Abstract
Context
Human epidermal growth factor (hEGF) has biological activities and can be used in medicines and cosmetics. A high level of effectiveness of hEGF can be obtained when three disulfide bonds fold perfectly. Extracellular secretion from E. coli BL21 using the PelB signal peptide is a new way to obtain hEGF with a structure that folds appropriately.
Object
This study aimed to determine the activity and effectiveness of recombinant hEGF excreted by E. coli BL21 on wound healing in induced diabetic mice.
Methods
Cell proliferation and migration tests were performed on NIH3T3 cells, followed by wound healing tests in induced diabetic mice, along with histological and endotoxin test at various hEGF concentrations (25, 50, and 75 µg/mL).
Results
Based on the results, hEGF at a level of 50 μg/mL showed optimal proliferation and migration activities. Wound healing in induced diabetic mice showed faster-wound closure within 12 days at hEGF 50 and 75 µg/mL with a percentage wound closure of 95% and 98.5%, respectively, which was significant versus control. In the histology test, the number of fibroblasts showed an increase and was significant at hEGF 75 µg/mL compared to the control group. The single test vial (STV) showed that hEGF solution was free of endotoxin.
Conclusion
Recombinant hEGF produced by extracellular secretion using E. coli BL21 has optimal diabetic wound healing activity through increased fibroblast proliferation.
Introduction
Various types of growth factors (GFs) such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF) have wound healing activities.Citation1–Citation8 Under normal circumstances, when a wound occurs, the body responds in several stages, namely, hemostasis, inflammation, proliferation, and remodeling. At the proliferation stage, GFs are released to help wound healing, including VEGF, FGF, and EGF. Among these GFs, EGF has a function that includes the role of all GFs, including accelerating the regeneration of keratinocytes, fibroblasts, and endothelial cells.Citation4,Citation9–Citation12 Based on this role, EGF has been widely used for treating diabetic wounds.Citation13–Citation17 In diabetic ulcers, EGF can stimulate the formation of new blood vessels to overcome the lack of nutrients that reach the wound area due to blockage by thickened blood. With adequate nutrition, EGF also stimulates the division of fibroblasts and keratinocytes to accelerate the healing of diabetic ulcers.Citation18,Citation19 It has been reported that the healing of diabetic foot ulcers (DFU) with hEGF therapy occurs five weeks faster than with conventional treatment.Citation20 Research conducted by Tsang et al in 21 patients given 0.04% hEGF showed total wound closure in 12 weeks.Citation13 However, the effectiveness of the hEGF is influenced by its structure, which contains three disulfide bonds that should fold perfectly.Citation21–Citation23 The process of protein recombination strongly influences this structure.Citation23–Citation28
One way to produce recombinant hEGF with three disulfide bonds that perfectly fold is by using Escherichia coli (E. coli) with the PelB signal peptide.Citation29–Citation31 The use of E. coli is also beneficial because recombinant hEGF is secreted directly into the periplasmic space and does not mix with cytoplasmic components, making it easy to purify and streamline production costs.Citation29,Citation32–Citation34 In this study, the hEGF used was obtained based on the results of optimization in previous studies using E. coli BL21 (DE3) as a recombinant vector.Citation31,Citation35–Citation38 The total levels of hEGF produced reached 416 μg/mL. However, the activity and effectiveness of the obtained hEGF on diabetic wound healing was not yet known. For this reason, this study aimed to examine the activity and effectiveness of hEGF on wound healing in induced diabetic mice and to study the histopathology and endotoxin potential of the hEGF.
Methods
Materials
NIH3T3 fibroblasts (ATCC® CRL-1658™, ATCC Inc., Manassas, Virginia), a water-soluble tetrazolium assay kit (WST)-8 (Dojindo Molecular Technologies Inc., USA), and a single test vial (STV) LAL test kit (Cape Cod Inc., East Falmouth, USA) were used. Male white mice, 8 weeks old with an average weight of 30–35 grams, were obtained from the Pharmacology Laboratories at Universitas Padjadjaran, Indonesia. Alloxan monohydrate (Sigma Aldrich, USA), Mallory-azan, Dulbecco’s modified Eagle’s medium (GibcoTM DMEM, Fisher Scientific, England), fetal bovine serum (FBS) (Sigma Aldrich, USA), trypan blue solution (Sigma Aldrich, USA), penicillin-streptomycin (PSM) (GibcoTM DMEM, Fisher Scientific, England), trypsin serine protease enzyme (Sigma Aldrich, USA), trichloroacetic acid (TCA) 20% (Sigma Aldrich, USA), alcohol 70% (Sigma Aldrich, USA), and Valisanbe® injection (PT, Sanbe Farma, Indonesia) were used in this study.
