Abstract
Background
Several studies have shown anti-inflammatory, anti-microbial, antifungal, and antioxidant effects from Citrus limon–peel essential oil (Cl-PEO). Cl-PEO can be developed as topical drugs for oral ulceration because of its potential active components. There have been no studies on the topical application of Cl-PEO inducing type IV hypersensitivity reaction.
Purpose
To investigate the potential of Cl-PEO from Batu City to induce type IV hypersensitivity reactions based on clinical changes, lymphocytes, macrophages, IFNγ, andIL10 expression.
Methods
This study was adapted from a guinea pig maximization-test method in Indonesia and the guidance of ISO 10,993-10:2010, and conducted on 20 guinea pigs (Cavia cobaya) divided into a control group and a treatment group. The treatment group was given Cl-PEO and the control group CMC-Na. Clinical changes were observed, then tissue specimens taken for hematoxylin–eosin and immunohistochemistry staining.
Results
There were no clinical changes after exposure. Lymphocyte and macrophage numbers and IFNγ and IL10 expression increased in the treatment group compared to the control group (p=0).
Conclusion
Cl-PEO can induce type IV hypersensitivity reactions in guinea pigs based on cellular and molecular cytokines, but there are no clinical changes after topical application.
Introduction
Lemon (Citrus limon) is one of the plants that is very useful for health and comes from Southeast Asia, with its tropical and subtropical climates.Citation1 One of the places for lemonade plantations in Indonesia is Batu City, East Java Province, located in an active volcanic area, such as Bromo, Tengger, and Semeru, which make land in this area fertile. Batu is located on a plateau, so it has low temperatures and high humidity. Such climate conditions are very suitable for agricultural development, especially horticulture.Citation2
C. limon is one of the many herbal ingredients that have been studied for use in alternative medicine, including in Indonesia, and has been widely tested for antibacterial, antifungal, anti-inflammatory, antiviral, antiseptic, anticancer, anti-diabetes, and wound-healing properties.Citation3–Citation6 C. limon peel (CI-P) contains 6% essential oil (EO). These EOs contain bioactive compounds, such as limonene, up to 64%–90%, which are believed to have anti-inflammatory activity and low toxicity.Citation1,Citation5,Citation7–Citation10
According to the World Health Organization (WHO), plants are the best source for various types of drugs. The WHO estimates that around 3–4 billion people use herbal medicine and as many as 80% of individuals from developing countries use traditional medicines with basic ingredients from plants. Therefore, various types of plants are examined to investigate their usefulness, safety, and efficiency. Every material to be used as medicine must go through the biocompatibility-test phase to assess whether the material causes detrimental effects on the body.Citation11–Citation13
One of the biocompatibility tests as per the US Food and Drug Administration (FDA) and International Organization for Standardization (ISO) that can be done is to assess the safety level of a material applied topically. This is a sensitization test that looks at the potential of the material to cause type IV hypersensitivity reactions. The sensitization test recommended by the FDA and ISO for research is the guinea pig maximization test using Freund’s complete adjuvant. Cl-PEO has shown the ability to inhibit fungal growth, eg, that of Candida albicans, and increasehealing of oral ulcers,Citation14,Citation15 but the potential to induce hypersensitivity has not been observed. There have been no studies done on the topical use of Cl-PEO effecting type IV hypersensitivity reactions by observing the clinical changes and expression of cytokines involved.
The aims of this research were to investigate the potential of Cl-PEO to induce type IV hypersensitivity reactions. Type IV reactions depend on the interaction of antigens with T lymphocytes, called delayed-type hypersensitivity. IFNγ is one of the cytokines that play a role at the beginning of the type IV hypersensitivity process, and involves macrophages and lymphocytes as indicators of erythema and edema. Analysis of the potential of Cl-PEO to cause type IV hypersensitivity reactions is based on clinical changes, such as erythema and edema, then further alteration in the number of macrophages and lymphocytes by hematoxylin–eosin staining and IFNγ and IL10 expression by immunohistochemistry staining.Citation12,Citation16
Methods
This study was performed in strict accordance with the Guide for the Care and Use of Laboratory Animals, and the National Health Research and Development Ethics Standards and Guidelines Council (2017), Ministry of Health, Republic of Indonesia. Ethical clearance was obtained by the Universitas Airlangga Faculty of Dental Medicine from the Health Research Ethical Clearance Commission ((022/HRECC.FODM/III/2018).
