Abstract
Acute GVHD (aGVHD) is a significant complication after allogeneic hematopoietic cell transplantation (HCT), occurring in up to 70% of HCT recipients. Steroid-refractory aGVHD represents a subset of patients failing initial therapy and is particularly morbid, with only 30% of patients surviving long term. Better therapies are urgently required for these patients. Here, we discuss recent advancements in the management of SR-aGVHD. We review the currently available therapies for SR-aGVHD including the results of the REACH1 and REACH2 trials, which provide the basis for the use of ruxolitinib for the treatment of SR-aGVHD. We additionally discuss newer agents under clinical investigation and will highlight the niche these agents may fill to further improve outcomes in aGVHD patient care.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) represents an important and potentially curative procedure for a variety of malignant and non-malignant conditions.Citation1–Citation3 Alongside benefit, allogeneic transplant recipients often face significant complications, including graft-versus-host disease (GVHD). Acute GVHD (aGVHD) is a frequent problem, occurring in up to 70% of HCT recipients.Citation4,Citation5 Upon development of aGVHD, 2-year treatment-related mortality (TRM) among allogeneic transplant recipients is approximately 40%.Citation5
Primary therapy for acute GVHD is systemic high-dose corticosteroids.Citation5 While steroid therapy is mostly effective, nearly 40% of acute GVHD cases fail to respond to high-dose corticosteroid therapy and are considered steroid-refractory.Citation5,Citation6 Steroid-refractory aGVHD (SR-aGVHD) represents a particularly morbid complication post-transplant, with an estimated 6-month survival of only 40% after development.Citation5 As a significant contributor to transplant-related mortality, the development of more effective therapies for SR-aGVHD is an area of need for allogeneic HCT recipients. In this review, we will discuss emerging pharmacologic therapies under investigation for SR-aGVHD.
Acute GVHD: Presentation and Pathophysiology
Acute GVHD typically develops within the first 100 days after allogeneic HCT, however can occur in a delayed manner, particularly in the setting of a reduced intensity conditioning transplant.Citation7 Pathologically, the development of aGVHD occurs following a three-step process. First, the HCT recipient incurs tissue injury from transplant conditioning, from infection, or pre-transplant from underlying disease or treatment of disease. Injured host cells subsequently release pro-inflammatory cytokines including tumor necrosis factor (TNF) – alpha, interleukin (IL)-1, and IL-6 resulting in the activation of host and donor antigen-presenting cells (APCs). Furthermore, mucosal injury, particularly within the GI tract, may result in barrier disruption, and subsequent bacterial translocation or leakage of pathogen-associated molecular patterns (PAMP) across the barrier. These inflammatory alterations, along with alterations due to antibiotics administered as prophylaxis or as treatment, can lead to dysbiosis, or deleterious alterations to the microbiome within the gut. Increasingly, there is evidence that microbial shifts, particularly to an enterococcal bias, may facilitate GVHD development.Citation8 Second, APCs engage and activate donor T cells. These activated T cells proliferate; CD4+ T cells differentiate into T-helper1 (Th1) cells, Th2, Th17 cells, while CD8+ T cells differentiate into cytotoxic T cells. Activated T cells additionally produce cytokines, such as IL-1, IL-2, TNF that promote activation and survival of these cells. Third, T cells migrate to and attack host target organs. At the site of GVH, the proliferation of cytotoxic T cells and activation of NK cells lead to host cell apoptosis.Citation7
Clinically, aGVHD most frequently involves the skin, liver, and GI tract. Skin manifestations include a maculopapular rash. GI aGVHD may involve both the upper or lower GI tract, and may present as anorexia and weight loss, or cramping with diarrhea. Finally, liver involvement most frequently manifests as a conjugated hyperbilirubinemia; however, severe cases may also demonstrate the development of coagulopathy or hyperammonemia.Citation5 Upon aGVHD development, staging is then performed. Traditionally, Glucksberg or Consensus criteria have been applied for staging; however, more recently, in the setting of clinical trials, MAGIC consortium criteria are increasingly being utilized, in line with the recent EBMT, NIH and CIBMTR recommendation.Citation9,Citation10 Generally, involved sites are assigned an initial stage, ranging from 1 to 4, depending on the magnitude of aGVHD involvement. From the perspective of SR-aGVHD, initial staging is of importance as it may help predict which patients ultimately fail initial steroid therapy. Using the Minnesota GVHD risk score, a prospectively validated tool, high-risk patients, representing about 16% of aGVHD cases, have about a 60% probability of ultimately being steroid-refractory.Citation6 Furthermore, there is evidence that serum biomarker studies drawn at the onset of aGVHD can additionally predict for patients who will fare poorly on steroid therapy.Citation11
Acute GVHD: Initial Treatment and Diagnosis of Steroid-Refractoriness
The initial treatment for aGVHD is systemic corticosteroids; prednisone is often used at a dose of 2 mg/kg daily.Citation5 Corticosteroids combat aGVHD by downregulating the production of inflammatory cytokines, inhibiting activation and proliferation of T cells, and by potentially modulating antigen presentation at the site of disease. Typically, responding patients demonstrate signs of improvement within about 72 hours after steroid initiation. Patients failing to improve or progressing on steroids are those that are considered to have SR-aGVHD.
