Abstract
The coronavirus infectious disease-2019 (COVID-19) has overwhelmed like a shock wave in a completely unprepared world. Despite coronavirus infections were involved in previous epidemic outbreaks, no antiviral agent was developed for specific treatment. As a consequence, since the beginning of this pandemic, both repositioned and experimental drugs were used to treat the infected patients without evidence of clinical efficacy. Just based on experience coming from the use of antiviral agents to treat other viruses (eg, lopinavir/ritonavir, remdesivir) and supposed antiviral or immunomodulatory activities of drugs with no approved antiviral indications (eg hydroxychloroquine, tocilizumab), clinicians have faced the ongoing pandemic. Currently, after about 9 months from the COVID-19 spread, there is still no antiviral agent capable of ensuring the cure of this syndrome. Clinical trials are beginning to confirm the benefits of some drugs, while for other compounds, efficacy and safety have not yet been confirmed. Randomized clinical trials (RCT) have denied or downsized the beneficial effects attributed to certain molecules, such as aminoquinolines, largely used in clinical practice at the beginning of COVID-19 spread. Conversely, at the same time, they have provided evidence for unexpected effectiveness of other agents that have been underutilized, such as steroids, which were not used in SARS treatment because of the threatened effect on viral replication. Evidence deriving from pathologic studies have demonstrated that the prothrombotic effects of SARS-CoV-2 can be prevented by heparin prophylaxis, underlining the need for personalized treatment for patients with severe disease. The main aim of this review is to synthesize the available information and evidence on both repositioned and experimental drugs for the treatment of COVID-19, focusing on the need to exercise caution on the use of unproven medical therapies.
Introduction
The Coronavirus Infectious Disease-2019 (COVID-19) pandemic is a novel challenge for the scientific community due to the high diffusion rate via respiratory droplets and contact with aerosol infected surfaces, and the absence of standard therapy. COVID-19 has multiple clinical presentations ranging from asymptomatic or mild disease to critical illness with rapid clinical deterioration and death. Its most frequent symptoms include fever, malaise, dry cough, shortness of breath, and respiratory distress. Severe cases need mechanical ventilation and report high mortality rates.Citation1,Citation2
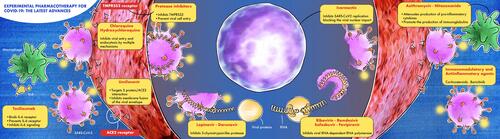
Various “repositioned” drugs (indicated for other uses) have been proposed for COVID-19 treatment. Remdesivir (active against Ebola), lopinavir/ritonavir (anti-HIV), chloroquine and hydroxychloroquine (antimalarial agents), interferon-β (treatment for multiple sclerosis), and tocilizumab (antirheumatic drug) are among the drugs proposed due to their antiviral or immunomodulatory effects, but none of them had sufficient efficacy and large availability to ensure a rapid and effective cure. highlights the mechanisms proposed for the most common drugs suggested for COVID-19 treatment.
The purpose of this review is to synthesize the information and evidence available on both repositioned and experimental drugs for the treatment of COVID-19.
Methods
Relevant papers and trials until July 1st, 2020 were searched on Medline, Google Scholar, and Clinicaltrials.gov. We performed a search considering the following keywords: “chloroquine”, “hydroxychloroquine”, “remdesivir”, “favipiravir”, “lopinavir/ritonavir”, “darunavir/cobicistat”, “umifenovir”, “sofosbuvir”, “ribavirin”, “penciclovir”, “camostat mesilate”, “nafamostat mesilate”, “azithromycin”, “enoxaparin”, “nitazoxanide”, “ivermectin”, “famotidine”, “corticosteroids”, “tocilizumab”, “ baricitinib” and matching each of them with the terms ‘COVID-19ʹ AND ‘SARS-CoV-2ʹ. A manual search on the reference and citations on each paper retrieved by a PubMed search completed the literature search. Study in English language “in vitro” or in a human population with COVID-19 infection, reporting data on each drug efficacy, were included in the review. This systematic review adopted the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
The authors independently reviewed each paper. Once a paper was identified as likely to be included, the full-text version was read, and a further search was performed considering references list and citations. Conflict about the inclusion of a paper was resolved by further evaluation by the senior author (PP).
Agents with Anti-Malarial Activity
Chloroquine-Hydroxychloroquine
Chloroquine (CQ) and Hydroxychloroquine (HCQ) are aminoquinolones well-known as anti-malarial agents, which have activity against several RNA viruses including Zika virus, Chikungunya-virus, SARS-CoV, and MERS-CoV.Citation3 According to in vitro studies, HCQ and CQ can interfere with the early phase of SARS-CoV-2 replication modifying the pH on the surface of the cell membrane and inhibiting the fusion of the virus with the host cell, finally interfering with important viral functions including attachment, assembly, transport of new particles, accumulation in lysosomes, and release into intracellular space. Moreover, HCQ and CQ bind to the sialic acids of the respiratory tract cells, interacting with the N-terminal domain of the SARS-CoV-2 spike protein, finally inhibiting cell/virus fusion during the early phase of the infection.Citation4 When HCQ or CQ are administered to patients with COVID-19 associated pneumonia, lung concentration rises up to 200–700 times than plasma concentration, posing these drugs as possible anti-COVID-19 agents for both treatment and post-exposure prophylaxis.Citation5,Citation6
On September 1st 2020, 335 trials investigating the efficacy of COVID-19 treatment with aminoquinolines were registered on clinicaltrials.gov. Definitive data are lacking for many of these studies. Only 8 studies registered are interventional clinical trials and only 2 have published their results, one is currently on a ‘preprint status’.Citation7,Citation8
Data on aminoquinolines administration are conflicting and the efficacy proposed, based on in vitro studies, is not confirmed by unequivocal clinical investigations. At the beginning of the pandemic diffusion of SARS-CoV-2, preliminary clinical data suggested the efficacy of CQ in avoiding exacerbation of pneumonia, promoting the disappearance of the virus from the respiratory tract, and reducing disease duration.Citation9 Similar results were proposed by a French study, which highlighted that treatment with HCQ 200 mg TID resulted in a greater number of negative nasal swabs after 3–6 days, compared to historical control.Citation10 However, another French study failed to demonstrate an advantage in patients receiving oxygen, compared to untreated controls.Citation11 Treatment with HCQ (400 mg/die) led to an improvement in lesions at the chest CT scan without disease progression in 62 mild COVID-19 patients while a study from Brazil reported Q-T prolongation in 25% of the severe COVID-19 patients treated with CQ (600 mg bid for 10 days), but with a high incidence of arrhythmias in those receiving CQ and no clinical benefit.Citation8,Citation12 A large study on the data deriving from a multi-national register analysis (published by The Lancet), which reported the absence of HCQ/CQ efficacy on COVID-19, was retracted due to the lack of control on the quality of the collected data.