Preparation of hEGF Solution
The hEGF powder obtained from previous studies was dissolved in distilled water and made in 3 variations of concentration, namely, 25 μg/mL, 50 μg/mL, and 75 μg/mL. The preparations were stored in a refrigerator (2–8°C) after each use.
Proliferation Test Using the WST-8 Method
This test determined the effect of hEGF on cell proliferation in NIH3T3 cells. NIH3T3 (1x105 cells) were prepared in a 12-well plate. hEGF at 25 µg/mL, 50 µg/mL, and 75 µg/mL was added to each well in triplicate. Then, the plate was rinsed using PBS (270 µL twice), and 30 µL of WST-8 reagent was added to the plate and incubated at 37°C for 4 h. The absorbance of the solution was measured at a wavelength of 450 nm, with a reference wavelength at 665 nm using a microplate reader (Nanoquant Tecan Infinite m200pro). The higher the absorbance of the solution obtained, the greater the viability of NIH3T3 cells.
Cell Migration Test
The cell migration test was performed on NIH3T3 cells using the scratch method described by Li et al with a few modifications.Citation39 Twelve-well plates containing NIH3T3 cells were scratched using a sterile 0.1–10 µL tip in a straight line. The NIH3T3 monolayer was washed with PBS to remove floating cells or other impurities, then the results of the scratch were observed under a microscope, and the width of the scratch was recorded. Then, hEGF (25 µg/mL, 50 µg/mL, and 75 µg/mL) was added to each well in triplicate. Scratch closure was monitored using a microscope (ZEISS Axio) and photographed at time intervals (6, 12, 18, and 24 h). The size of the wound for each culture condition and time point was recorded by taking an average of 3 measurements per field of view. The width of the scratch is expressed as a percentage of the initial wound area.Citation40
Wound Healing Effectiveness in Induced Diabetic Mice
A total of 40 healthy mice were prepared for diabetes induction. Before being induced, mice fasted for 8 h. Then, the mice were weighed to determine the dose of alloxan to be given. Alloxan monohydrate stock solution was made at a concentration of 10 mg/mL using aqua pro injection. Mice were given an intraperitoneal injection of alloxan at a dose of 120 mg/kg. One hour later, the mice were given feed and 5% glucose solution ad libitum for 24 h. After 24 h, the glucose solution was replaced with distilled water. Three days after induction, glucose levels in mice were measured (diabetes: >200 mg/dl). The living mice were divided into four groups consisting of five mice per group. Then, the tail of each mouse was swabbed with 70% alcohol and wounded to draw blood from the base of the tail vein. Blood sugar levels were measured using a glucometer strip. All experiments were performed following the guidelines of OIE animal welfare standards and were approved by the local Ethical Committee Faculty of Medicine, Universitas Padjadjaran, Bandung (No:1296/UN6.C10/PN2017).
After induced diabetic mice were available, hair removal was performed on the back of the mice. Valisanbe® injection with a dose of 2.5 mg/kg body weight was given intramuscularly as an anesthetic. Then, a full-thickness wound was made using a sterile 8 mm diameter biopsy punch on the back of the mouse after sterilization with 70% alcohol. After that, a 1 mL hEGF solution was applied to the wound once a day for 12 days. The experimental groups consisted of:
The control group: wounds were not given hEGF
Test group I: wounds were given a dosage of 25 μg/mL
Test group II: the wound is given a dosage of 50 μg/mL
Test group III: wounds were given a dosage of 75 μg/mL
The image of the wound area was taken digitally using a camera (Canon EOS 1200D), and the area of the wound was calculated using ImageJ software on days 0, 4, 6, 9, and 12.
Histology Test
Histological testing was performed by taking the middle part of the wound. The tissue was prepared in 10% formalin (BNF) to prevent spoilage in the preparations. The clean tissue was cut and stored in a tissue cassette, dehydrated automatically using a dehydration machine, then dried with a vacuum machine. The tissue was blocked in paraffin liquid and then cut into 3–5 µm sections using a microtome, and parts were placed on slides. After that, Mallory-azan staining was performed.
Endotoxin Test
Endotoxin test was performed to determine the presence of endotoxin content in hEGF. This test used the Single Test Vial (STV) method. In this test, positive control was made by adding the bacterium Bacillus subtilis as a standard positive control STV, where the results would give positive results with the formation of the gel. The gel formed will be seen when the tube is rotated 180°, which indicates that the concentration of endotoxin in the tube is higher than the sensitivity of the Limulus Amebocyte Lysate (LAL) reagent contained in the STV.
Statistical Analysis
The significance of wound closure in each group over time was analyzed with one-way ANOVA followed by Scheffe’s test with a significance level of 5%. The viability test was analyzed using Dunnett’s test. The histological test was analyzed using the post hoc Fisher’s Least Significant Difference (LSD) method. The software Statistical Product and Service Solutions (SPSS), version 22 (IBM Corporation, New York) was used to run the statistical analysis.
