that 6,3′dinitroflavone exhibited the highest affinity for GABA type A receptors composed of α1β2γ2 subunits and a 2–20-fold lower affinity for homologous receptors containing α2, α3, or α5 subunits. 6,3′-dinitroflavone did not elicit currents in the absence of GABA in X. laevis oocytes expressing any of the recombinant GABA type A receptors tested. However, 6,3′-dinitroflavone was slightly able to modulate GABA-elicited currents, similar to other benzodiazepine site ligands.Citation99
Relatively extensive pharmacological studies on our synthetic flavonoids have been conducted by other authors, and the results obtained confirmed and extended our findings.Citation100,Citation101 Recently, flavone analogs, each varying only in the 6-position substituent, were compared. Whole-cell patch-clamp and animal behavior experiments demonstrated 6-bromoflavone to be a positive modulator at GABA type A receptors, acting via a flumazenil-sensitive high-affinity benzodiazepine binding site. In contrast, 6-fluoroflavone and 6-chloroflavone were found to be neutralizing modulators. In patch-clamp studies, 6-hydroxyflavone displayed a significant preference for α2-containing and α3-containing subtypes, which were thought to mediate the anxiolytic effect, compared with α1-and α5-containing subtypes expressed in HEK 293T cells. In mice, 6-hydroxyflavone exhibited anxiolytic-like effects unaccompanied by the sedative, cognitive impairment, myorelaxant, motor incoordination, and anticonvulsant effects commonly associated with classical benzodiazepines.Citation102 In addition, in vitro electrophysiological and in vivo animal experiments showed that 2′-hydroxyflavone was an antagonist, different in efficacy from its structural analog, 6,2′-dihydroxyflavone, a negative modulator of GABA type A receptors. The fact that flavone derivatives differing only at position 6 showed drastically different pharmacological properties clearly points to 6-substitution being an important determinant of efficacy. All the results suggest that a large width of the first atom on the 6-substituent favors a high binding affinity of the 6-substituted flavone, whereas a large overall volume of the 6-substituent favors positive modulator activity, which could be modified by, eg, 2′-hydroxyl substitution.Citation101
6-Methylflavone was found to be a positive allosteric modulator at α1β2γ2L and α1β2 GABA type A receptors, with no significant difference between the enhancement seen at either receptor subtype, at ionotropic GABA type A receptors expressed in X. laevis oocytes and at sites independent of the flumazenil-sensitive benzodiazepine binding site.Citation103 Subsequently, 6-methylflavanone () was also found to be a flumazenil-insensitive positive modulator of recombinant GABA type A receptors that, unlike 6-methylflavone, was subtype-selective, being a more efficacious positive modulator at α2β2γ2L receptors that at α1β2γ2L and α1β2 receptors.Citation104 6-Methylflavanone differs from 6-methylflavone in having a single rather than a double bond at C2–C3; hence, this bond is crucial to the observed subtype-selective efficacy of 6-methylflavanone. Two new 6-methylflavone derivatives were recently reported, ie, 2′-methoxy-6-methylflavoneCitation105 and 3-hydroxy-2′-methoxy-6-methylflavone.Citation106 2′-Methoxy-6-methylflavone potentiated GABA at α2β1γ2L and all α1-containing GABA type A receptor subtypes. However, it directly activated α2β2/3γ2L GABA type A receptors without potentiating GABA. This activation was attenuated by bicuculline and gabazine, but not flumazenil, indicating a novel site. In mice, when administered intraperitoneally, it displayed anxiolytic-like effects, and at higher doses induced sedation. Like the in vitro data, the anxiolytic effects of 2′-methoxy-6-methylflavone were not blocked by flumazenil, a benzodiazepine antagonist, but were attenuated by pentylenetetrazole, a GABA type A channel blocker. These data imply that the anxiolytic effects are mediated via the GABAergic system, but not through the classical benzodiazepine binding site, despite 2′-methoxy-6-methylflavone weakly displacing flunitrazepam binding. These data would indicate that 2′-methoxy-6-methylflavone could bind to a novel as yet unidentified site on α2β2/3γ2L GABA type A receptors. 2′-Methoxy-6-methylflavone could serve as a tool to study the complex nature of the activation and modulation of GABA type A receptor subtypes. In addition, the synthetic flavonoid, 3-hydroxy-2′-methoxy-6-methylflavone was reported to be an anxiolytic without sedative and myorelaxant effects when administered intraperitoneally in mice, acting through positive allosteric modulation of the α2β2/3γ2L and direct activation of α4β2/3δ GABA type A receptor subtypes ().