Steroid-refractory aGVHD is a clinical diagnosis, with varying criteria between trials. Traditionally, the following are often included as criteria for SR-aGVHD: 1) aGVHD progression after 72 hours on steroids, or 2) aGVHD which fails to respond to after 7 days on high-dose steroids. Increasingly, studies have additionally begun to consider the following as SR-aGVHD: 3) aGVHD developing in a new site while on >1 mg/kg prednisone, or 4) aGVHD recurrence during steroid taper, with the inability to increase steroids.Citation12 Upon diagnosis of SR-aGVHD, second-line immunosuppressive therapies are indicated but historically have had limited efficacy.
SR-aGVHD: Conventional Therapies
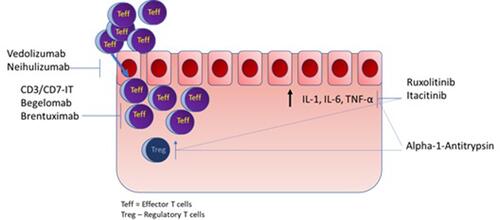
Recently, ruxolitinib phosphate received approval by the US FDA for the treatment of SR-aGVHD. Prior to this, no agent had specific indications for SR-aGVHD, however several agents demonstrated efficacy in limited studies and are used in clinical practice. These agents include traditional oral immunosuppressive agents targeting T-cell activation such as sirolimus or mycophenolate mofetil, and lymphodepletive agents including pentostatin or horse or anti-thymocyte globulin.Citation13–Citation16 Based on either Phase I/II study results or retrospective analyses, off-label use of monoclonal antibodies targeting cytokines, such as the anti-TNF-alpha antibody agents infliximab, etanercept, the anti-IL-6 receptor antibody tocilizumab, and anti-IL2 receptor antibody inolimomab, is occasionally employed.Citation17–Citation19 Finally, extracorporeal photopheresis represents a frequent non-pharmacologic intervention.Citation20 While these options all appear to elicit responses, with reported responses ranging from 30% to 70%, a limited survival benefit with all these options is seen with long-term survival in the range of only 20–40% (). Lack of initial response or subsequent loss of response, frequent infectious complications, and/or excess relapse contribute to these dismal outcomes.Citation5 Given these poor outcomes, clinical trials for SR-aGVHD represent an area of significant activity. More recently, experimental agents in clinical development have taken a targeted approach to limit infectious risks and excess relapse, while treating specific disorders leading to steroid resistance. illustrates the proposed mechanisms of these novel agents.
Table 1 Conventional Second-Line Therapies for SR-aGVHD
Experimental Therapy: Ruxolitinib Phosphate
Ruxolitinib phosphate is an oral multi-kinase inhibitor of the Janus kinases JAK1 and JAK2, and tyrosine kinase 2 (Tyk2).Citation21,Citation22 These enzymes play a critical role in cytoplasmic cytokine signaling and growth factor receptor signaling; JAK1 and Tyk2 modulate proinflammatory cytokine signaling, and JAK2 is involved in erythropoietin and thrombopoietin signaling.Citation22
In GVHD, pre-clinical studies demonstrate that ruxolitinib therapy rapidly decreases proinflammatory cytokine including TNF-alpha, IL-6, and IL-12.Citation23 In murine models, these cytokine reductions were mostly seen to be early events seen within a week of dosing. Further, ruxolitinib exposure was shown to decrease proliferation of activated T cells in vivo and resulted in an expansion of CD4+FoxP3+ regulatory T cells (Tregs).Citation24 In all, these findings provided a mechanistic basis for exploring ruxolitinib clinically.