Results
Proliferation Test Using the WST-8 Method
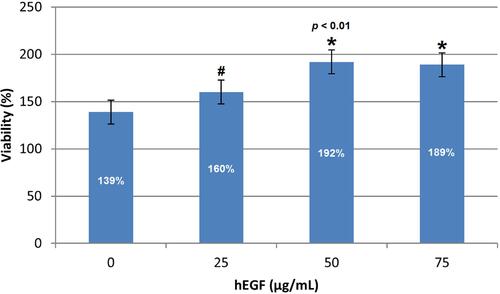
The percentage of cell viability shows that the addition of hEGF relatively increases cell viability compared to controls (see ). The control group had a regular proliferation activity with cell viability of 139%. An optimal increase occurs in the addition of hEGF 50 µg/mL by 192%. Still, it tends to be constant in the addition of 75 µg/mL hEGF caused by hEGF having equilibrium regulation in proliferation so that it is set to increase, not excessive proliferation. Based on the test results, it is proven that the hEGF produced has cell proliferation activity in vitro. Thus, hEGF at a dose of 50 µg/mL has the most optimal cell proliferation activity compared to other control and test samples.
Cell Migration Test
Cell migration was observed by measuring the width of the stroke in the same field of view with a microscope magnification 200 times. The initial stroke represented by A0 and the width of the final stroke represented by A1, so the percentage of cell migration can be measured using the formula:
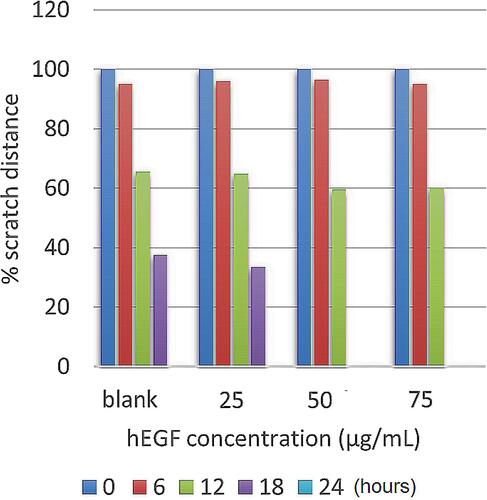
The control group and the test group were given a scratch in the middle of the Petri dish, which was then marked. The cell would divide and move to fill the space from scratch. The results of the measurement of migration distances are shown in .
As shown in , the overall test and control sample showed the same scratch distance between 95% and 100% at 0–6 h, and mobilization was seen to be significant in 12 h. However, neither the test nor the control sample showed a significant difference. The difference was seen to be significant at the 18th hour, where the distance of the scratches in the hEGF 50 and 75 µg/mL groups had closed entirely. Whereas both the control and hEGF 25 µg/mL closed entirely in 24 h. Thus, hEGF at a dose of 50 µg/mL has the most optimal cell migration activity compared to other control and test samples.
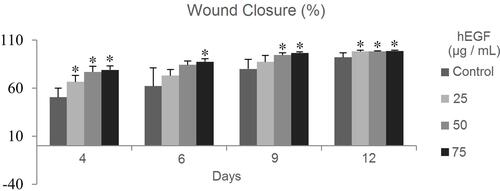
Healing Effectiveness of hEGF on Induced Diabetic Mice
The development of wound closure can be seen in . This wound healing test was calculated based on the percent area of wound closure using ImageJ software. Percentage of wound closure at each time interval was calculated using the following formula:
Figure 3 Observation of wound closure in all groups of test animals on days 0, 4, 6, and 9 and 12.
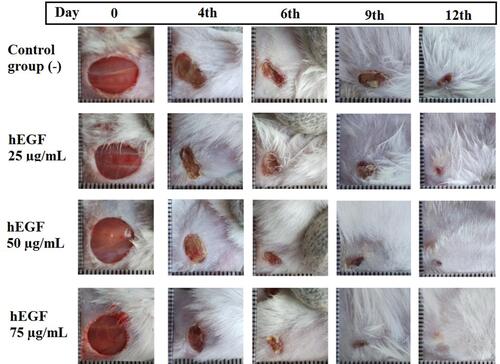
Based on , it was seen that the mice of the 75 µg/mL hEGF group had better-wound closure compared to other groups. With the addition of exogenous hEGF as much as 25, 50, and 75 µg/mL, it was seen that it significantly accelerated the healing of the wounds of mice (see ). Based on the result of the Scheffe test, there are no significant differences among the treated groups.