Citrus limon Peel Essential Oil
The C. limon was bought at Balai Penelitian Tanaman Jeruk dan Buah SubtropikaBatu. EO was extracted from its peel using steam distillation at the Biochemistry Laboratory, Faculty of Science and Technology, Universitas Airlangga. Five kilograms C. limon peel was washed and put in a distillation flask, transferred to an Erlenmeyer flask to separate the EO (300 mL) and water (70 mL). Steam distillation was done for 10 hours. The of distillation then had n-hexane (100 mL) solvent added through a separating funnel to extract the EO in the distillation mixture, the water removed, then put in a rotary vapor and heated at 50°C until the hexane had evaporated completely. The final volume of Cl-CEO obtained was 270 mL. The Cl-PEO was obtained at a concentration of 100%, then diluted using 3% carboxymethyl cellulose–sodium (CMC-Na) powder until concentrations reached 1.56%, 0.78%, and 0.39%.
Component Analysis of Cl-PEO
To find out the compounds contained in Cl-PEO used in this study, gas chromatography–mass spectrometry (GC-MS) was performed at the Faculty of Pharmacy, Airlangga University. Cl-PEO was analyzed after being extracted twice with dichloromethane (pure analysis; Merck)The 0.4 μL obtained was then analyzed using GC-MS model 6890N equipped with an inert MSD 5973 MS detector (Agilent Technologies, Santa Clara, CA, USA). A HP-5MS UI column detector (polyethylene glycol, 30 m × 0.25 mm, 0.25 μm; J&W Scientific). The temperature of the injector was 230°C. The carrier gas was helium at a constant flow rate of 1 mL/min. The initial oven temperature of the column was raised from 40° to 300°C at 10°C/min, then maintained for 4 minutes at 300°C. The MS-detector conditions were a capillary direct–interface temperature of 270°C, MS source temperature 230°C, and MS quad temperature 150°C. Identification of the individual constituents was based on comparison of mass spectra and retention-time indices of authentic reference compounds stored in the NIST17 MS data library.
Type IV Hypersensitivity Model
This research used healthy young (2–3 months), maleadult guinea pigs (Cavia cobaya) weighting 300–500 g that had been acclimated for 2 weeks and were placed individually in single cages. Cl-PEO testing was done in two phases: called preliminary and main tests. The preliminary test was done with injected Cl-PEO to determine challenge and induction doses. The main test was done with applied topical Cl-PEO (). Twenty C. cobaya were divided into treatment and control groups based on the minimum number of samples according to the of ISO guidance 10,993-10:2010for in vivo non-clinical toxicity testing. At 24 hours before intradermal induction, the intrascapular region was shaved, then an injection of Freund’s complete adjuvant was made on point C bilaterally. There were three points injected, shown in A.
Figure 2 Type IV hypersensitivity model induced FCA in intrascapular region. (A) Point (AC) in main test. (B) applied topically Cl-PEO 0.78% (treatment group) and physiological solution (NaCl) and CMC 3% (control group) in point (D).