The first report on ruxolitinib use in SR-aGVHD was a retrospective series from a number of European sites.Citation25 In this study, 54 patients with SR-aGVHD were treated with ruxolitinib, and an 81.5% ORR was reported, with a nearly 50% complete response to therapy. Most impressively, the 6-month survival in these patients was 79%. These results were subsequently replicated in several retrospective studies where ruxolitinib was used off label for SR-aGVHD.
These promising findings led to the development of the REACH trials to formally evaluate the efficacy and safety of ruxolitinib for GVHD. REACH1 was a single-arm interventional study evaluating the efficacy and safety of ruxolitinib in SR-aGVHD. REACH2 was next performed to assess ruxolitinib efficacy in a randomized fashion for SR-aGVHD, while REACH3 evaluated ruxolitinib in a randomized study for corticosteroid chronic GVHD.Citation26
REACH1, and open-label Phase II study evaluating the safety and efficacy of ruxolitinib in SR-aGVHD reported favorable findings.Citation27 Overall, 71 patients were enrolled in this study; patients were 12 or older and could have undergone allogeneic HCT from any donor source. Among ruxolitinib-treated patients, an estimated ORR of 54.9% was seen by day 28, with complete responses in estimated 26.8% patients. Beyond 28 days, an estimated 73% of patients ultimately achieved a response on ruxolitinib, including complete responses in an estimated 56.3% of patients. Responses were seen in all types of SR-aGVHD; however, a greater proportion of responses were seen in SR-aGVHD involving the skin, compared to patients with SR-aGVHD involving the liver or the GI tract. Among all patients, 6-month survival was 51%, and among responders, 6-month survival was 70%. Based on these results, and available retrospective data, the US FDA approved ruxolitinib for the management of SR-aGVHD.
Recently, the results of the REACH2 were published and demonstrated benefit with ruxolitinib compared against best available care.Citation28 REACH 2 was a randomized, Phase 3 trial comparing Ruxolitinib (at 10 mg twice daily) against best available therapy (BAT). The primary endpoint for this study was the overall response at day 28. Overall, the study met its primary endpoint; compared against BAT, ruxolitinib yielded a significantly improved ORR at 28 days, with 62.3% responding, compared with 39.4% (p<0.001). Furthermore, subgroup analysis revealed that a greater proportion of patients with Grade 3 or 4 aGVHD responded to ruxolitinib compared to control, and all organ sites saw improved responses with ruxolitinib. Finally, among responders, at 6 months, nearly 90% of ruxolitinib treated patients maintained their response compared to 60% receiving BAT. From a safety standpoint, ruxolitinib appeared to have a similar safety profile compared with control; severe adverse events were reported in 38% of patients receiving ruxolitinib compared to 34% receiving BAT. Thrombocytopenia and anemia remained the most common severe adverse events; neutropenia was also observed but at a lower rate. Infections occurred at nearly an equal rate among ruxolitinib-treated patients compared to control patients (grade 3+: 22% vs 19%). At one-year, an estimated one-year survival of 49% was reported on ruxolitinib, compared to 44% among patients receiving BAT. Overall, these results demonstrated that ruxolitinib represents a reasonable first option for patients with SR-aGVHD. summarizes the outcomes of these studies.
Table 2 Ruxolitinib in SR-aGVHD. BAT: Best Available Therapy (Sirolimus, MMF, MSC, MTX, Infliximab, ECP, Etanercept, ATG)
Despite these encouraging results, the REACH2 trial also revealed groups for which, outcomes remain suboptimal. REACH2 subgroup analysis revealed that among patients with Grade 3+ aGVHD at randomization, about 55% responded to therapy, as opposed to 76% response rates among patients with Grade 2 aGVHD.Citation29 Additionally, looking at aGVHD site, the overall response rate in patients with liver aGVHD was about 44%, and lower GI aGVHD responded in about 57% of cases.Citation29 This is in comparison to upper GI and skin aGVHD, which demonstrated responses to ruxolitinib in about 68% and 72% of cases.Citation29 Finally, in terms of safety, ruxolitinib did not appear to spare patients from infectious complications. A previous study also reported that almost 70% of patients developed infection during ruxolitinib therapy for SR-aGVHD.Citation30 Further, additional toxicities may be emerging with increased use; drug-induced microangiopathy has recently been reported with ruxolitinib use.Citation31 Given the limitations of ruxolitinib in these settings, nearly 22% of patients treated with ruxolitinib still died due to aGVHD compared to 25% of control patients. With that, the cumulative incidence of non-relapse-related death at one-year was 49% among ruxolitinib-treated patients compared to 51% in the control group.