Histology Test
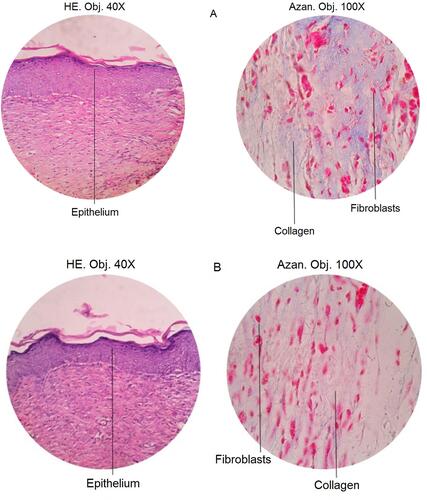
In this test, we only selected the 75 μg hEGF group to be compared with the control group because of its significance in healing progress by proliferation, cell migration, and in vivo test. Individually, the number of fibroblasts, collagen, and epithelium on the surface of the skin was assessed on a scale from 0 to 3, where 0 = weak staining; 1 = moderate staining; 2 = intense staining; and 3 = robust staining. Microscopic observations of mice wound tissue can be seen in .
Figure 5 The results of skin histopathology analysis using Mallory-azan staining at 40x and 100x magnification in (A) control mice and (B) mice treated with 75 µg/mL hEGF.
The test results were then statistically analyzed using the post hoc LSD method to determine the significance of the number of fibroblasts, collagen, and epitheliums between the hEGF 75 µg/mL and control groups. shows the statistical results of fibroblasts, collagen, and epithelium score.
Figure 6 The number of epithelial cells and fibroblasts, as well as the collagen area in control mice group and mice treated with 75 µg/mL hEGF (n = 3).
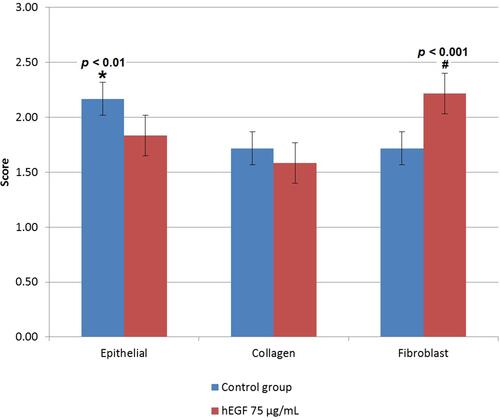
From the results of histopathological observations, the 75 μg hEGF group had a more significant number of fibroblasts than the control group. The amount of collagen and epithelium in the hEGF 75 μg/mL group was less than the control group.
Endotoxin Test
All preparations were tested for endotoxin by inserting 0.2 mL of the preparation into STV, and the preparation was allowed to stand for 60 ± 2 min at 37°C ± 1°C. The three formulas tested did not form a gel based on the STV when the tube was turned 180°. This phenomenon shows that all test samples with various hEGF concentrations did not contain endotoxin or their endotoxin concentrations were lower than the sensitivity of the LAL reagent, which is 0.125 EU/mL. Endotoxin test results are shown in .
Table 1 Endotoxin Test Results
Discussion
In this study, the effectiveness of hEGF obtained through recombination using E. coli BL21 on cell proliferation and migration, wound healing in induced diabetic mice, as well as histological and endotoxin aspects, were investigated. Based on the known mechanism, ulcer healing is a complex and dynamic process of restoring cell structure and tissue layers. hEGF plays a vital role in the proliferation phase. The phase is divided into three processes, namely, reepithelialization, neovascularization, and granulation tissue formation. The role of endogenous growth factors that work in mouse wound experiments was seen in the control group where the wound healing process continued. To determine the effect of an exogenous growth factor (hEGF) on wound healing, cell proliferation, and migration tests were performed.