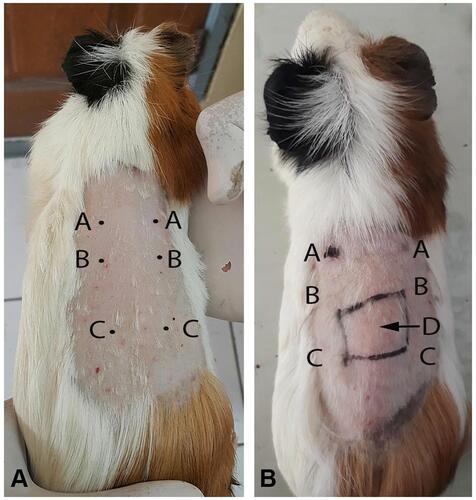
Seven days later, points A, B, and C had physiological solution (NaCl) and CMC 3% (control group) and Cl-PEO 1.56% (treatment group) applied topically, then covered with occlusive dressing and elastic bandage for 24 hours (). Fourteen days later (21 days after injection of points A, B, and C), the challenge stage was carried out on point D (B) with topically applied Cl-PEO 0.78% (treatment group), and physiological solution (NaCl) and CMC 3% (control group), then covered with occlusive dressings and elastic bandages. After 24 hours, occlusive dressings and elastic bandages were opened, then observed for 72 hours. After 72 hours, skin tissue at point D was biopsied. Histopathology examination was carried out to analyze the number of macrophages and lymphocytes with hematoxylin (CI 75,290; Merck, Germany) and eosin staining (C.I. 45,380, Merck, Germany). The immunohistochemistry was carried out to analyze IFNγ (purified antimouse IFNγ antibody; BioLegend, San Diego, CA, USA) and IL10 (purified antihuman IL10 antibody; BioLegend) expression. All analysis used light microscopy with 400× magnification.
Statistical Analysis
Data are expressed as mean ± SD for each measurement. Data were then analyzed using independent t-tests (SPSS 24.0 for Windows) to determine significant differences (p<0.05).
Results
Taxonomic Tests
Taxonomic tests were carried out on lemons at the Biochemistry Laboratory, Faculty of Science and Technology, Universitas Airlangga as the first step before all other tests were performed.
Gas Chromatography–Mass Spectrometry
On GC-MS testing, 24 components were identified in Cl-PEO (). The dominant component was D-limonene (75.02%), followed by linalool (5.63%), citral A (4.41%), citral B (3.68%), and limonene oxide (0.94%), and 19 other components with concentrations <1% ().
Table 1 Components Analysis of Citrus limon Using GC-MS
Clinical Features
Clinical intrascapular assessment of the in both groups at point D assessed using the Magnusson–Kligman test after 21 days showed normal features. After topical application of Cl-PEO 0.78%, point D showed no form of erythema or edema, indicators of type IV hypersensitivity reactions, at 48 and 72 hours.
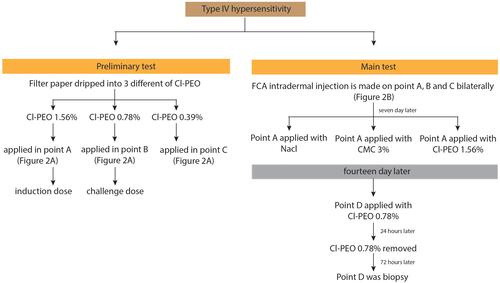
Number of Lymphocytes and Macrophages
Lymphocyte numbers in the control group (9.80±2.25, ) were lower than the treatment group (16.1±3.90, ). Macrophages numbers in the control group (10.5±1.84, ) were lower than the treatment group (16.2±1.81, ). The number of cells in the treatment group ( and ) was higher than the control group ( and ). There were significant differences among lymphocytes and macrophages between the control and treatment groups ().
Figure 3 Number of lymphocytes using hematoxylin-eosin staining. (A) control group; (B) treatment group (observed using a light microscope with 400x magnification).
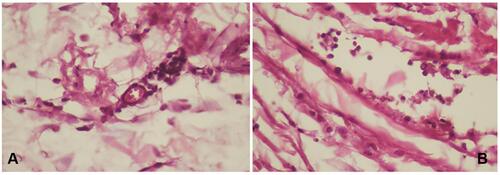
Figure 4 Number of macrophages using hematoxylin-eosin staining. (A) control group; (B) treatment group (observed using a light microscope with 400x magnification).