A recent systematic review highlighted that in two recent trials and one cohort study there was no reduction in hospitalization rate in COVID-19 patients who received HCQ before their hospitalization. Of these, RECOVERY trial demonstrated that no benefit could be achieved using high-dose HCQ and SOLIDARITY trial was terminated due to the lack of efficacy.Citation13
Recently, a Belgian study analyzed 8075 COVID-19 patients receiving or not low-dose HCQ associated with standard therapy. Those receiving low-dose HCQ therapy reported a reduction of the risk of death as assessed by multivariate analysis.Citation14
In a double-blind, placebo-controlled RCT, HCQ did not show efficacy in preventing COVID-19 when used as post-exposure prophylaxis within 4 days after high- or moderate-risk exposure to SARS-COV-2.Citation15
Main studies investigating CQ/HCQ efficacy are reported in .
Table 1 Main Studies Investigating CQ/HCQ and Remdesivir Efficacy Against COVID-19
Remdesivir
Remdesivir () is a prodrug metabolized into an analogue of adenosine triphosphate that inhibits viral RNA-dependent RNA polymerase, interrupting the growing RNA chain. This antiviral agent has shown efficacy in vitro against filoviruses (eg, Ebola), Marburg virus, Paramyxoviridae, and Pneumoviridae, as well as against other Coronaviruses (eg, SARS-CoV and MERS-CoV).Citation16,Citation17
Clinical conditions of the first patient diagnosed with COVID-19 in the USA improved after remdesivir administration and the oropharyngeal swab turned steadily negative.Citation18 The evidence demonstrating remdesivir efficacy was reported in April 2020 after the analysis of the data from a compassionate use program. The study evaluated only 61 hospitalized patients, enrolled from USA, Canada, Europe, and Japan, with 94% oxygen saturation or less, who received a 10-day course of remdesivir (200 mg intravenously on day 1, 100 mg daily from day 2 to day 10). Remdesivir administration resulted in a significant improvement in respiratory outcome in 36 out of 53 patients (68%) and a 13% decrease in mortality rate was shown.Citation17 In a multicenter trial (NCT04257656) conducted in 10 hospitals in Hubei-China, 237 patients with COVID-19 associated pneumonia and 94% oxygen saturation or less were randomized to receive remdesivir or placebo. No difference was reported in terms of mortality, but those receiving remdesivir had a small (not statistically significant) reduction of recovery time (median 18·0 days [IQR 12·0–28·0] vs 23·0 days [15·0–28·0]; HR 1·52 [0·95–2·43]).Citation19 In the SIMPLE1 Phase III trial, among 397 patients with severe COVID-19, a clinical improvement was obtained in 64% receiving a 5-day remdesivir treatment and in 54% scheduled to receive a 10 days of remdesivir with no significant difference related to the adopted schedule.Citation20 Moreover, the ACTT trial demonstrated that remdesivir was superior to placebo in shortening the time to recovery (median time 11 days in the remdesivir group and 15 days in the placebo group, HR 1.32, 95% CI 1.12–155 [p<0.001]), in addition, the number of deaths was inferior in the remdesivir group, but the difference was not statistically different (HR 0.70; 95% CI 0.47–1.04).Citation21 Remdesivir is being evaluated in the WHO-sponsored trial called SOLIDARITY, which considered patients with severe COVID-19 randomized to one of the following four treatments: standard of care, remdesivir, lopinavir/r plus interferon (IFN) beta-1a and HCQ, presently, no significant efficacy was reported.Citation22 A Gilead Sciences-sponsored Phase II–III study enrolling pediatric patients from the age of 28 days to under 18 years old with laboratory-confirmed COVID-19, is still ongoing in order to evaluate the safety, tolerability, and pharmacokinetics (PK) of remdesivir.Citation23 So far (accessed 01/09/2020), 49 studies proposing remdesivir for COVID-19 treatment have been registered on clinicaltrials.gov. Among them, 16 are phase III (both profit and nonprofit) studies.
Favipiravir
Favipiravir (called Favilavir in China and Avigan in Europe) is a viral RNA-dependent RNA-polymerase inhibitor approved in Japan for the treatment of influenza. The recommended loading dose is 2400 mg BID, while the maintenance dose is 1200–1800 mg QD. Preliminary data from a non-randomized control study have shown that among 340 patients, those who received favipiravir improved respiratory conditions. Favipiravir shortened the duration of fever and increased viral clearance rate. Based on a Kaplan-Meier, those receiving favipiravir had a median time to viral clearance of 4 days (IQR: 2.5–9) as compared to 11 days (IQR: 8–13) in those receiving placebo (P < 0.001).Citation24
Favipiravir was compared to umifenovir in an RCT enrolling 240 patients with COVID-19 associated pneumonia. No difference in terms of outcome was reported in patients with severe disease, but the seven-day recovery rate was significantly higher in those receiving favipiravir compared to those treated with umifenovir (71% Vs 56%, p = 0.0199).Citation25 Favipiravir is proposed as an experimental treatment for COVID-19 in 34 studies registered on clinicaltrials.gov (accessed 01/09/2020). Among them, 13 are phase III trials.