Experimental Therapy: Itacitinib
The most frequent adverse events associated with ruxolitinib were related to thrombocytopenia and anemia. In previous studies, this has led to dose reductions and occasionally treatment discontinuation with ruxolitinib, potentially limiting efficacy in aGVHD. As discussed above, JAK2 signaling appears to be more heavily implicated in thrombopoiesis and erythropoiesis, while JAK1 signaling appears to play a greater role in inflammatory cytokine signaling. On this basis, itacitinib, a selective JAK1 inhibitor, was studied in both patients developing aGVHD and SR-aGVHD.
Recently, a Phase I study of itacitinib in combination with corticosteroids for steroid-naïve and SR-aGVHD was performed.Citation32 Patients received itacitinib at either 200 mg or 300 mg daily. In all, 17 patients with SR-aGVHD were enrolled in this study. Of these patients, 13 had grade 3+ aGVHD, 11/16 had lower GI aGVHD, and 5/16 patients had liver aGVHD. In terms of safety, thrombocytopenia and anemia were both seen in about 30% of cases, but the rates of Grade 3+ cytopenias appeared lower than what is reported with ruxolitinib. In terms of response, the day 28 ORR reported was 70.6%, with 30% of patients in CR by day 28. Non-relapse mortality at 6 months was high at 52.9% resulting in an estimated 6-month survival of 47.1%.
Moving forward regarding SR-aGVHD, given the tolerability of itacitinib monotherapy, an institutional phase I trial in combination with tocilizumab is nearing enrollment to evaluate the safety and efficacy of this approach (NCT04070781).
Experimental Therapy: Anti-CD3/CD7 Immunotoxins (T-Guard)
Among SR-aGVHD patients with grade 3+ disease, or with GI involvement, combination anti-CD3/CD7 immunotoxin (CD3/CD7-IT) therapy has demonstrated promising preliminary results.Citation33 CD3/CD7-IT consists of a 1:1 mixture of monoclonal antibodies against CD3 and CD7, each conjugated ricin toxin A. In vivo studies demonstrate that CD3/CD7-IT therapy induces apoptosis in both T-cell and NK cells, and may reduce T cell activation.
A single-arm Phase I/II study with CD3/CD7-IT was conducted in patients SR-aGVHD, of which 17/20 patients had Grade 3+ aGVHD, and 18/20 patients had either GI or liver involvement.Citation33 Treatment consisted of four weekly 4-hour infusions of CD3/CD7-IT at 4mg/m2. In this high-risk cohort, the ORR by day 28 was 60%, with 50% of patients achieving a CR. The most common grade 3 or greater toxicities related to treatment included thrombocytopenia in 8/20 patients, and hyperbilirubinemia in 2/20 patients, and nearly all responses were complete responses. Overall, 6-month survival was estimated at 60%. Furthermore, 12 patients with SR-aGVHD were treated with CD3/CD7-IT on an expanded access program, and these patients appeared to also farewell with an estimated 6-month survival of 75%.Citation34
These promising results led the US FDA to designate CD3/CD7-IT fast track status, and presently the BMT-CTN is planning a multi-center study to examine CD3/CD7-IT versus ruxolitinib in grade 3+ SR-aGVHD (NCT04128319).
Experimental Therapy: Begelomab
Another monoclonal antibody therapy with promising results in severe SR-aGVHD is Begelomab.Citation35 Begelomab is a monoclonal antibody against CD26. CD26 is a marker for T cell activation and CD26+ T cells accumulate in inflamed tissues.