Using 6-methylflavanone and EGCG as lead compounds, structure-activity studies led to the discovery of Fa131 (2S,3R-3-acetoxy-4′-methoxyflavan) () as the most efficacious compound in a series of flavonol esters with positive modulatory activity of GABA type A receptors.Citation107,Citation108 Interestingly, similar to barbiturates, Fa131 also acts as a weak partial agonist α1β2γ2L receptor and at higher doses exerts a negative modulatory effect.Citation107 Fa131 is the first positive modulator to distinguish between the α2-subunit and α3-subunit containing GABA type A receptors, highlighting the potential of targeting flumazenil-insensitive allosteric sites in the search for new anxioselective drugs. In mice, when administered intraperitoneally, it induced an anxiolytic-like action with no sedative or myorelaxant effects, and only weak barbiturate-potentiating effects on the loss of the righting reflex test. Recently there has been a report of a new flavan, Fa173 (2S;3S-3 acetoxy-3′,4′-dimethoxyflavan) (), which neutralizes the potentiating actions of Fa131, etomidate, and loreclezole at α1β2 and α1β2γ2L GABA type A receptors expressed in X. laevis oocytes. Furthermore potentiation of high, but not low, concentrations of diazepam can be blocked by Fa173.Citation109
A series of isoflavone derivatives was recently synthesized, and their modulatory effect was evaluated on the α1β2γ2L GABA type A receptors expressed in X. laevis oocytes. This set of isoflavones acted as positive modulators of GABA type A receptors and it was demonstrated that substitution of the A, B, and C rings plays an important role in determining GABA type A modulation activity. Flumazenil-insensitive modulation by the isoflavones suggested that these compounds might not bind to the benzodiazepine binding site.Citation118
Since the first discovery of a flavonoid as a benzodiazepine binding site ligand almost 30 years ago, an extensive collection of natural and synthetic flavonoids has been reported providing potential leads for new GABA type A receptor agents and they have already become drugs with beneficial effects in the central nervous system. The in vivo and in vitro actions of some flavonoids are more complex than a single action at the benzodiazepine binding site, and some evidence of a flavonoid site in GABA type A receptors is emerging. More studies are required in order to determine the precise site of action of these bioactive molecules on GABA type A receptors and to understand fully their mechanisms of action as modulators of brain function. Also, further investigations of type specificity of flavonoids might lead to identification of flavonoids with selective pharmacological activity, thus providing a clinically interesting lead structure. Further, the concentrations of dietary flavonoids encountered in vivo are high enough to have pharmacological activity at receptors, and the evidence also supports localization of flavonoids within the brain.
In summary, flavonoids are prominent drugs in the treatment of mental disorders, and can also be used as tools to study modulatory sites at GABA type A receptors and to develop GABA subtype-selective agents further.
Acknowledgments
We are grateful to the International Foundation for Science, Stockholm, Sweden, the National Research Council of Argentina, the University of Buenos Aires, and the Ministry of Health and the National Agency for Promotion of Science and Technology, Argentina, for financial support.
Disclosure
The authors report no conflicts of interest in this work.