Lopinavir/Ritonavir
Despite the lack of scientific evidence, lopinavir/ritonavir (lopinavir/r) was used for the treatment of COVID-19 patients at the beginning of the pandemic. This drug is an HIV protease inhibitor, used in the treatment of HIV-1 infection in patients over 14 years old, which was considered potentially active due to the similarity between coronavirus and HIV proteases.Citation26 Up to date, 83 studies on lopinavir/r have been registered on clinicaltrials.gov (accessed 01/09/2020). The use of this drug is also investigated in two ongoing experimental protocols: one of them is the SOLIDARITY trial, promoted by the WHO, which aims to show the effectiveness of four treatment options against COVID-19 (local standard of care, or local standard of care plus one of Remdesivir, Lopinavir/r, Lopinavir/r plus IFN beta-1);Citation22 the second one is the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, which aims to evaluate potential treatments for people hospitalized with confirmed or suspected COVID-19, analyzing lopinavir/r, low-dose dexamethasone, azithromycin, tocilizumab, and convalescent plasma.Citation27
In an open-label RCT by Cao et al, 199 severe COVID-19 adult patients with an oxygen saturation of 94% were randomized to receive standard care or lopinavir/r plus standard care. There was no difference between the two treatments regarding the time to reach clinical improvement and mortality rate.Citation28 Conversely, a Phase II RCT conducted in Hong Kong, involving 127 mild to moderate COVID-19 patients, highlighted a shorter median time to test negative nasopharyngeal swab (7 days [IQR 5–11]) for those receiving triple therapy with lopinavir/r, ribavirin, and IFN beta 1b compared to those receiving lopinavir/r alone (12 days [8–15]; hazard ratio 4·37 [95% CI 1·86–10·24], p=0·0010).Citation29
It is important to remember that use of lopinavir/r can be dangerous in those receiving azithromycin due to the increase of risk of Q-T prolongation as established by a recent review on the argument.Citation30
Darunavir/Cobicistat
Darunavir/cobicistat is another combination used for COVID-19 and investigated in 6 trials registered on cinicaltrials.gov (accessed 01/09/2020). Darunavir is an HIV-1 protease inhibitor and its activity is enhanced by the booster cobicistat. Data from preclinical studies showed inhibitory effects of this combination on SARS-CoV-2;Citation31 moreover, the computational-based analysis demonstrated a potential inhibition of SARS-CoV-2 replication and activity on viral spike protein.Citation32
In an open-label RCT, mild COVID-19 patients were enrolled and treated with 5 days of darunavir/cobicistat, but no increase in the proportion of negative swab as compared to standard of care alone was demonstrated.Citation33 Currently, no evidence can support darunavir administration in patients affected by COVID-19.
Umifenovir
Umifenovir (also known as Arbidol), currently approved in Russia and China, showed antiviral activity against influenza A and B viruses, by inhibiting viral envelope cellular membrane fusion, preventing S protein/ACE2 interaction, and affecting activation of macrophages and induction of interferons.Citation34 It showed in vitro activity against HBV, HCV, Ebola, poliovirus, and HHV-8 and was well tolerated, except for common gastrointestinal adverse reactions.Citation35–Citation37 Seventy-seven COVID-19 patients enrolled in an observational study were treated for 9 days with umifenovir plus aerosolized IFN-a 2b. Patients receiving such a combination of IFN-a 2b/umifenovir shortened the time to viral clearance and reported a reduction of IL-6 levels.Citation38 Two more studies are available on the “preprint platform”. One of these is an RCT enrolling 86 COVID-19 patients, which highlights that adding umifenovir to standard therapy does not improve the patients’ condition in terms of positive-to-negative conversion, and radiologic outcomes. The second is an observational study on 504 COVID-19 patients which reports a decrease in mortality rate after umifenovir administration as assessed by a multivariate analysis.Citation39,Citation40 At the moment (accessed 01/09/2020), 10 studies investigating the role of umifenovir in COVID-19 therapy have been registered on clinicaltrials.gov.
Sofosbuvir
Sofosbuvir is a prodrug nucleotide uridine analog acting as an inhibitor of the RNA-dependent RNA polymerase and as a specific RNA chain terminator from the template strand to the primer strand, which has been introduced for the treatment of HCV. The active triphosphate form is incorporated by SARS-CoV-2 polymerase and blocks further nucleotides incorporation.Citation41,Citation42 Sofosbuvir could be combined with HCV protease inhibitors. Among these, fixed antiviral combinations with ledipasvir or velpatasvir may be particularly attractive as they can inhibit both the polymerase and the anchorage point of SARS-CoV-2. An open-label multicentre study enrolling patients with moderate/severe COVID-19 patients in Iran reported a shorter average duration of hospitalization after sofosbuvir/daclatasvir combination treatment [6 days (IQR 4–8)] than the standard treatment group [8 days (IQR 5–13)] (P = 0.029).Citation43 Eight trials on sofosbuvir treatment are officially registered on clinicaltrials.gov, one of them investigating the efficacy of sofosbuvir/ledipasvir combination was completed in July 2020, but no data is currently available.
Nucleoside Analogues (Polymerase Inhibitors)
Ribavirin is a guanosine analogue used against HCV, respiratory syncytial virus, and viral hemorrhagic fevers.Citation44 It was administered together with IFN-a in MERS-CoV patientsCitation45 but demonstrated inefficacy in severe patients.Citation46 Ribavirin limits SARS-COV-2 infection in vitro,Citation47 but it should be considered with caution in COVID-19 routine use due to the risk of hemolytic anemia, hemoglobin reduction, fatigue, rash, leukopenia, and teratogenic effects.Citation48 In a Phase 2 trial the triple combination of IFN beta 1b, ribavirin, and lopinavir/r was superior to lopinavir/r alone in a population of hospitalized patients with COVID-19.Citation29 Twelve trials (accessed 01/09/2020) are registered on clinicaltrials.gov, most of them are now in early phases.
Penciclovir
The efficacy of penciclovir against SARS-CoV-2 was assessed in vitro by Wang et al and another group who demonstrated its activity on the SARS-CoV-2 RNA-polymerase. However, the limited pieces of evidence on its safety in pregnancy, during breastfeeding, and the side effects do not prompt the use in COVID-19.Citation47,Citation49,Citation50
Protease Inhibitors
Camostat mesylate and nafamostat mesylate are serine protease inhibitors, the former used for oral squamous cell carcinoma and dystrophic epidermolysis and the latter for acute pancreatitis, disseminated intravascular coagulation and anticoagulation in the extracorporeal circulation.Citation51–Citation54
Nafamostat mesylate was able to inhibit Transmembrane Serine Protease 2 (TMPRSS2) activity in MERS-CoV by preventing spike protein-mediated viral fusion to lung Calu-3 host cells.Citation55 This mechanism of action could justify its efficacy against COVID-19, given the similar characteristics with both SARS-COV-2 and MERS-CoV spike proteins and investigated in simian Vero E6 cells.Citation48 No clinical trial is currently planned for these drugs.