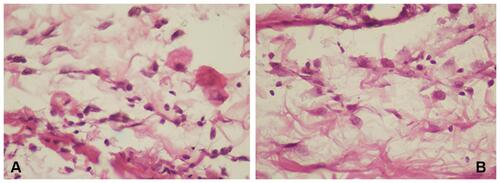
Table 2 Mean and Standard Deviation and Significant Level
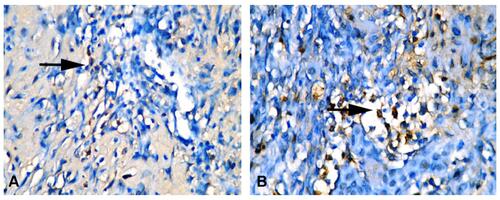
IFNγ Expression
IFNγ expression appears as a blackish-brown image in macrophage cells. IFNγ expression in the control group (3.40±0.69, ) was significantly lower than the treatment group (8.50±1.36, p=0, ).
IL10 Expression
IL10 expression also appears as a blackish-brown image in macrophage cells. IL10 expression in the control group (9.60±1.65, ) was lower than the treatment group (20.40±4.40, p=0, ). There were significant differences in IFNγ and IL10 expression between the control and treatment groups ().
Discussion
C. limon is part of the Rutaceae family. EOs in lemon are obtained from the outer portion of the skin.Citation17 It has been shown in various studies that EOs of lemon peel have antimicrobial, anti-inflammatory, and antifungal effects.Citation5,Citation6,Citation14,Citation18,Citation19 Although the EOs of lemon peel come from plants and are considered a natural ingredient, a common misunderstanding is that the product is truly safe. Some unwanted reactions from this oil that occur on the skin have been reported, especially when this oil has been used often.Citation13,Citation20,Citation21
In this research, the highest bioactive-compound concentration in Cl-PEO was D-limonene (75.02%), followed by linalool (5.63%), citral A (4.41%), citral B (3.68%), and limonene oxide (0.94%). These concentrations maydifferamong lemons. Variations in composition andconcentration of constituent chemical compounds, both qualitatively and quantitatively, are caused by intrinsic and extrinsic factors. Intrinsic factors are related to such variables as time of planting and fertilization, interactions with the environment, including the type of soil and climatic conditions, maturity, and harvest time of the plant. Extrinsic factors are related to extraction methods, environment, duration of extraction procedures, storage methods, and dehydration procedures.Citation21
D-limonene, the main component, had an important role in this research. D-limonene is a chemicals from the terpene class contained in most cosmetics and detergents sold throughout the world. In its unoxidized form, this substance has been reported to be a weak allergen. However, when contaminated with free air, this substance will auto-oxidize, making it highly allergenic.Citation22 In various studies, it has been shown that D-Limonene, citral, and linalool have greater effect in individuals with dermatitis or a history of allergies such that they can cause type IV hypersensitivity reactions.Citation23
Type IV hypersensitivity occurs in two phases: the sensitization phase involving activation of innate immunity, and the elicitationphase in the form of adaptive immunoactivity. In the sensitization phase, allergens will be recognized and phagocytosed by dendritic cells, which act as antigen-presenting cells. Dendritic cells that carry allergens migrate to regional lymph nodes and cause recruitment of specific T cells into the bloodstream and sensitization processes. At this stage, specific T cells will become memory T cells that will be sensitive to subsequent exposure to the antigen. The sensitization phase usually lasts 1–2 weeks after antigen exposure.Citation24 In the next phase (elicitation), dendritic cells phagocytose allergens, process these allergens, and present them on the surface of major histocompatibility–complex class II molecules, and will then be introduced to T-helper cells. These cells will produce IFNγ, which will activate more macrophages. Macrophages will also produce proinflammatory cytokines, such as IL1, IL6, and TNF-α, which trigger the manifestation of type IV hypersensitivity reactions in skin exposed to antigens, namely erythema and edema. Therefore, macrophages, lymphocytes, and IFNγ were selected as the main indicators in this study.Citation25,Citation26
In this study, the application of Cl-PEO led to numbers of lymphocytes and macrophages and IFNγ expression increasing. In the elicitation phase of hypersensitivity, increased numbers of lymphocytes and macrophages, and higher expression of IFNγ cytokines will manifest in such clinical signs as edema and erythema. However, we observed no signs of inflammationin C. cobaya after application of Cl-PEO. This was probably due to the activity of proinflammatory cytokines being inhibited well by anti-inflammatory cytokine activity, in this case IL10.