References
- TapasARSakarkarDMKakdeRBFlavonoids as nutraceuticals: a reviewTrop J Pharm Res2008710891099
- BuerCSIminNDjordjevicMAFlavonoids: new roles for old moleculesJ Integr Plant Biol2010529811120074144
- SpencerJPEBeyond antioxidants: the cellular and molecular interactions of flavonoids and how these underpin their actions on the brainProc Nutr Soc20106924426020158941
- TaylorLPGrotewoldEFlavonoids as developmental regulatorsCurr Opin Plant Biol2005831732315860429
- GonzálezRBallesterILópez-PosadasREffects of flavonoids and other polyphenols on inflammationCrit Rev Food Sci Nutr20115133136221432698
- ShanmugamMKKannaiyanRSethiGTargeting cell signaling and apoptotic pathways by dietary agents: role in the prevention and treatment of cancerNutr Cancer20116316117321294053
- XiaoZPPengZYPengMJYanWBOuyangYZZhuHLFlavonoids health benefits and their molecular mechanismMini Rev Med Chem20111116917721222576
- TuñónMJGarcía-MediavillaMVSánchez-CamposSGonzález-GallegoJPotential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathwaysCurr Drug Metab20091025627119442088
- WilliamsonGManachCBioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studiesAm J Clin Nutr200581243S255S15640487
- ManachCWilliamsonGMorandCScalbertARémésyCBioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studiesAm J Clin Nutr200581230S242S15640486
- SpencerJPEAbd El MohsenMMMinihaneAMMathersJCBiomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition researchBr J Nutr200899122217666146
- SpencerJPEMetabolism of tea flavonoids in the gastrointestinal tractJ Nutr2003133Suppl3255S3261S14519823
- DayAJCanadaFJDiazJCDietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolaseFEBS Lett200046816617010692580
- WalgrenRALinJTKinneRKWalleTCellular uptake of dietary flavonoid quercetin 4′-beta-glucoside by sodium-dependent glucose transporter SGLT1J Pharmacol Exp Ther200029483784310945831
- WolfframSBlockMAderPQuercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestineJ Nutr200213263063511925453
- NemethKPlumbGWBerrinJGDeglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humansEur J Nutr200342294212594539
- Radominska-PandyaALittleJMPandyaJTUDP-glucuronosyltransferases in human intestinal mucosaBiochim Biophys Acta199813941992089795217
- O’LearyKADayAJNeedsPWMellonFAO’BrienNMWilliamsonGMetabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolismBiochem Pharmacol20036547949112527341
- MatsukawaNMatsumotoMHaraHHigh biliary excretion levels of quercetin metabolites after administration of a quercetin glycoside in conscious bile duct cannulated ratsBiosci Biotechnol Biochem2009731863186519661706
- SchelineRRMetabolism of oxygen heterocyclic compoundsHandbook of Mammalian Metabolism of Plant CompoundsBoca Raton, FLCRC Press Inc1999
- UnwinNThe structure of ion channels in membranes of excitable cellsNeuron198936656762484344
- BarnardEASkolnickPOlsenRWInternational Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: Classification on the basis of subunit structure and receptor functionPharmacol Rev1998502913139647870
- WhitingPMcKernanRMIversenLLAnother mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation siteProc Natl Acad Sci U S A199087996699701702226
- McKinleyDDLennonDJCarterDBCloning, sequence analysis and expression of two forms of mRNA coding for the human beta 2 subunit of the GABAA receptorMol Brain Res1995281751797707873
- OlsenRWSieghartWInternational Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. UpdatePharmacol Rev20086024326018790874
- BenkeDFakitsasPRoggenmoserCMichelCRudolphUMohlerHAnalysis of the presence and abundance of GABAA receptors containing two different types of alpha subunits in murine brain using point-mutated alpha subunitsJ Biol Chem2004279436544366015304513