Other Antiviral Agents
Other drugs are under investigation for their potential effect against SARS-COV-2 infection, but clinical data are not currently available. Among them, oseltamivir, acyclovir, and ganciclovir have not shown any effect in COVID-19 patients.Citation56 Another anti-influenza drug, baloxavir marboxil, inhibits the protein synthesis blocking viral replication. Baloxavir marboxil has demonstrated a synergistic antiviral effect when combined with oseltamivir without adverse drug reactions.Citation57,Citation58
Compounds with Antiviral Activity
Other compounds potentially active against SARS-CoV-2 have been screened into in vitro studies. The cysteine protease inhibitors ONO 5334 and MDL 28,170 and the FYVE finger-containing phosphoinositide kinase (PIKfyve) inhibitor, named apilimod, showed antiviral activity in human pluripotent stem cell-derived pneumocyte-like cells reducing the number of infected cells.Citation59 Apilimod showed antiviral efficacy in primary human lung explant model infected with SARS-CoV-2.Citation59 PIKfyve maintains endomembrane homeostasis in early endosomes; therefore, apilimod seems to inhibit viral replication during virus insert.Citation60 Apilimod is an experimental compound, investigated in phase II clinical trials for the treatment of Crohn’s disease, rheumatoid arthritis, and common variable immunodeficiency.Citation61,Citation62 Moreover, it has also been shown to inhibit in vitro Ebola, Lassa, and Marburg viruses.Citation63,Citation64
Non-Antiviral Agents
Azithromycin
Macrolides are bacteriostatic antibiotics widely used against many Gram-positive and intracellular bacteria causing respiratory infections. In addition to their antibacterial effects, macrolides demonstrate immunomodulatory and anti-inflammatory effects.Citation65–Citation67 Preclinical and clinical studies have shown their ability in regulating the inflammatory response, attenuating the production of pro-inflammatory cytokines, and promoting the production of immunoglobulins. These regulatory effects on immune response can reduce complications of respiratory viral infections.Citation68 Among macrolides, azithromycin is considered the most suitable agent in therapeutic combinations. The reasons are related to the better profile of potential drug–drug interactions and the relatively low impact on QTc elongation.Citation66
Severity and mortality caused by respiratory viral infections, including COVID-19, is associated with the host’s excessive inflammatory response characterized by hyper-production of cytokines.Citation69 Based on preliminary investigations in vitro HCQ/Azithromycin seemed to have a synergistic effect against SARS-CoV2. Clinical investigations on small series of COVID-19 patients suggested that adding azithromycin to HCQ led to significant improvement, regardless of the absence of direct effect on viral load.Citation10 Further investigations failed in demonstrating such favorable effects.Citation70
One hundred and twelve trials registered on clinicaltrials.gov have been planned to investigate the efficacy of azithromycin alone or in combination with other agents in COVID-19 patients. The majority of the studies investigated azithromycin/HCQ combination, but no result is currently available by checking the results section on “www.clinicaltrials.gov”. Despite no study can ultimately assess the efficacy of azithromycin in COVID-19, we believe that the data on its favorable effects on other viral pneumonia can support its use, but several doubts arise on HCQ/azithromycin combination due to an excess in terms of mortality and the increased risk of arrhythmias suggested by Fiolet et al meta-analysis.Citation70 Moreover, a retrospective study evaluating 323,122 non-COVID-19 patients demonstrated that adding azithromycin to rheumatologic disease patients on HCQ treatment seemed to increase the risk of 30-day cardiovascular mortality, chest pain or angina and heart failure.Citation71
Heparins
COVID-19 patients very often present a state of hypercoagulability, which exposes them to an increased risk of thrombosis.Citation72 In COVID-19 patients with pulmonary thromboembolism, there is an essential elevation of D-dimer neither associated with thrombocytopenia, nor with clotting time prolongation (PT, PTT). Postmortem autopsy data confirm this hypothesis, considering that emboli and, even more often, intrapulmonary microvascular thrombosis have been found in autoptic investigations.Citation73 Consistent with this hypothesis, a Chinese clinical study has reported that patients receiving a prophylactic dose of heparin showed a significantly higher survival rate than those who did not receive heparin. In this retrospective analysis conducted in Wuhan, the authors examined 449 patients affected by severe COVID-19. In such subjects, they reviewed and compared the parameters of coagulation tests and clinical characteristics between survivors and non-survivors to evaluate the effects of heparin therapy: 94 patients received LMWH (40–60 mg enoxaparin/day), and five received unfractionated heparin (UFH, 10,000–15,000 U/day), without other anticoagulants. Heparin therapy significantly reduced mortality in patients with sepsis-induced coagulopathy (SIC) score ≥4 (40.0%vs 64.2%, p<0.05), but not in those with SIC score <4 (29.0% vs 22.6%, p>0.05). Stratifying according to D-dimer values of the study population, the authors reported a rise of mortality linked to the rising D-dimer and 20% reduction of mortality for patients on heparin. Therefore, heparin treatment appears to be associated with better prognosis in severe COVID-19 patients with coagulopathy.Citation74,Citation75
Currently, 64 clinical trials are registered on “clinicaltrials.gov” (accessed 01/09/2020). Only 4 trials have been completed, but none of them released the results before publication. Instead, performing an extensive search on PUBMED, we found a study investigating the efficacy of platelet inhibition in a very small series of patients affected by severe respiratory failure who underwent treatment with continuous positive airway pressure (CPAP). Platelet inhibition resulted in a better outcome in respect to treatment with fondaparinux as assessed by improvement in ventilation/perfusion ratio.Citation76
Nitazoxanide
Nitazoxanide, traditionally used as an anthelmintic agent, has also broad antiviral activity against rotavirus, norovirus, HBV, and RSV.Citation77–Citation80 Nitazoxanide showed in vitro antiviral activity against human coronavirus causing MERS and COVID-19, and against murine and bovine coronavirus through inhibition of the viral N protein expression. In addition, nitazoxanide demonstrates an anti-inflammatory activity suppressing the production of IL-2, IL6, and IL-10.Citation80 No clinical data can be retrieved after a PUBMED search using the keywords ‘Nitazoxanide and COVID-19ʹ, but the safety profile of the drug and its activity (assessed in vitro) justify the current interest. Nineteen ongoing trials investigating nitazoxanide safety and efficacy in COVID-19 either alone or in combination are registered on clinicaltrials.gov, but no result is currently available (accessed 01/09/2020).