Two clinical trials have been performed with begelomab, a pilot study (EudraCT 2007–005809-21), and a dose-finding study (EudraCT No. 2012–001353-19). Results from these studies, as well as patients treated with begelomab on a compassionate use basis, were recently published, reporting on the outcomes of 69 patients.Citation35 Various dosing schemas were applied to patients, including the recommended dosing schedule of 3mg/m2/day x 5 days followed by 2 weekly administrations for three consecutive weeks. Of the 69 patients, 61 had Grade 3+ aGVHD; 51 of 69 patients had Stage 3+ GI GVHD, and 45 of 69 patients had Stage 3+ liver GVHD. In this study, the ORR at day 28 was approximately 69% among patients with Grade 3+ aGVHD, with responses in 56% and 68%, respectively, for severe liver and GI GVHD. In terms of toxicities, no treatment-related severe adverse events were reported; however, infections, including CMV reactivation, were common events during treatment. Overall, estimated one-year survival was 50%; among responders, an estimated 75% of patients were alive at 1 year.
With these findings, begelomab was granted orphan drug designation by the US FDA, and in the EU and Switzerland, and a multinational phase II/III trial was planned for 2020; this trial is presently on hold.
Experimental Therapy: Brentuximab Vedotin
A third monoclonal antibody targeting activated T cells is Brentuximab Vedotin (BV). CD30 is thought to be overexpressed on activated CD8+ T cells, and BV is an antibody–drug conjugate against CD30.
BV was studied in a single-arm, multicenter, phase I study in patients with SR-aGVHD. The maximum tolerated dose (MTD) and dose that most patients received were 0.8 mg/kg IV every 2 weeks for 4 doses. A total of 34 patients were treated, and the ORR with BV at day 28 was 38.2%. By day 56, additional 7 patients responded; in total 18/34 (53%) responded to therapy. Responses were seen in 42% of patients with refractory GI GVHD, and 45% of patients with refractory liver GVHD. The commonest toxicities seen with therapy were related to cytopenias; thrombocytopenia and neutropenia frequently occurred. Peripheral neuropathy, a common adverse event among Hodgkin lymphoma patients receiving BV, was not observed in this study. Among all patients, 41% were alive at 6 months, and 38% at 1 year. Among responders, 12/18 (67%) were alive at 1 year.
Going forward, a phase II study was planned but terminated; there are no plans to combine this agent or move this agent into the frontline for aGVHD.
Experimental Therapy: Alpha-1-Antitrypsin
In addition to the persistence of activated T cells, suppression of Treg populations is thought to play a role in the development of SR-aGVHD. Alpha1-antitrypsin (AAT) is a serine protease inhibitor with anti-inflammatory properties and has been shown to increase Tregs.
Alpha1-antitrypsin was studied in a single-arm, multi-center study for SR-aGVHD.Citation36 Enrolled patients were administered AAT at a dose of 60mg/kg per day on Days 1,4,8,12,16,20,24, and 28. In all, 40 patients were enrolled, of which 70% had Grade 3+ aGVHD. By day 28, an ORR of 65% was reported; 35% had achieved a CR at this timepoint. Among patients with GI aGVHD, an ORR of 64% was reported, and among liver aGVHD patients, a 57% ORR was reported by day 28. AAT was well tolerated, with no Grade 3+ adverse events attributed to drug. Overall, a modest 50% survival was seen at 6 months among responding patients.
While further development of AAT is not currently underway in the setting of SR-aGVHD, with the safety profile demonstrated in for this drug, and with the signal in GI aGVHD, a randomized trial is underway studying corticosteroids versus corticosteroids + AAT in steroid-naïve high-risk aGVHD (BMT-CTN 1705).
Experimental Therapy: Vedolizumab
Targeting T cell migration is a newer area of investigation in GVHD. Vedolizumab, an antibody against α4β7 integrin, has been investigated as a treatment that impairs T cell homing to the GI endothelium.
Vedolizumab is an approved agent in the management of inflammatory bowel disorders and initially was used off label for the treatment of SR-aGVHD. Two retrospective studies reported promising activity in SR-aGVHD. The first reported on 29 patients receiving Vedolizumab, 300 mg IV every 2 weeks for 3 doses, followed by maintenance dosing every 8 weeks. All patients had SR-aGVHD of the GI tract, and 12 patients had concurrent GVHD involving the liver. At 28 days, all patients receiving Vedolizumab as second-line therapy responded, and reportedly 66% of patients receiving Vedolizumab as third-line therapy responded. Overall, among patients receiving Vedolizumab as second-line therapy, 6-month OS was 42%. Drug-related toxicities were not reported, but 90% of treated patients had an infectious complication. A second retrospective study reported similar findings; among 29 patients with SR-aGVHD, after 6 weeks, 64% of patients responded. A 6-month OS at 54% was reported in this cohort. This study reported on adverse events, and infection again was frequently observed, as well as one possible case of ileus related to drug.