An interesting result of this study was that examination of the expression ofthe anti-inflammatory cytokine IL10 showed a significant increase. The increase in the number of macrophages here not only functioned in the proinflammatory process but also in the anti-inflammatory process by producing IL10. In previous studies, it has been found that D-limonene and linalool induces macrophages to produce even more IL10.Citation27,Citation28 Limonene can increase the amount of Treg cells in tissue, which function to modulate both proinflammatory and anti-inflammatory cytokines. Treg can induce monocyte differentiation into the M2-macrophage phenotype (anti-inflammatory) and inhibit induction of the M1 phenotype (proinflammatory) such that the inflammatory process can be stopped. Linalool can induce macrophages to produce IL10.Citation29
It can be concluded that even though Cl-PEO was able to induce type IV hypersensitivity reactions in guinea pigs, based on cellular and molecular cytokines, there were no clinical feature changes after topical application. Cellular and molecular cytokines in a hypersensitivity reaction, with increased lymphocyte and macrophage numbers and IFNγ, can be suppressed by the activation of the mainly anti-inflammatory cytokine IL10. This is the reason no change clinical examination after topical application both erythema and edema which usually occurs in type IV hypersensitivity reactions.
Disclosure
All contributing authors declare no conflicts of interest.
Additional information
Funding
References
- Dev C, Shrivastava R, Raj S, Nidhi. Basketful benefit of Citrus Limon. Int Res J Pharm. 2016;7(6):1–4. doi:10.7897/2230-8407.07653
- Kambuaya B, Yuwono A, Rachmawaty E, Mulyanto HS, Widayati T. Climate Change Risk and Adaptation Assessment Greater Malang Synthesis Report. Jakarta: Ministry of Environment Jalan; 2012:9–14.
- Tanjung KA, Sudarno SL. Efektivitas ekstrak kulit jeruk lemon (Citrus limonum) terhadap daya hambat pertumbuhan Aeromonas hydrophila secara in vitro. Berk Ilm Perikan. 2008;3(1):89–93.
- Ahmad M, Ansari M, Alam A, Khan T. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pakistan J Biol Sci. 2013;16(20):1086–1094. doi:10.3923/pjbs.2013.1086.1094
- Surboyo MDC, Mahdani FY, Savitri DS, et al. Number of macrophages and transforming growth factor β expression in Citrus limon L. Tlekung peel oil-treated traumatic ulcers in diabetic rats. Trop J Pharm Res. 2019;18(7):1427–1433.
- Ganesha R, Hernawan I, Hendarti HT, et al. Expression of FGF-2 and fibronectin in citrus limon fruit peel malang essential oil gel treated traumatic ulcer in diabetic wistar rats (Rattus novergicus). Res J Pharm Technol. 2019;12(July):5958.
- Sun J. D-limonene: safety and clinical applications. Altern Med Rev. 2007;12(3):259–264.
- Huang Y, Ho S. Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel. Food Chem. 2010;119(3):868–873. doi:10.1016/j.foodchem.2009.09.092
- Lorente J, Vegara S, Martí N, et al. Chemical guide parameters for Spanish lemon (Citrus limon (L.) Burm.) juices. Food Chem. 2014;162(2014):186–191. doi:10.1016/j.foodchem.2014.04.042
- Gosslau A, Yu K, Ho C, Li S. Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones Anti-inflammatory effects of characterized orange peel extracts enriched with bioactive polymethoxyflavones. Food Sci Hum Wellness. 2014;3(1):26–35. doi:10.1016/j.fshw.2014.02.002
- WHO. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine World Health Organization. Geneva: World Health Organization; 2000:1–18 p.