Ivermectin
Ivermectin is an antiparasitic drug for which 37 trials investigating its efficacy against COVID-19 are registered (clinicaltrials.gov, accessed 01/09/2020). In vitro investigations highlighted its antiviral activity against HIV-1, dengue virus,Citation81,Citation82 West Nile and influenza viruses which prompted further investigations in investigating its anti-SARS-CoV-2 activity, which can be related to the ability in blocking the viral nuclear import.Citation83–Citation85 When Ivermectin is administered in non-COVID-19 patients, important adverse drug reactions such as ataxia, psychosis, depression, confusion, and convulsions by increasing GABAergic transmission can be reported in just a few patients.Citation86 Ivermectin resulted in a SARS-Cov-2 RNA reduction of 93% after 24 h and of 99.8% after 48 h in Vero/hSLAM cells. No clinical data are currently available on ivermectin treatment for COVID-19.Citation85
Famotidine
Famotidine is a histamine-2 (H2) receptor antagonist used to treat peptic ulcer, Zollinger-Ellison syndrome, and symptomatic mild reflux oesophagitis.Citation87 A potential role in COVID-19 patients was suggested by an in silico study showing famotidine ability to target the 3C-like protease (3CLpro) of coronavirus, which is the main protease used during viral assemblage.Citation88 In a case series, ten COVID-19 patients improved their symptoms and their peripheral oxygen saturation 24 hours after high-dose famotidine administration without relevant side-effects.Citation89 In addition, famotidine resulted in a reduction of mortality and use of mechanical ventilation assistance in a retrospective cohort study investigating 1620 hospitalized COVID-19 patients. The proportion of cases who died or needed mechanical ventilation was reported to be significantly lower in 84 cases receiving famotidine within 24 hours from hospital admission.Citation90 Five trials were registered on clinicaltrials.gov, two of them have been completed, but the results are not yet available (accessed 01/09/2020).
Immunomodulatory and Anti-Inflammatory Agents
Corticosteroids
Corticosteroids act within the lungs reducing the host inflammatory response, which can lead to acute lung injury and ARDS. Russell, examining the literature data in favor or against the use of corticosteroids in respiratory disease caused by SARS-CoV-2, highlighted that the studies were mainly observational and that use of corticosteroids has been reserved for the most critical patients only.Citation91
In a review on the use of corticosteroids in SARS-CoV, 4 out of 29 studies have underlined a probable worsening effect of such drugs on acute illness. In comparison, the other 25 studies have provided no evidence, either favorable or against.Citation92 However, according to accredited expert opinions, such a lack of definitive clinical evidence is not enough to drop out the use of corticosteroids in SARS-CoV-2 pneumonia.
Data favoring steroids use are relevant when we consider researches conducted for other virus-associated respiratory diseases, such as pneumonia after influenza virus infection due to the ability in reducing the threatened effects of inflammation on the organisms.Citation93 In a prospective cohort study evaluating 2141 patients with viral influenza A pneumonia (H1N1), low/moderate doses of corticosteroids (25–150 mg/day of methylprednisolone) reduced mortality in patients with SaO2/FiO2 ratio <300 mmHg.Citation94 Corticosteroids reduced mortality and the need for mechanical ventilation in patients with severe community pneumonia, as assessed in a recent systematic review.Citation95 However, it is believed that short cycles (≤7 days) of corticosteroids at low/moderate doses (≤0.5–1 mg/kg per day of methylprednisolone) are a suitable therapeutic option in selected critical patients with SARS-CoV-2 pneumonia.Citation96
A retrospective study involving 201 Chinese patients with COVID-19 found that a treatment with methylprednisolone was associated with a decreased risk of death for subjects who developed ARDS (23/50 [46%] with steroids vs 21/34 [62%] without steroids; HR, 0.38 [95% CI, 0.20–0.72]).Citation97 However, the authors noted that bias and residual confounding factors between the patients who did or did not receive steroids might exist. Further data deriving from an open-label trial on dexamethasone use in COVID-19 (RECOVERY trial) highlighted that a daily dose of 6 mg resulted in an improvement in the 28-day mortality rate. The size of the sample evaluated was consistent (over 2000 patients) and permitted to establish that dexamethasone use was associated with reduction of the mortality rate with an age-adjusted advantage approaching 12% for those receiving invasive mechanical ventilation (29.3% vs 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81), but that treatment of those not receiving oxygen therapy at the time of randomization did not give a significant advantage.Citation98 Based on suggestions released by the National Institute of Health, dexamethasone use at 6 mg per day for up to 10 days or until hospital discharge can be proposed for the treatment of COVID-19 in hospitalized patients who are “mechanically ventilated” (AI) and in hospitalized patients who require supplemental oxygen but who are not mechanically ventilated (NIH COVID-19 guidelines available at https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf, accessed on 16/09/2020). Moreover, the European Society of Intensive Care Medicine, for mechanically ventilated adult patients suffering from COVID-19 and ARDS, suggests using systemic corticosteroids; for those without ARDS, it suggests against the routine use of systemic corticosteroids; for adult patients with COVID-19 and refractory shock, it suggests using low-dose corticosteroid therapy.Citation99
Tocilizumab
Tocilizumab (TCZ) is a humanized anti-interleukin-6 receptor monoclonal antibody able to inhibit interleukin 6 activity which is commonly used in patients with rheumatoid arthritis. Its role in the treatment of COVID-19 was based on the efficacy in decreasing serum values of interleukins IL-6, IL-2, IL-7, IL-10, and TNF, which are crucial in determining pulmonary damage after SARS-CoV-2 infection.Citation100,Citation101
Data on Tocilizumab efficacy are conflicting. TOCIVID-19 RCT, a Multicenter Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients with Severe COVID-19 Pneumonia, showed statistically significant results in improving the 30-day mortality rate (22.4% vs >30%) without relevant toxicity.Citation102 At the same time, two multicenter clinical trials are ongoing in China, under the local clinical trials registry control.Citation103,Citation104 Instead, the open-label, multicentre RCT to evaluate the effectiveness of early administration of Tocilizumab in patients with COVID-19 pneumonia (RCT-TCZ-COVID-19) was prematurely interrupted after the enrollment of 126 patients because the early administration of TCZ in Covid-19 pneumonia patients did not provide any relevant clinical benefit. Clinical data on TCZ efficacy derive from the CHIC study, which examines the efficacy of steroids followed by TCZ administration. In the small group considered sequential steroids/TCZ therapy was associated with better respiratory outcome and lower mortality rate.Citation105,Citation106
Main clinical studies, investigating the efficacy of drugs against COVID-19 described in this review, are reported in .