These results led to a prospective dose-finding study of Vedolizumab in SR-aGVHD (NCT02993783). This study was terminated due to lack of efficacy. Going forward, Vedolizumab was studied in a Phase Ib study as a prophylactic agent against aGVHD, and a lower incidence of lower-intestinal aGVHD was observed. These results may lead to further investigation in this setting.
Experimental Therapy: Neihulizumab
Neihulizumab is another novel agent targeting T cell migration. Neihulizumab is a monoclonal antibody against P-selectin glycoprotein ligand-1 (PSGL-1). At sites of inflammation, the endothelium of blood vessels expresses P-selectin, and activated T cells bind to these sites and migrate through the expression of PSGL-1. In that manner, Neihulizumab is thought to target activated T cells and inhibit trafficking to sites of inflamed tissue.
A multicenter, single-arm phase I trial is underway, and interim analysis demonstrated that after one dose, among enrolled patients, all of which were required to have SR-aGVHD involving the skin, 10/11 patients experienced an improvement in aGVHD by at least 1 stage.Citation37 This study is ongoing for SR-aGVHD involving any site, now with a weekly dosing schedule for 4 weeks (NCT03327857).
Experimental Therapy: Non-Pharmacologic Therapies
Mesenchymal Stem Cells (MSC)
Mesenchymal stem cells (MSCs) are a type of multipotent stem cells capable of differentiating into osteocytes, adipocytes, and chondroblasts. Proliferation of MSCs has been shown to inhibit cytotoxic T cell activity and T cell proliferation, and thus have been studied for many years as a therapy for aGVHD with promising results. Despite activity, variable manufacturing practices limited the use of MSCs, given inconsistent efficacy between manufactured batches of product. Remestemcel-L, a culture-expanded mesenchymal stem cell product, represents a manufactured product with more consistent results among various studies. Among 309 pediatric patients treated with remestemcel-L on three studies and on an expanded access protocol, an overall response rate of 66% was reported at 28 days.Citation38 Additionally, a favorable response rate of 68% at 6-month was also reported. On this basis, remestemcel-L is under review by the US FDA for use in pediatric SR-aGVHD.
Fecal Microbiota Transplantation (FMT)
Increasing awareness of the interplay between the gut microbiota, its metabolites, and the immune system has led to the study of modifying the gut in the prevention and management of aGVHD. Fecal microbiota transplantation (FMT) represents a therapy under investigation which allows for fecal suspension from a healthy individual to be given to patients with GVHD to facilitate the restoration of a healthy microbiota in patients with disease. In SR-aGVHD, a handful of small studies appear to demonstrate the benefit of this approach. In a pilot study in which all patients had some degree of steroid-refractory aGVHD involving the gut, four patients received two FMT infusions resulting in responses for all patients.Citation39 Additional small studies have shown equally promising results, and larger prospective studies are underway (NCT04059757, NCT04139577, NCT04285424).Citation40,Citation41
Conclusion
Steroid-refractory aGVHD remains a significant complication after allogeneic HCT with a considerable risk of death after development. Fortunately, incremental progress is now being realized. The results of the REACH studies and approval of ruxolitinib represent a step forward. With ruxolitinib, compared against standard options, more patients are anticipated to respond to second-line therapy.
Going forward, progress is needed for patients with higher grade aGVHD and GI aGVHD. GVHD remained the leading cause of non-relapse death among ruxolitinib-treated patients on the REACH trials, and these patients, in particular, still achieved limited responses. There is still a need for novel therapies; CD3/CD7-IT and possibly Neihulizumab have the potential to help these patients. outlines active studies for SR-aGVHD. Further, building on the success of JAK inhibitors, combination trials with complementary therapies will be important to pursue. In addition to improving GVHD responses in these higher risk patients, these trials must also balance increasing immunosuppression with infectious risk, excess relapse risk, and long-term tolerability. Overall, the development of novel therapeutics for SR-aGVHD will likely remain an active area in the future.