- LuscherBFuchsTKilpatrickCLGABAA receptor trafficking-mediated plasticity of inhibitory synapsesNeuron20117038540921555068
- KorpiERGründerGLüddensHDrug interactions at GABA(A) receptorsProg Neurobiol20026711315912126658
- SieghartWStructure and pharmacology of gamma-aminobutyric acid A receptor subtypesPharmacol Rev1995471812347568326
- IversenLGABA pharmacology – what prospects for the future?Biochem Pharmacol2004681537154015451396
- SieghartWStructure, pharmacology, and function of GABAA receptor subtypesAdv Pharmacol20065423126317175817
- OlsenRWSieghartWGABAA receptors: Subtypes provide diversity of function and pharmacologyNeuropharmacology20095614114818760291
- RudolphUMohlerHAnalysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse geneticsAnnu Rev Pharmacol Toxicol20044447549814744255
- WhitingPJGABAA-receptors: a viable target for novel anxiolytics?Curr Opin Pharmacol20066242916359919
- RudolphUCrestaniFBenkeDH. Benzodiazepine actions mediated by specific gammaaminobutyric acid(A) receptor subtypesNature199940179680010548105
- McKernanRMRosahlTWReynoldsDSSedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtypeNat Neurosci2000358759210816315
- LowKCrestaniFKeistRMolecular and neuronal substrate for the selective attenuation of anxietyScience200029013113411021797
- DiasRSheppardWFFradleyRLEvidence for a significant role of alpha3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepinesJ Neurosci200525106821068816291941
- CollinsonNKuenziFMJarolimekWEnhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha5 subunit of the GABAA receptorJ Neurosci2002225572558012097508
- MalherbePDraguhnAMulthaupGBeyreutherKMöhlerHGABAA-receptor expressed from rat brain α- and β-subunit cDNAs displays potentiation by benzodiazepine receptor ligandsMol Brain Res199081992081977069
- WaltersRJHadleySHMorrisDWAminJBenzodiazepines act on GABAA receptors via two distinct and separable mechanismsNat Neurosci200031274128111100148
- LukKCSternLWeigeleMO’BrienRASpirstNIsolation and identification of “diazepam-like” compounds from bovine urineJ Nat Prod1983468528616330305
- MedinaJHDanelonJLWasowskiCLevi de SteinMPaladiniACProduction of benzodiazepine-like compounds in bovine rumenBiochem Biophys Res Commun1991181104810551764056
- NielsenMFrokjaerSBraestrupCHigh affinity of naturally-occurring biflavonoid, amentoflavone, to brain benzodiazepine receptors in vitroBiochem Pharmacol198837328532872840912
- HansenRSPaulsenIDaviesMDeterminants of amentoflavone interaction at the GABA(A) receptorEur J Pharmacol200551919920716129428
- HanrahanJRChebibMDavucheronNMHallBJJohnstonGARSemisynthetic preparation of amentoflavone: A negative modulator at GABAA receptorsBioorg Med Chem Lett2003132281228412824018
- ButterweckVNahrstedtAEvansJIn vitro receptor screening of pure constituents of St John’s wort reveals novel interactions with a number of GPCRsPsychopharmacology200216219320212110997
- MedinaJHPeñaCLevi de SteinMWolfmanCPaladiniACBenzodiazepine-like molecules as well as other ligands for the brain benzodiazepine receptor are relatively common constituents of plantsBiochem Biophys Res Commun19891655475532557016
- MedinaJHViolaHWolfmanCOverview – flavonoids: a new family of benzodiazepine receptor ligandsNeurochem Res1997224194259130252
- PaladiniACMarderMViolaHWolfmanCWasowskiCMedinaJHFlavonoids and the central nervous system: from forgotten factors to potent anxiolytic compoundsJ Pharm Pharmacol19995151952610411210
- MarderMPaladiniACGABA(A)-receptor ligands of flavonoid structureCurr Top Med Chem2002285386712171576
- MedinaJHPaladiniACWolfmanCChrysin (5,7 di-OH flavone) a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant propertiesBiochem Pharmacol199040222722322173925
- WolfmanCViolaHPaladiniACDajasFMedinaJHPossible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coeruleaPharmacol Biochem Behav199447147906886