Table 2 Main Studies Investigating Clinical Efficacy of Drugs Against COVID-19
Other Immunomodulatory Drugs
Baricitinib is a JAK 1/2 inhibitor, it inhibits AP2-associated protein kinase 1, that enables coronavirus to entry in the host cells and favors the intracellular assembly of the virus.Citation107–Citation109 Apart from its activity on virus replication, it can inhibit the cytokine storm associated with COVID-19, but its administration requires caution in immunocompromised patients as several adverse reactions can occur.Citation110 No clinical data are currently available.
Other immunomodulatory drugs have also been investigated, such as sarilumab, another anti-IL6, and various combinations such as emapalumab/anakinra and ruxolitinib/eculizumab. On the basis of a recent open-label study, the ruxolitinib/eculizumab combination administered to patients with severe COVID-19 resulted in a significant reduction of mortality rate and rapid improvement of respiratory parameters.Citation111
Other compounds judged to be attractive for COVID-19 treatment are reported in . Even if they are attractive for their very limited toxicity, data are not sufficient to introduce further investigations in this literature review.Citation112–Citation114
Table 3 Molecules Showing Therapeutic Potential in Small Clinical Trials (Curcumin, Resveratrol) or in vitro (AR-12)
Conclusions
Since the beginning of the emergency, the scientific community has immediately engaged in searching for the best therapy to stem the COVID-19 outbreak through repositioned drugs use or conducting clinical trials on potentially active drugs. Apart from SARS-CoV-2 direct effects, it is evident that other mechanisms can determine a poor outcome after COVID-19, due to its ability to cause damage, related to immunologic activation, in many sites of the body, including the nervous system.Citation115 Some clinical trials are beginning to confirm the benefits of some drugs; however, for others, the evidence of efficacy and safety is still unclear due to several risks of bias and the heterogenicity of the study populations. Current pandemic stressed the importance to allowing a large access to highly attractive investigational drugs in international programs and to authorize a more rapid extension of the approved drugs indications as performed in clinical settings other than COVID-19.Citation116,Citation117 Surely, clinical research will lead us to find a successful therapy to overcome the COVID-19 pandemic.
Disclosure
The authors report no conflicts of interest for this work.
References
- Mehta N, Mazer-Amirshahi M, Alkindi N, Pourmand A. Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am J Emerg Med. 2020;38(7):1488–1493.
- Fathizadeh H, Maroufi P, Momen-Heravi M, et al. Protection and disinfection policies against SARS-CoV-2 (COVID-19). Infez Med. 2020;28(2):185–191.
- Alim Al-Bari A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5(1):e00293.
- Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55(5):105960. doi:10.1016/j.ijantimicag.2020.105960
- Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15):732–739.
- Pagliano P, Piazza O, De Caro F, Ascione T, Filippelli A. Is hydroxychloroquine a possible postexposure prophylaxis drug to limit the transmission to healthcare workers exposed to Coronavirus disease 2019? Clin Infect Dis. 2020;71(15):887–888.
- Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020. doi:10.7326/M20-4207.
- Chen Z, Hi J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. 2020. doi:10.1101/2020.03.22.20040758
- Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73.
- Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
- Mahévas M, Tran VT, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi:10.1136/bmj.m1844
- Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J Zhejiang Univ (Med Sci). 2020;49(2):215–219.
- Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. doi:10.1001/jamanetworkopen.2020.8857
- Catteau L, Dauby N, Montourcy M, et al. Belgian Collaborative Group on COVID-19 hospital surveillance. Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants. Int J Antimicrob Agents. 2020;56(4):106144.
- Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–525.
- Brown AJ, Won JJ, Graham RL, et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi:10.1016/j.antiviral.2019.104541
- Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336.
- Holshue M, DeBolt C, Lindquis S, et al. First case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936.
- Wang Y, Zhou F, Zhang D, et al. Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a Phase 3 randomized, double-blind, placebo-controlled, multicentre trial. Trials. 2020;21(1):422.
- Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;NEJMoa2015301.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
- Solidarity clinical trial. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments. Accessed December 14, 2020.
- Study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of remdesivir (GS-5734™) in participants from birth to < 18 years of age with Coronavirus disease 2019 (COVID-19) (CARAVAN). Available from: https://clinicaltrials.gov/ct2/show/NCT04431453?term=NCT04431453&draw=2&rank=1. Accessed December 14, 2020.
- Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study [published online ahead of print, 2020 Mar 18]. Engineering (Beijing). 2020. doi:10.1016/j.eng.2020.03.007
- Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020. doi:10.1101/2020.03.17.20037432
- Uzunova K, Filipova E, Pavlova V, Vekov T. Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed Pharmacother. 2020;131:110668. doi:10.1016/j.biopha.2020.110668
- Recovery trial. Available from: https://www.recoverytrial.net/. Accessed December 14, 2020.
- Cao B, Wan Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799.
- Hung I, Lung KC, Tso EYK, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704.
- Sciaccaluga C, Cameli M, Menci D, et al. COVID-19 and the burning issue of drug interaction: never forget the ECG [published online ahead of print, 2020 Aug 20]. Postgrad Med J. 2020.
- Omotuyi OI, Nash O, Ajiboye BO, et al. Molecular modeling evaluation of the binding effect of ritonavir, lopinavir and darunavir to severe acute respiratory syndrome coronavirus 2 proteases. Bio-Rxiv. 2020. doi:10.1101/2020.01.31.929695
- Talluri S. Molecular Docking and Virtual Screening based prediction of drugs for COVID-19. Comb Chem High Throughput Screen. 2020. doi:10.2174/1386207323666200814132149
- Chen J, Xia L, Liu L, et al. Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infect Dis. 2020;7(7):ofaa241.
- Liu Q, Xiong HR, Lu L, et al. Antiviral and anti-inflammatory activity of arbidol hydrochloride in influenza A (H1N1) virus infection. Acta Pharmacol Sin. 2013;34(8):1075–1083.