Table 3 Active Trials in SR-aGVHD
Disclosure
Mehdi Hamadani reports consultancy for Incyte during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
- Kanate AS, Majhail NS, Savani BN, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American society for transplantation and cellular therapy. Biol Blood Marrow Transplant. 2020;26(7):1247–1256. doi:10.1016/j.bbmt.2020.03.002
- Majhail NS, Farnia SH, Carpenter PA, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American society for blood and marrow transplantation. Biol Blood Marrow Transplant. 2015;21(11):1863–1869. doi:10.1016/j.bbmt.2015.07.032
- D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides. 2018. Available from: https://www.cibmtr.org. Accessed January 20, 2020.
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828.
- Martin PJ, Rizzo JD, Wingard JR, et al. First and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American society of blood and marrow transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150–1163. doi:10.1016/j.bbmt.2012.04.005
- MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761–767. doi:10.1016/j.bbmt.2015.01.001
- Zeiser R, Blazar BR. Acute graft-versus-host disease — biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167–2179. doi:10.1056/NEJMra1609337
- Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. (1523-6536 (Electronic)).
- Schoemans HA-O, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. (1476-5365 (Electronic)).
- Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transplant. 2016;22(1):4–10. doi:10.1016/j.bbmt.2015.09.001
- Levine JE, Braun TM, Harris AC, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. (2352-3026 (Electronic)).
- Martin PJ. How I treat steroid-refractory acute graft-versus-host disease. Blood. 2020;135(19):1630–1638. doi:10.1182/blood.2019000960
- Hoda D, Pidala J, Salgado-Vila N, et al. Sirolimus for treatment of steroid-refractory acute graft-versus-host disease. Bone Marrow Transplant. 2010;45(8):1347–1351. doi:10.1038/bmt.2009.343
- Pidala J, Kim J, Perkins J, et al. Mycophenolate mofetil for the management of steroid-refractory acute graft vs host disease. Bone Marrow Transplant. 2010;45(5):919–924. doi:10.1038/bmt.2009.252
- Hsu B, May R, Carrum G, Krance R, Przepiorka D. Use of antithymocyte globulin for treatment of steroid-refractory acute graft-versus-host disease: an international practice survey. Bone Marrow Transplant. 2001;28(10):945–950. doi:10.1038/sj.bmt.1703269
- Ragon BK, Mehta RS, Gulbis AM, et al. Pentostatin therapy for steroid-refractory acute graft versus host disease: identifying those who may benefit. Bone Marrow Transplant. 2018;53(3):315–325. doi:10.1038/s41409-017-0034-z
- Yalniz FF, Hefazi M, McCullough K, et al. Safety and efficacy of infliximab therapy in the setting of steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23(9):1478–1484. doi:10.1016/j.bbmt.2017.05.001
- Drobyski WR, Pasquini M, Kovatovic K, et al. Tocilizumab for the treatment of steroid refractory graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17(12):1862–1868. doi:10.1016/j.bbmt.2011.07.001
- Socié G, Vigouroux S, Yakoub-Agha I, et al. A phase 3 randomized trial comparing inolimomab vs usual care in steroid-resistant acute GVHD. Blood. 2017;129(5):643–649. doi:10.1182/blood-2016-09-738625
- Abu-Dalle I, Reljic T, Nishihori T, et al. Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: results of a systematic review of prospective studies. Biol Blood Marrow Transplant. 2014;20(11):1677–1686. doi:10.1016/j.bbmt.2014.05.017
- Yang LPH, Keating GM. Ruxolitinib. Drugs. 2012;72(16):2117–2127. doi:10.2165/11209340-000000000-00000
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–3117. doi:10.1182/blood-2009-04-214957
- Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832–3842.
- Choi J, Cooper ML, Alahmari B, et al. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS One. 2014;9(10):e109799. doi:10.1371/journal.pone.0109799
- Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi:10.1038/leu.2015.212
- Jagasia M, Zeiser R, Arbushites M, Delaite P, Gadbaw B, Bubnoff N. Ruxolitinib for the treatment of patients with steroid-refractory GVHD: an introduction to the REACH trials. Immunotherapy. 2018;10(5):391–402. doi:10.2217/imt-2017-0156
- Jagasia M, Perales M-A, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label Phase 2 trial. Blood. 2020;135(20):1739–1749. doi:10.1182/blood.2020004823
- Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800–1810. doi:10.1056/NEJMoa1917635
- Zeiser R, Niederwieser D, Mohty M, et al. Ruxolitinib versus best available therapy in patients with steroid-refractory acute graft-versus-host disease: overall response rate by baseline characteristics in the randomized phase 3 REACH2 trial. EHA Annual Meeting. 2020.