- Services USD of H and H. Use of International Standard ISO 10993-1. Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing Within a Risk Management Process. Rockville, USA: Food and Drug Administration; 2016.
- WHO. Who Global Report on Traditional and Complementary Medicine 2019. Ghebreyesus TA, editor. Luxemburg; 2019.
- Hernawan I, Radhitia D, Hadi P, Ernawati DS. Fungal inhibitory effect of citrus limon peel essential oil on candida albicans. Dent J. 2015;48(2):84–88.
- Prabajati R, Hernawan I, Hendarti HT. Effects of citrus limon essential oil (Citrus limon L.) on cytomorphometric changes of Candida albicans. Dent J. 2017;50(1):43. doi:10.20473/j.djmkg.v50.i1.p43-48
- Vinay Kumar AK, Abbas JCA. Robbins Basic Pathology. Vinay Kumar AK, Abbas JCA, editor. 10th Editi. Philadelphia, Pennsylvania:Elsevier; 2018.
- Jain N, Sharma M. Evaluation of citrus lemon essential oil for its chemical and biological properties against fungi causing dermatophytic infection in human beings. Anal Chem Lett. 2017;7(3):402–409. doi:10.1080/22297928.2017.1349620
- Khazal N, Hindi K, Adil Z, Chabuck G. Antimicrobial activity of different aqueous lemon extracts. J Appl Pharm Sci. 2013;3(06):8–74.
- Kummer R, Fachini-Queiroz FC, Estevão-Silva CF, et al. Evaluation of anti-inflammatory activity of citrus latifolia Tanaka essential oil and limonene in experimental mouse models. Evidence-Based Complement Altern Med. 2013;2013:1–8. doi:10.1155/2013/859083
- Plumlee KH, Ms DVM, Dacvim D. Citrus Oils. In: Small Animal Toxicology. Third ed. UK: Wiley-Blackwell, A John Wiley & Sons, Inc., Publication; 2013:513–515.
- Tjahyono N, Parmadianti AE, Mahdani FY. Cytotoxicity test of citrus limon peel essential oil in human gingival fibroblast. Oral Med Dent J. 2017;9(1):53–60.
- Audrain H, Kenward C, Lovell CR, et al. Allergy to oxidized limonene and linalool is frequent in the. Br J Dermatol. 2014;171(2014):292–297. doi:10.1111/bjd.13037
- Tisserand R, Young R. Essential Oil Safety. 2nd ed. Edinburg: Churchill Livingstone Elsevier; 2014:187–482.
- Schmidt M, Goebeler M. Immunology of metal allergies. J Ger Soc Dermatology. 2015;653–659.
- Geha R, Notarangelo L. Case studies in immunology: a clinical companion. In: Pediatric and Developmental Pathology. Vol. 9. 6th ed. New York: Garland Science, Taylor & Francis Group; 2012.
- Coico R, Sunshine G. Immunology: A Short Course. 7th ed. Vol. 53. Wiley online library United Kingdom: Wiley Blackwell; 2015:183,259.
- Ramalho TR, Filgueiras LR, De Oliveira MTP, et al. Gamma-Terpinene modulation of LPS-stimulated macrophages is dependent on the PGE2/IL-10 Axis. Planta Med. 2016;82(15):1341–1345. doi:10.1055/s-0042-107799
- Plastina P, Apriantini A, Meijerink J, Witkamp R, Gabriele B, Fazio A. In vitro anti-inflammatory and radical scavenging properties of chinotto (Citrus myrtifolia Raf.) essential oils. Nutrients. 2018;10(6):1–13. doi:10.3390/nu10060783
- Turabelidze A, DiPietro A. Inflammation and Wound Healing. In: Larjava H, editor. Oral Wound Healing: Cell Biology and Clinical Management. UK: Wiley-Blackwell, A John Wiley & Sons, Inc., Publication; 2012:47–48.