The cell proliferation test determined the effect of exogenous hEGF on the number of cells that grow and divide in cell culture medium in vitro. This process can be assessed by cell viability, confluence, and abnormalities in cultured cells. Viability is defined as the number of cells capable of developing in a culture medium. Confluent is the even distribution of cells as a monolayer cells. Abnormalities include cells larger than normal cell sizes and changes in shape. The acceleration of growth in the number of cells displayed by the viability of rat embryo fibroblasts (NIH3T3 cells) was conducted out over a 4-hour incubation period using the WST-8 kit.Citation41–Citation44 NIH3T3 fibroblasts were chosen as model cells because fibroblasts are directly involved in the process of wound healing and the regeneration of skin tissue.Citation45 The in vitro cell migration test was performed to determine the effect of hEGF on cell motility, which aids the wound healing process. The cell migration test was done using the scratch assay. Cells migrate to the scratch-made by scraping a cell monolayer using a pipette tip. The number of cells covering scratch is a parameter of the successful wound healing process.Citation46–Citation51
Cell proliferation and migration tests showed that the optimal hEGF dose of 50 µg/mL significantly increase cell viability and motility compared to controls. These results indicate that exogenous hEGF helps increase epidermal cell movement, the formation of collagen, proteoglycans, and fibronectin, and reduces the production of protease enzymes that damage the matrix. More specifically, hEGF plays a role in pleiotropic cell motility and proliferation.Citation52
In the healing process, fibroblasts bind to fibers in the fibrin matrix and begin producing collagen, which is mainly collagen type 1. Collagen formation starts from the creation of procollagen in the form of a triple helix after being secreted into the extracellular space. It is then hydroxylated and cleaved by lysyl oxidase, which allows for more stable crosslinking. Healthy collagen in the skin is arranged regularly and has a muscular stretch strength.Citation53–Citation55
The re-epithelialization process occurs within a few h after injury. The cytokines that play a role are endogenous, and exogenous EGF and TGF-α produced by platelets, macrophages, and keratinocytes. Because this process has a high metabolic activity, there will be an increase in oxygen and nutrient requirements. A decrease in pH and oxygen tension will trigger the formation of new blood vessels. This phenomenon is commonly known as angiogenesis, which is mainly influenced by VEGF, bFGF, and TGF-β. This process is vital in the continuity of the next process, which is the formation of granulation tissue on days 4 to 7.Citation56–Citation59
Based on the in vivo wound healing test shown in and , the conclusion is that the group of mice given the 75 µg/mL hEGF preparation experienced a faster wound closure process than the other groups. Significant differences in the acceleration of healing between groups given hEGF versus the control group occurred from day 4, then day 6 to day 12 for groups given hEGF 50 and 75 µg/mL. It confirms the results of the cell proliferation and migration tests that exogenous hEGF can increase the effectiveness of wound healing.
A histological test was performed to determine the effect of exogenous hEGF on wound mucosal tissue. The 75 µg/mL hEGF group was selected because it would show any significant difference compared to the control group. The Mallory-azan staining method is used to analyze connective tissue, mainly to show collagen, erythrocytes, muscle tissue, epithelium, and fibroblasts.Citation60 The process of destaining in Mallory-azan staining will produce a different color, ie, the cell nucleus will look dark red, collagen will look blue and red fibers indicate the epithelium.Citation61
The histological observation in this study began with the process of fixation. Fixation was performed by soaking the skin tissue in a 10% formaldehyde solution. This process prevents tissue digestion by enzymes in cells (autolysis) or by bacteria. This solution is also useful for preserving the structure and molecular components in cells. The fixed tissue was then dehydrated by immersion in a series of alcohol solutions in the concentration range of 96–100%. This dehydration process is useful for removing all water contained in the tissue. The tissue was then embedded in a solid medium to facilitate cutting. The immersion material used was paraffin. The tissue was then cut to a size of 3–5 µm using a microtome.
To be observed under a microscope, the tissue must be stained because most tissue is colorless. Tissue components with an anionic content are more stained daubed with basic dyes, while cationic components such as proteins with many ionized amino groups have an affinity for acid dyes. Of all dyes, the combination of hematoxylin and eosin (H&E) is most commonly used. Hematoxylin stains plasma DNA and the cartilage matrix blue. Conversely, eosin turns the cytoplasm and collagen pink. Mallory-azan staining is included in the trichrome-staining technique. This staining technique can also distinguish extracellular tissue components similar to the H&E method. The length of the entire procedure, from the time of fixation to the process of observing tissue under a microscope, can take from 12 h to 3 days, depending on the size of the tissue, fixation material, immersion media, and sectioning process.
The results of histological observations showed that the amount of collagen did not differ significantly versus control. Fibroblasts are stimulated by hEGF to continue to divide so that the production of collagen by fibroblasts is delayed.Citation62 Based on these results, it can be concluded that hEGF has more of a role in increasing the number of fibroblasts to accelerate the wound healing process in mice.
Based on the endotoxin testing, hEGF did not show any reaction to the LAL reagent. This indicates that the procedure of recombination and extracellular isolation of hEGF from E. coli BL21 succeeded in producing endotoxin-free hEGF.
Conclusion
The determination of cell proliferation induced by hEGF in cultured NIH3T3 cells using the WST-8 method showed an effective dose of hEGF at 50 μg/mL with a proliferation induction of 192%. Likewise, in the cell migration test, hEGF at 50 µg/mL showed complete scratch closure after 18 h. The results of the wound healing test in induced diabetic mice showed hEGF at 50, and 75 µg/mL effectively closed wounds by 95% and 98.5% on day 12, which was significant against control. Histological observations of mouse wound tissue using microscopic methods showed a significant increase in fibroblasts compared to control. The results of the endotoxin test using the LAL method did not show the presence of endotoxin in hEGF.