- Pécheur E-I, Borisevich V, Halfmann P, et al. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J Virol. 2016;90(6):3086–3092.
- Boriskin YS, Pécheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol J. 2006;3:56.
- Meng-zhao W, Long-yun C, Ln AA, et al. Efficacy and safety of arbidol in treatment of naturally acquired influenza. Zhongguo Yi XueKeXue Yuan XueBao. 2004;26(3):289–293.
- Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b Treatment for COVID-19. Front Immunol. 2020;11:1061.
- Li Y, Xie Z, Lin W, et al. An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) [preprint]. MedRxiv. 2020. doi:10.1101/2020.03.19.20038984
- Liu Q, Fang X, Tian L, et al. The effect of arbidol hydrochloride on reducing mortality of COVID-19 patients: a retrospective study of real world data from three hospitals in Wuhan [preprint]. MedRxiv. 2020. doi:10.1101/2020.04.11.20056523
- Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi:10.1016/j.lfs.2020.117477
- Chen YW, Yiu CB, Wong KY. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL (pro)) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Res. 2020;9:129.
- Eslami G, Mousaviasl S, Radmanesh E, et al. The impact of sofosbuvir/daclatasvir or ribavirin in patients with severe COVID-19. J Antimicrob Chemother. 2020;dkaa331.
- Snell NJC. Ribavirin - Current status of a broad spectrum antiviral agent. Expert Opin Pharmacother. 2001;2:1317–1324.
- Morra ME, Van Thanh L, Kamel MG, et al. Clinical outcomes of current medical approaches for Middle East respiratory syndrome: a systematic review and metaanalysis. Rev Med Virol. 2018;28(3):e1977.
- Al-Tawfiq JA, Momattin H, Dib J, et al. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46.
- Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271.
- Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095.
- Dey S, Saini M, Dhembla C, et al. “Suramin, penciclovir and anidulafungin bind Nsp12, which governs the RNA-dependent-RNA polymerase activity of Sars-cov-2, with similar interaction energy as remdesivir-triphosphate, indicating potential in the treatment of COVID-19 infection”. OSF. 2020. doi:10.31219/osf.io/urxwh
- Mubareka S, Leung V, Aoki FY, Vinh DC. Famciclovir: a focus on efficacy and safety. Expert Opin Drug Saf. 2010;9(4):643–658.
- Sai JK, Suyama M, Kubokawa Y, et al. Efficacy of camostat mesilate against dyspepsia associated with non-alcoholic mild pancreatic disease. J Gastroenterol. 2010;45:335–341.
- Hirota M, Shimosegawa T, Kitamura K, et al. Continuous regional arterial infusion versus intravenous administration of the protease inhibitor nafamostatmesilate for predicted severe acute pancreatitis: a multicenter, randomized, open-label, phase 2trial. J Gastroenterol. 2020;55:342–352.
- Iwaki M, Ino Y, Motoyoshi A, et al. Pharmacological studies of FUT-175, nafamostat mesilate V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats. Jpn J Pharmacol. 1986;41:155–162.
- Ohkoshi M, Oka T. Clinical experience with a protease inhibitor [N, Ndimethylcarbamoylmethyl 4-(4-guanidinobenzoyloxy)-phenylacetate] methanesulfate for prevention of recurrence of carcinoma of the mouth and in treatment of terminal carcinoma. J Maxillofac Surg. 1984;12:148–152.
- Yamamoto M, Matsuyama S, Li X, et al. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60:6532–6539.
- Li H, Wang YM, Xu JY, Cao B. Potential antiviral therapeutics for 2019 novel coronavirus. Zhonghua Jie He He Hu Xi ZaZhi. 2020;43:E002.
- Kitano M, Yamamoto A, Noshi T, et al. Synergistic antiviral activity of S-033188/S-033447, a novel inhibitor of influenza virus cap-dependent endonuclease, in combination with neuraminidase inhibitors in vitro. Infect Dis. 2017;4:S371.
- Kawaguchi N, Koshimichi H, Ishibashi T, et al. Evaluation of drug–drug interaction potential between baloxavir marboxil and oseltamivir in healthy subjects. Clin Drug Investig. 2018;38:1053–1060.
- Riva L, Yuan S, Yin X, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020. doi:10.1038/s41586-020-2577-1
- Rutherford AC, Traer C, Wassmer T, et al. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119(Pt 19):3944–3957.
- Sbrissa D, Naisan G, Ikonomov OC, Shisheva A. Apilimod, a candidate anticancer therapeutic, arrests not only PtdIns(3,5)P2 but also PtdIns5P synthesis by PIKfyve and induces bafilomycin A1-reversible aberrant endomembrane dilation. PLoS One. 2018;13:e0204532–e0204532.
- Billich A. Drug evaluation: apilimod, an oral IL-12/IL-23 inhibitor for the treatment of autoimmune diseases and common variable immunodeficiency. IDrugs. 2007;10(1):53–59.
- Nelson EA, Dyall J, Hoenen T, et al. The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection. PLoS Negl Trop Dis. 2017;11(4):e0005540.
- Qiu S, Leung A, Bo Y, et al. Ebola virus requires phosphatidylinositol (3,5) bisphosphate production for efficient viral entry. Virology. 2018;513:17–28.
- Amsden GW. Anti-inflammatory effects of macrolides an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2004;55(1):10–21.
- Kanoh S. Rubin BK Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23(3):590–615.
- Zarogoulidis P, Papanas N, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68(5):479–503.
- Lin SJ, Kuo ML, Hsiao HS, Lee PT. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4+ T cells. Int Immunopharmacol. 2016;40:318–326.
- Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75.
- Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2020.
- Fossa AA, Wisialowski T, Duncan JN, Deng S, Dunne M. Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. Am J Trop Med Hyg. 2007;77(5):929–938.
- Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18(5):1020–1022.
- Calabrese F, Pezzuto F, Fortarezza F, et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477(3):359–372.
- Coppola A, Lombardi M, Tassoni MI, et al. COVID-19, thromboembolic risk and thromboprophylaxis: learning lessons from the bedside, awaiting evidence. Blood Transfus. 2020;18(3):226–229. doi:10.2450/2020.0113-20
- Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of. Thromb Haemost. 2020;18(5):1094–1099.