- ZhaiKHuLChenJFuCYChenQChrysin induces hyperalgesia via the GABAA receptor in micePlanta Med2008741229123418612941
- ViolaHWasowskiCLevi de SteinMApigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effectsPlanta Med1995612132167617761
- AvalloneRZanoliPPuiaGKleinschnitzMSchreierPBaraldiMPharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomillaBiochem Pharmacol2000591387139410751547
- ZanoliPAvalloneRBaraldiMBehavioral characterization of the flavonoids apigenin and chrysinFitoterapia200071S117S12310930722
- DekermendjianKKahnbergPWittMRSternerONielsenMLiljeforsTStructure–activity relationships and molecular modeling analysis of flavonoids binding to the benzodiazepine site of the rat brain GABAA receptor complexJ Med Chem1999424343435010543878
- GoutmanJDWaxembergMDDonate-OliverFPomataPECalvoDJFlavonoid modulation of ionic currents mediated by GABAA and GABAC receptorsEur J Pharmacol2003461798712586201
- CampbellELChebibMJohnstonGARThe dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABAA receptorsBiochem Pharmacol2004681631163815451406
- ViolaHWolfmanCLevi de SteinMIsolation of pharmacologically active benzodiazepine receptor ligands from Tília tomentosa (Tiliaceae)J Ethnopharmacol19944447537990504
- ViolaHMarderMWolfmanCWasowskiCMedinaJHPaladiniACSedative and hypnotic properties of Salvia guaranítica St Hil and of its active principle, cirsiliolPhytomedicine199744550
- MarderMViolaHWasowskiCCirsiliol and caffeic acid ethyl ester isolated from Salvia guaranitica, are competitive ligands for the central benzodiazepine receptorsPhytomedicine19963293123194857
- VissiennonaCNieberKKelberOButterweckVRoute of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin – are they prodrugs?J Nutr Biochem2011811 Epub ahead of print
- GrundmannONakajimaJKamataKSeoSButterweckVKaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in micePhytomedicine20091629530219303276
- HuiKMHuenMSWangHYAnxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis GeorgiBiochem Pharmacol2002641415142412392823
- ParkHGYoonSYChoiJYAnticonvulsant effect of wogonin isolated from Scutellaria baicalensisEur J Pharmacol200757411211917692312
- HuenMSLeungJWNgW5,7-Dihydroxy-6-methoxyflavone, a benzodiazepine site ligand isolated from Scutellaria baicalensis Georgi, with selective antagonistic propertiesBiochem Pharmacol200366125113212818372
- ShenXLNielsenMWittMRSternerOBergendorffOKhayyalMInhibition of [methyl-3H]diazepam binding to rat brain membranes in vitro by dinatin and skrofuleinZhongguo Yao Li Xue Bao1994153853887717057
- KavvadiasDMonscheinVSandPRiedererPSchreierPConstituents of sage (Salvia officinalis) with in vitro affinity to human brain benzodiazepine receptorPlanta Med20036911311712624814
- KavvadiasDSandPYoudimKAThe flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effectsBr J Pharmacol200414281182015231642
- ShenXLWittMRNielsenMSternerOInhibition of [3H] flunitraze-pam binding to rat brain membranes in vitro by puerarin and daidzeinYao Xue Xue Bao19963159628762462
- HäberleinHTschierschKPSchäferHLFlavonoids from Leptospermum scoparium with affinity to the benzodiazepine receptor characterized by structure activity relationships and in vivo studies of a plant extractPharmazie1994499129227838881
- WasowskiCMarderMViolaHMedinaJHPaladiniACIsolation and identification of 6-methylapigenin, a competitive ligand for the brain GABA(A) receptors, from Valeriana wallichiiPlanta Med20026893493612391561
- MarderMViolaHWasowskiCFernandezSMedinaJHPaladiniAC6-Methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNSPharmacol Biochem Behav20037553754512895671
- AdachiNTomonagaSTachibanaTDenbowDMFuruseM(−)-Epigallocatechin gallate attenuates acute stress responses through GABAergic system in the brainEur J Pharmacol200653117117516457806
- VignesMMauriceTLantéFAnxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG)Brain Res2006111010211516859659
- CampbellELChebibMJohnstonGAThe dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABA(A) receptorsBiochem Pharmacol2004681631163815451406
- PaladiniACMarderMViolaHWolfmanCWasowskiCMedinaJHFlavonoids and the central nervous system: from forgotten factors to potent anxiolytic compoundsJ Pharm Pharmacol19995151952610411210
- MarderMPaladiniACGABA(A)-receptor ligands of flavonoid structureCurr Top Med Chem2002285386712171576
- ShenYLindemeyerAKGonzalezCDihydromyricetin as a novel anti-alcohol intoxication medicationJ Neurosci20123239040122219299
- ColetaMCamposMGCotrimMDLimaTCCunhaAPAssessment of luteolin (3′,4′,5,7-tetrahydroxyflavone) neuropharmacological activityBehav Brain Res2008189758218249450
- RainesTJonesPMoeNDuncanRMcCallSCeremugaTEInvestigation of the anxiolytic effects of luteolin, a lemon balm flavonoid in the male Sprague-Dawley ratAANA J200977333619263826
- WangFXuZRenLTsangSYXueHGABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalinNeuropharmacology2008551231123718723037
- YoonSYde la PeñaICShinCYConvulsion-related activities of Scutellaria flavones are related to the 5,7-dihydroxyl structuresEur J Pharmacol201165915516021440538
- de CarvalhoRSDuarteFSde LimaTCInvolvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in miceBehav Brain Res2011221758221377498
- HuenMSHuiKMLeungJWNaturally occurring 2′-hydroxyl-substituted flavonoids as high-affinity benzodiazepine site ligandsBiochem Pharmacol2003662397240714637197
- ChoSKimSJinZIsoliquiritigenin, a chalcone compound, is a positive allosteric modulator of GABAA receptors and shows hypnotic effectsBiochem Biophys Res Commun201141363764221945440
- JamalHAnsariWHRizviSJEvaluation of chalcones – a flavonoid subclass, for, their anxiolytic effects in rats using elevated plus maze and open field behaviour testsFundam Clin Pharmacol20082267368119049672
- SternbachLHThe benzodiazepine storyProg Drug Res19782222926630117
- MarderMViolaHWasowskiC6 Bromoflavone, a high affinity ligand for the benzodiazepine receptors is a member of a family of active flavonoidsBiochem Biophys Res Commun19962233843898670291
- ViolaHMarderMWolfmanCWasowskiCMedinaJHPaladiniAC6-Bromo-3′-nitroflavone, a new high affinity benzodiazepine receptor agonist recognizes two populations of cerebral cortical binding sitesBioorg Med Chem Lett19977373378
- WolfmanCViolaHMarderMPharmacological characterization of 6-bromo-3′-nitroflavone, a synthetic flavonoid with high affinity for the benzodiazepine receptorPharmacol Biochem Behav1998612392469768558
- ViolaHMarderMNuñezJ6-Methyl-3′-bromoflavone, a high-affinity ligand for the benzodiazepine binding site of the GABAA receptor with some antagonistic propertiesBiochem Biophys Res Commun199926264364610471378
- ViolaHWolfmanCMarderM6-Chloro-3′-nitroflavone is a potent ligand for the benzodiazepine binding site of the GABA(A) receptor devoid of intrinsic activityPharmacol Biochem Behav20006531332010672984
- ViolaHMarderMWasowskiCGiorgiOPaladiniACMedinaJH6,3′-Dibromoflavone and 6-nitro-3′-bromoflavone: new additions to the 6,3′-disubstituted flavone family of high-affinity ligands of the brain benzodiazepine binding site with agonistic propertiesBiochem Biophys Res Commun200027369469810873666
- MarderMViolaHWasowskiC6,3′-Dinitroflavone, a novel high affinity ligand for the benzodiazepine receptor with potent anxiolytic propertiesBioorg Med Chem Lett1995527172720
- WolfmanCViolaHMarderMAnxioselective properties of 6,3′-dinitroflavone, a high-affinity benzodiazepine receptor ligandEur J Pharmacol199631823299007508
- FurtmuellerRFurtmuellerBRamerstorferJ6,3′-Dinitroflavone is a low efficacy modulator of GABAA receptorsEur J Pharmacol200859114214618639544