- Abedin S, McKenna E, Chhabra S, et al. Efficacy, toxicity, and infectious complications in ruxolitinib-treated patients with corticosteroid-refractory graft-versus-host disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(8):1689–1694. doi:10.1016/j.bbmt.2019.04.003
- Gavriilaki E, Bousiou Z, Chatzikonstantinou T, et al. Transplant-associated thrombotic microangiopathy is independently associated with ruxolitinib administration in patients with graft-versus-host-disease. EBMT. 2020;2020.
- Schroeder MA, Khoury HJ, Jagasia M, et al. A Phase 1 trial of itacitinib, a selective JAK1 inhibitor, in patients with acute graft-versus-host disease. Blood Adv. 2020;4(8):1656–1669. doi:10.1182/bloodadvances.2019001043
- Groth C, van Groningen LFJ, Matos TR, et al. Phase I/II trial of a combination of anti-CD3/CD7 immunotoxins for steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2019;25(4):712–719. doi:10.1016/j.bbmt.2018.10.020
- Groningen LFJ, Groth C, Bremmers MEJ, et al. Results from an expanded access program of anti-CD3/CD7 immunotoxin combination (T-guard®) for the treatment of steroid-refractory acute Gvhd. Blood. 2019;134(Supplement_1):4553. doi:10.1182/blood-2019-122923
- Bacigalupo A, Angelucci E, Raiola AM, et al. Treatment of steroid resistant acute graft versus host disease with an anti-CD26 monoclonal antibody—Begelomab. Bone Marrow Transplant. 2020;55(8):1580–1587. doi:10.1038/s41409-020-0855-z
- Magenau JM, Goldstein SC, Peltier D, et al. α1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease. Blood. 2018;131(12):1372–1379. doi:10.1182/blood-2017-11-815746
- S HM A, Holtan SG, Anand S, et al. Neihulizumab (ABGN-168H) in patients with steroid-refractory acute graft-versus-host-disease (SR-aGVHD): preliminary results of a phase I study. Paper presented at: EHA Annual Meeting; June 12, 2020.
- J MP K, Prockop SE, Burke E, Segal K. Aggregate results of remestemcel-L treatment for steroid-refractory acute graft-versus-host disease in pediatric patients. Orlando, FL:TCT; 2020.
- Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128(16):2083–2088. doi:10.1182/blood-2016-05-717652
- van Lier YF, Davids M, Haverkate NJE, et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med. 2020;12(556):eaaz8926. doi:10.1126/scitranslmed.aaz8926
- Zhao Y, Li X, Zhou Y, et al. Fecal microbiota transplantation for grade IV steroid refractory GI-GvHD: interim results a non-randomized, open-label, phase 1 clinical study. Research Square; 2020.
- Benito AI, Furlong T, Martin PJ, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease1. Transplantation. 2001;72(12):1924–1929. doi:10.1097/00007890-200112270-00010
- Bolaños-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23(12):2661–2668. doi:10.1200/JCO.2005.06.130
- Macmillan ML, Couriel D, Weisdorf DJ, et al. A phase 2/3 multicenter randomized clinical trial of ABX-CBL versus ATG as secondary therapy for steroid-resistant acute graft-versus-host disease. Blood. 2007;109:2657–2662. doi:10.1182/blood-2006-08-013995
- Yucebay F, Matthews C, Puto M, et al. Tocilizumab as first-line therapy for steroid-refractory acute graft-versus-host-disease: analysis of a single-center experience. Leuk Lymphoma. 2019;60(9):2223–2229. doi:10.1080/10428194.2019.1573996
- Jagasia M, Greinix H, Robin M, et al. Extracorporeal photopheresis versus anticytokine therapy as a second-line treatment for steroid-refractory acute GVHD: a multicenter comparative analysis. Biol Blood Marrow Transplant. 2013;19(7):1129–1133. doi:10.1016/j.bbmt.2013.04.018