- Viecca M, Radovanovic D, Forleo GB, Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. 2020;158:104950.
- Keeffe EB, Rossignol JF. Treatment of chronic viral hepatitis with nitazoxanide and second generation thiazolides. World J Gastroenterol. 2009;15:1805–1808.
- Rossignol JF. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9(3):227–230.
- Dang W, Xu L, Ma B, et al. Nitazoxanide inhibits human norovirus replication and synergizes with ribavirin by activation of cellular antiviral response. Antimicrob Agents Chemother. 2018;62(11):e00707–e00718.
- Hong S, Kim H, Song C, et al. Nitazoxanide suppresses IL-6 production in LPS stimulated mouse macrophages and TG-injected mice. Int Immunopharmacol. 2012;13(1):23–27.
- Tay M, Fraser J, Chan W, et al. Nuclear localization of dengue virus (DENV) 1–4 nonstructural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 2013;99(3):301–306.
- Wagstaff KM, Sivakumaran H, Heaton SM, et al. Ivermectin is a specific inhibitor of importinα/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443(3):851–856.
- Yang SNY, Atkinson SC, Wang C, et al. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. doi:10.1016/j.antiviral.2020.104760
- Götz V, Magar L, Dornfeld D, et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep. 2016;6:23138. doi:10.1038/srep23138
- Caly L, Druce JD, Catton MG, et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;104787. doi:10.1016/j.antiviral.2020.104787.
- Chandler RE. Serious neurological adverse events after ivermectin-do they occur beyond the indication of onchocerciasis? Am J Trop Med Hyg. 2018;98(2):382–388.
- Malone RW, Tisdall P, Fremont-Smith P, et al. COVID-19: famotidine, histamine, mast cells, and mechanisms. Res Sq. 2020. doi:10.21203/rs.3.rs-30934/v2
- Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766–788.
- Janowitz T, Gablenz E, Pattinson D, et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020. doi:10.1136/gutjnl-2020-321852
- Freedberg DE, Conigliaro J, Wang TC, et al. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020;159(3):1129–1131.e3.
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475.
- Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452.
- Pagliano P, Spera AM, Ascione T, Esposito S. Infections causing stroke or stroke-like syndromes. Infection. 2020;48(3):323–332. doi:10.1007/s15010-020-01415-6
- Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395(10225):683–684.
- Siemieniuk RA, Meade O, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(7):519–528.
- Stanley P. Another decade, another coronavirus. N Engl J Med. 2020;382(8):760–762.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11.
- Horby P, Lim WS, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020.
- Available from: https://emergency.cdc.gov/coca/ppt/2020/V4_Combined_Critically-Ill-Adults-COCA-4.2.2020.pdf. Accessed December 14, 2020.
- Esposito S, Noviello S, Pagliano P. Update on treatment of COVID-19: ongoing studies between promising and disappointing results. Infez Med. 2020;28(2):198–211.
- Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818.
- AIFA. Executive summary studio TOCIVID-19. Available from: https://www.aifa.gov.it/documents/20142/1127901/executive_summary_Toci_Nazionale.pdf. Accessed December 14, 2020.
- ClinicalTrials.gov Available from: https://clinicaltrials.gov/ct2/show/NCT04320615. Accessed December 14, 2020.
- Chinese Clinical Trials Registry. Available from http://www.chictr.org.cn/searchprojen.aspx?title=Tocilizumab&officialname=&subjectid=&secondaryid=&applier=&studyleader=ðicalcommitteesanction=&sponsor=&studyailment=&studyailmentcode=&studytype=0&studystage=0&studydesign=0&minstudyexecutetime=&maxstudyexecutetime=&recruitmentstatus=0&gender=0&agreetosign=&secsponsor=®no=®status=0&country=China&province=&city=&institution=&institutionlevel=&measure=&intercode=&sourceofspends=&createyear=0&isup-loadrf=&whetherpublic=&btngo=btn&verify-code=&page=1. Accessed December 14, 2020.
- AIFA Covid 19: studio randomizzato italiano, nessun beneficio dal tocilizumab 18 giugno 2020. Available from: https://www.aifa.gov.it/-/covid-19-studio-randomizzato-italiano-nessun-beneficio-dal-tocilizumab. Accessed December 14, 2020.
- Ramiro S, Mostard RLM, Magro-Checa C, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79(9):1143–1151.
- Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31.
- Richardson PJ, Corbellino M, Stebbing J. Baricitinib for COVID-19: a suitable treatment? - Authors’ reply. Lancet Infect Dis. 2020;20(9):1013–1014.
- Rademaker M, Baker C, Foley P, Sullivan J, Wang C. Advice regarding COVID-19 and use of immunomodulators, in patients with severe dermatological diseases. Australas J Dermatol. 2020;61(2):158–159.
- Lee YH, Song GG. Relative efficacy and safety of tofacitinib, baricitinib, upadacitinib, and filgotinib in comparison to adalimumab in patients with active rheumatoid arthritis. Z Rheumatol. 2020. doi:10.1007/s00393-020-00750-1
- Giudice V, Pagliano P, Vatrella A, et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS-CoV-2-related acute respiratory distress syndrome: a controlled study. Front Pharmacol. 2020;11:857.
- Rayner JO, Roberts RA, Kim J, et al. AR12 (OSU-03012) suppresses GRP78 expression and inhibits SARS-CoV-2 replication. Biochem Pharmacol. 2020;182:114227.
- Mittra I, de Souza R, Bhadade R, et al. Resveratrol and Copper for treatment of severe COVID-19: an observational study (RESCU 002). medRxiv. 2020. doi:10.1101/2020.07.21.20151423
- Nemati M, Asl ER, Pouya FD, Rasmi Y. Curcumin, an inhibitor of PAK1, potential treatment for COVID-19. J Infectiol. 2020;3(2):1–3.
- Brouwer MC, Ascione T, Pagliano P. Neurologic aspects of covid-19: a concise review. Infez Med. 2020;28(suppl1):42–45.
- Dzhafer N, Papathanasiou J. Compassionate drug use: an imperative challenge for Bulgarian health system during COVID-19. Health Policy Technol. 2020;9(3):274–275.
- Esposito V, Verdina A, Manente L, et al. Amprenavir inhibits the migration in human hepatocarcinoma cell and the growth of xenografts. J Cell Physiol. 2013;228(3):640–645.