Abbreviations
EGF, epidermal growth factor; GFs, growth factors; hEGF, human epidermal growth factor; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; STV, single test vial; LAL, limulus amebocyte lysate; WST-8, 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium; H&E, hematoxylin and eosin; bFGF, basic fibroblast growth factor; TGF-β, transforming growth factor-β.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Martí-Carvajal AJ, Gluud C, Nicola S, et al. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2015. doi:10.1002/14651858.CD008548.pub2
- Sridharan K, Sivaramakrishnan G. Growth factors for diabetic foot ulcers: mixed treatment comparison analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(3):434–444. doi:10.1111/bcp.13470
- Khanbanha N, Atyabi F, Taheri A, Talaie F, Mahbod M, Dinarvand R. Healing efficacy of an EGF impregnated triple gel based wound dressing: in vitro and in vivo studies. Biomed Res Int. 2014;2014:1–10. doi:10.1155/2014/493732
- Grazul-Bilska AT, Johnson ML, Bilski JJ, et al. Wound healing: the role of growth factors. Drugs Today. 2003;39(10):787. doi:10.1358/dot.2003.39.10.799472
- Bodnar RJ. Epidermal growth factor and epidermal growth factor receptor: the Yin and Yang in the treatment of cutaneous wounds and cancer. Adv Wound Care. 2013;2(1):24–29. doi:10.1089/wound.2011.0326
- Hardwicke J, Schmaljohann D, Boyce D, Thomas D. Epidermal growth factor therapy and wound healing — past, present and future perspectives. Surg. 2008;6(3):172–177. doi:10.1016/S1479-666X(08)80114-X
- Tarnuzzer RW, Macauley SP, Mast BA, et al. Epidermal growth factor in wound healing: a model for the molecular pathogenesis of chronic wounds. In: Ziegler T, Pierce GF, Herndon DN, editors. Growth Factors and Wound Healing. New York: Springer; 1997:206–228. doi:10.1007/978-1-4612-1876-0_12
- Yamakawa S, Hayashida K. Advances in surgical applications of growth factors for wound healing. Burn Trauma. 2019;7. doi:10.1186/s41038-019-0148-1.
- Wojtowicz AM, Oliveira S, Carlson MW, Zawadzka A, Rousseau CF, Baksh D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014;22(2):246–255. doi:10.1111/wrr.12154
- Seeger MA, Paller AS. The roles of growth factors in keratinocyte migration. Adv Wound Care. 2015;4(4):213–224. doi:10.1089/wound.2014.0540
- Schultz G, Clark W, Rotatori DS. EGF and TGF-α in wound healing and repair. J Cell Biochem. 1991;45(4):346–352. doi:10.1002/jcb.240450407
- Park J, Hwang S, Yoon I-S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules. 2017;22(8):1259. doi:10.3390/molecules22081259
- Tsang MW, Wong WKR, Hung CS, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers. Diabetes Care. 2003;26(6):1856–1861. doi:10.2337/diacare.26.6.1856
- Park KH, Han SH, Hong JP, et al. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: A Phase III multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res Clin Pract. 2018;142:335–344. doi:10.1016/j.diabres.2018.06.002
- Zhang J, Hu W, Diao Q, et al. Therapeutic effect of the epidermal growth factor on diabetic foot ulcer and the underlying mechanisms. Exp Ther Med. 2018. doi:10.3892/etm.2018.7133
- Berlanga-Acosta J, Mendoza-Mari Y, Garcia-Ojalvo A, Acosta-Buxado JA, Fernandez-Mayola M, Guillen Nieto G. Epidermal Growth Factor (EGF) intralesional infiltrations: from the bench to the diabetic ulcers cells. Integr Mol Med. 2019;6(1). doi:10.15761/IMM.1000354
- Berlanga-Acosta J, Fernández-Montequín J, Valdés-Pérez C, et al. Diabetic foot ulcers and epidermal growth factor: revisiting the local delivery route for a successful outcome. Biomed Res Int. 2017;2017:2923759. doi:10.1155/2017/2923759
- Tiaka EK, Papanas N, Manolakis AC, Georgiadis GS. Epidermal growth factor in the treatment of diabetic foot ulcers: an update. Perspect Vasc Surg Endovasc Ther. 2012;24(1):37–44. doi:10.1177/1531003512442093
- Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg. 2006;56(4):394–398. doi:10.1097/01.sap.0000198731.12407.0c
- Singla S, Singla S, Kumar A, Singla M. Role of epidermal growth factor in healing of diabetic foot ulcers. Indian J Surg. 2012;74(6):451–455. doi:10.1007/s12262-012-0447-2