- GriebelGPerraultGTanSSchoemakerHSangerDJPharmacological studies on synthetic flavonoids: comparison with diazepamNeuropharmacology19993896597710428415
- RenLChanWMWangFEffects of flavone 6-substitutions on GABAA receptors efficacyEur J Pharmacol201167012112921914441
- RenLWangFXuZChanWMZhaoCXueHGABA(A) receptor subtype selectivity underlying anxiolytic effect of 6-hydroxyflavoneBiochem Pharmacol2010791337134420067772
- HallBJChebibMHanrahanJRJohnstonGAFlumazenil-independent positive modulation of gamma-aminobutyric acid action by 6-methylflavone at human recombinant alpha1beta2gamma2L and alpha1beta2 GABAA receptorsEur J Pharmacol20044911815102527
- HallBJChebibMHanrahanJRJohnstonGA6-Methylflavanone, a more efficacious positive allosteric modulator of gamma-aminobutyric acid (GABA) action at human recombinant alpha2beta2gamma2L than at alpha1beta2gamma2L and alpha1beta2 GABA(A) receptors expressed in Xenopus oocytesEur J Pharmacol20055129710415840393
- KarimNCurmiJGavandeN2′-Methoxy-6-methylflavone: a novel anxiolytic and sedative with subtype selective activating and modulating actions at GABA(A) receptorsBr J Pharmacol2012165488089621797842
- KarimNGavandeNWellendorphPJohnstonGAHanrahanJRChebibM3-Hydroxy-2′-methoxy-6-methylflavone: a potent anxiolytic with a unique selectivity profile at GABA(A) receptor subtypesBiochem Pharmacol2011821971198321924247
- FernandezSPMewettKNHanrahanJRChebibMJohnstonGAFlavan-3-ol derivatives are positive modulators of GABA(A) receptors with higher efficacy for the alpha(2) subtype and anxiolytic action in miceNeuropharmacology20085590090718657554
- MewettKNFernandezSPPasrichaAKSynthesis and biological evaluation of flavan-3-ol derivatives as positive modulators of GABAA receptorsBioorg Med Chem2009177156717319783443
- FernandezSPKarimNMewettKNChebibMJohnstonGAHanrahanJRFlavan-3-ol esters: new agents for exploring modulatory sites on GABA(A) receptorsBr J Pharmacol201216596597721806603
- FacklamMSchochPHaefelyWERelationship between benzodiazepine receptor occupancy and potentiation of gamma-aminobutyric acid-stimulated chloride flux in vitro of four ligands of differing intrinsic efficaciesJ Pharmacol Exp Ther1992261110611121318370
- BraestrupCNielsenMHonoreTJensenLPetersenEBenzodiazepine receptor ligands with positive and negative efficacyNeuropharmacology198322145114576322039
- AiJDekermendjianKWangXNielsenMWittMR6-Methylflavone, a benzodiazepine receptor ligand with antagonistic properties on rat brain and human recombinant GABAA receptors in vitroDrug Dev Res19974199108
- MarderMZinczukJColomboMISynthesis of halogenated/nitrated flavone derivatives and evaluation of their affinity for the central benzodiazepine receptorBioorg Med Chem Lett1997720032008
- DekermendjianKKahnbergPWittMRSternerONielsenMLiljerforsTStructure-activity relationships and molecular modeling analysis of flavonoids binding to the benzodiazepine site of the rat brain GABA(A) receptor complexJ Med Chem1999424343435010543878
- MarderMViolaHBacigaluppoJADetection of benzodiazepine receptor ligands in small libraries of flavone derivatives synthesized by solution phase combinatorial chemistryBiochem Biophys Res Commun19982494814859712722
- MarderMEstiuGBlanchLBMolecular modeling and QSAR analysis of the interaction of flavone derivatives with the benzodiazepine binding site of the GABA(A) receptor complexBioorg Med Chem2001932333511249125
- WangFXuZYuenCT6,2′-Dihydroxyflavone, a subtype-selective partial inverse agonist of GABAA receptor benzodiazepine siteNeuropharmacology20075357458217681556
- GavandeNKarimNJohnstonGAHanrahanJRChebibMIdentification of benzopyran-4-one derivatives (isoflavones) as positive modulators of GABA(A) receptorsChem Med Chem201161340134621560249