- Alewood D, Nielsen K, Alewood PF, et al. The role of disulfide bonds in the structure and function of murine epidermal growth factor (mEGF). Growth Factors. 2005;23(2):97–110. doi:10.1080/08977190500096061
- Chang J-Y, Schindler P, Ramseier U, Lai P-H. The disulfide folding pathway of human epidermal growth factor. J Biol Chem. 1995;270(16):9207–9216. doi:10.1074/jbc.270.16.9207
- Feige MJ, Braakman I, Hendershot LM. Disulfide Bonds in Protein Folding and Stability. London: The Royal Society of Chemistry; 2018:1–33. doi:10.1039/9781788013253-00001
- Joseph BC, Pichaimuthu S, Srimeenakshi S. An overview of the parameters for recombinant protein expression in Escherichia coli. J Cell Sci Ther. 2015;6:5. doi:10.4172/2157-7013.1000221
- Okumura M, Shimamoto S, Hidaka Y. A chemical method for investigating disulfide-coupled peptide and protein folding. FEBS J. 2012;279(13):2283–2295. doi:10.1111/j.1742-4658.2012.08596.x
- Landgraf BJ, Ren G, Masuch T, Boyd D, Berkmen M. From biology to biotechnology: disulfide bond formation in escherichia coli. In: Escherichia Coli - Recent Advances on Physiology, Pathogenesis and Biotechnological Applications. InTech; 2017:359–382. doi10.5772/67393
- Berkmen M. Production of disulfide-bonded proteins in Escherichia coli. Protein Expr Purif. 2012;82(1):240–251. doi:10.1016/j.pep.2011.10.009
- Tyo KE, Liu Z, Petranovic D, Nielsen J. Imbalance of heterologous protein folding and disulfide bond formation rates yields runaway oxidative stress. BMC Biol. 2012;10(1):16. doi:10.1186/1741-7007-10-16
- Razis AFA, Ismail EN, Hambali Z, Abdullah MNH, Ali AM, Lila MAM. The periplasmic expression of recombinant human epidermal growth factor (hEGF) in Escherichia coli. Asia-Pacific J Mol Biol Biotechnol. 2006;14(2):41–45.
- Zheng X, Wu X, Fu X, Dai D, Wang F. Expression and purification of human epidermal growth factor (hEGF) fused with GB1. Biotechnol Biotechnol Equip. 2016;30(4):813–818. doi:10.1080/13102818.2016.1166984
- Indriyani A, Anggraeni NI, Maksum IP. Optimization extracellular secretion of recombinant human epidermal growth factor (hEGF) in Escherichia coli BL21 (DE3) pD881-OmpA-hEGF by using response surface method (RSM). Int J Res Pharm Sci. 2019;10(3):1824–1831. doi:10.26452/ijrps.v10i3.1378
- Abdull Razis AF, Ismail EN, Hambali Z, Abdullah MNH, Ali AM, Mohd Lila MA. Expression of recombinant human epidermal growth factor in Escherichia coli and characterization of its biological activity. Appl Biochem Biotechnol. 2008;144(3):249–261. doi:10.1007/s12010-007-8019-9
- Ma Y, Yu J, Lin J, Wu S, Li S, Wang J. High efficient expression, purification, and functional characterization of native human epidermal growth factor in Escherichia coli. Biomed Res Int. 2016;2016:1–7. doi:10.1155/2016/3758941
- Pouranvari S, Ebrahimi F, Javadi G, Maddah B. Cloning, expression, and cost effective purification of authentic human epidermal growth factor with high activity. Iran Red Crescent Med J. 2016;18:3. doi:10.5812/ircmj.24966
- Melati R, Indriyani A, Gaffar S, Maksum IP. Comparison of extracellular secretion of recombinant human epidermal growth factor using TORA and PELB signal peptides in Escherichia coli BL21 (DE3). Asian J Pharm Clin Res. 2019;81–84. doi:10.22159/ajpcr.2019.v12i11.35316
- Sriwidodo S, Subroto T, Maksum I, et al. Optimization of secreted recombinant human epidermal growth factor production using pectate lyase B from Escherichia coli BL21(DE3) by central composite design and its production in high cell density culture. J Pharm Bioallied Sci. 2019;11(8):562. doi:10.4103/jpbs.JPBS_207_19
- Maksum IP, Rostinawati NR, Subroto T. Extracellular secretion recombinant of human epidermal growth factor (hEGF) using pectate lyase B (PelB) signal peptide in Escherichia coli BL21(DE3). Int J Res Pharm Sci. 2017;8(1):33–40.
- Maksum IP, Utama E, Sriwidodo ST. Extracellular secretion of recombinant human epidermal growth factor by using trimethylamine N-Oxide reductase a (TORA) signal peptide in Escherichia coli BL21 (DE3). J Pharm Sci Res. 2017;9(6):1007–1016.
- Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105(49):19426–19431. doi:10.1073/pnas.0805230105
- Pratoomsoot C, Hou Q, Chau DYS, et al. A biosynthetic bandage for corneal wound repair. Abstract presented at: TERMIS-EU 2008 Porto Meeting; June 22–26; 2008; Porto Congress Center–Alfândega Portugal.
- Ginouves M, Carme B, Couppie P, Prevot G. Comparison of tetrazolium salt assays for evaluation of drug activity against leishmania spp. J Clin Microbiol. 2014;52(6):2131–2138. doi:10.1128/JCM.00201-14
- Aslantürk ÖS. Vitro cytotoxicity and cell viability assays: principles, advantages, and disadvantages. In: Genotoxicity - a Predictable Risk to Our Actual World. InTech; 2018:1–17. doi10.5772/intechopen.71923
- Tiwari K, Wavdhane M, Haque S, et al. A sensitive WST-8-based bioassay for PEGylated granulocyte colony stimulating factor using the NFS-60 cell line. Pharm Biol. 2015;53(6):849–854. doi:10.3109/13880209.2014.943248
- Chamchoy K, Pakotiprapha D, Pumirat P, Leartsakulpanich U, Boonyuen U. Application of WST-8 based colorimetric NAD(P)H detection for quantitative dehydrogenase assays. BMC Biochem. 2019;20(1):4. doi:10.1186/s12858-019-0108-1
- Riss TL, Moravec RA, Niles AL, Benink HA, Worzlla TJ, Minor L. Cell viability assays. Assay Guid Man. 2004;1–23. doi:10.1007/978-1-61779-108-6_6
- Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7):445–464. doi:10.1089/wound.2013.0473
- Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z, Falanga V. Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol. 2017;137(2):e11–e16. doi:10.1016/j.jid.2016.11.020
- Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4(1):21. doi:10.1186/1472-6750-4-21
- Liang -C-C, Park AY, Guan J. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi:10.1038/nprot.2007.30
- Cappiello F, Casciaro B, Mangoni ML. A novel in vitro wound healing assay to evaluate cell migration. J Vis Exp. 2018;133. doi:10.3791/56825.
- Jonkman JEN, Cathcart JA, Xu F, et al. An introduction to the wound healing assay using live-cell microscopy. Cell Adh Migr. 2014;8(5):440–451. doi:10.4161/cam.36224
- Nolte C, Kirchhoff F, Kettenmann H. Epidermal growth factor is a motility factor for microglial cells in vitro: evidence for EGF receptor expression. Eur J Neurosci. 1997;9(8):1690–1698. doi:10.1111/j.1460-9568.1997.tb01526.x
- Green H, Hamerman D. Production of hyaluronate and collagen by fibroblast clones in culture. Nature. 1964;201(4920):710. doi:10.1038/201710a0
- Narayanan AS, Page RC, Swanson J. Collagen synthesis by human fibroblasts. Regulation by transforming growth factor-β in the presence of other inflammatory mediators. Biochem J. 1989;260(2):463–469. doi:10.1042/bj2600463
- Cole MA, Quan T, Voorhees JJ, Fisher GJ. Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J Cell Commun Signal. 2018;12(1):35–43. doi:10.1007/s12079-018-0459-1
- Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3(10):647–661. doi:10.1089/wound.2013.0517
- Kumar P, Kumar S, Udupa EP, Kumar U, Rao P, Honnegowda T. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthetic Res. 2015;2(5):243. doi:10.4103/2347-9264.165438
- Ucuzian AA, Gassman AA, East AT, Greisler HP. Molecular mediators of angiogenesis. J Burn Care Res. 2010;31(1):158–175. doi:10.1097/BCR.0b013e3181c7ed82
- Sorg H, Tilkorn DJ, Mirastschijski U, Hauser J, Kraemer R. Panta Rhei: neovascularization, angiogenesis and nutritive perfusion in wound healing. Eur Surg Res. 2018;59(3–4):232–241. doi:10.1159/000492410
- Pfeiffer, H. Reinhard B. Dettmeyer. Forensic Histopathology: Fundamentals and Perspectives. Int J Legal Med. 2012;126:185. doi:10.1007/s00414-011-0645-7
- Rutland CS. Histological and histochemical methods 4th edition. J Anat. 2008;213(3):356. doi:10.1111/j.1469-7580.2008.00957.x
- Petreaca M, Martins-Green M. Cell–ECM interactions in repair and regeneration. Lanza R, Atala A, editors. In: Handbook of Stem Cells. Elsevier; 2013:191–226. doi:10.1016/B978-0-12-385942-6.00017-2