Abstract
Basal cell carcinoma (BCC) of the skin is the most common type of cancer and accounts for up to 40% of all cancers in the US, with a growing incidence rate over recent decades in all developed countries. Surgery is curative for most patients, although it leaves unaesthetic scars, but those that develop locally advanced or metastatic BCC require different therapeutic approaches. Furthermore, patients with BCC present a high risk of developing additional tumors. The increasing economic burden and the morbidity of BCC render primary interest in the development of targeted treatments for this disease. Among the molecular signals involved in the development of BCC, the critical role of the morphogenetic Hedgehog (Hh) pathway has become evident. This pathway is found altered and activated in almost all BCCs, both sporadic and inherited. Given the centrality of the Hh pathway in the pathophysiology of BCC, the primary efforts to identify molecular targets for the topical or systemic treatment of this cancer have focused on the Hh components. Several Hh inhibitors have been so far identified – from the first identified natural cyclopamine to the recently Food and Drug Administration-approved synthetic vismodegib – most of which target the Hh receptor Smoothened (either its function or its translocation to the primary cilium). Other molecules await further characterization (bisamide compounds), while drugs currently approved for other diseases such as itraconazole (an antimicotic agent) and vitamin D3 have been tested on BCC with encouraging results. The outcomes of the numerous ongoing clinical trials are expected to expand the field in the very near future. Further research is needed to obtain drugs targeting downstream components of the Hh pathway (eg, Gli) or to exploit combinatorial therapies (eg, with phosphatidylinositol 3-kinase inhibitors or retinoids) in order to overcome potential drug resistance.
Introduction
Basal cell carcinoma (BCC) of the skin is the most common type of cancer and accounts for up to 40% of all cancers in the US.Citation1 BCC, together with squamous cell carcinoma, is grouped into the so-called nonmelanoma skin cancers which present cells with keratinocytic morphology.Citation2
BCC is characterized by cells that morphologically resemble the undifferentiated cells that constitute the basal layer of the epidermis or hair follicle, but may present different growth patterns (infiltrating, superficial, nodular, sclerosing, and fibroepithelial), indicating that the cell of origin may be a stem or progenitor cell.Citation3,Citation4
BCC tumors are generally curable and thus frequently not reported in cancer registries, making it difficult to estimate the real incidence of the disease. A report estimated BCC occurrence in the US of more than 2 million cases in 2006, with some patients having multiple diagnoses.Citation5 In 95% of cases, the diagnosis is made in 40–79 year olds mainly of Caucasian origin, with a 30% higher incidence in men than in women.Citation6
Exposure to sunlight (ultraviolet rays) is the most important risk factor (in most cases the lesion is in a region of the body exposed to the sun); other well known risk factors are exposure to ionizing radiations, arsenic, and immunosuppressive conditions as well as genetic syndromes such as the basal cell nevus syndrome (BCNS) or Gorlin syndrome.Citation3,Citation6
Despite its very high frequency, this tumor rarely leads to death, partly because of the remarkable progress made in the field of diagnosis and treatment, but also for the fact that it rarely metastasizes. However, since BCC often infiltrates the skin locally and aggressively and involves disfiguring surgical resection, it is considered a malignant tumor. It is important to observe that metastatic patients, although very rare (0.003%–0.5% of cases), often present a poor prognosis with mean survival ranging from 8 months to 3.6 years.Citation7
A recent systematic review of the published data suggests that the incidence rate of BCC has grown in recent decades in developed countries, probably because of the now widespread practice of sunbathing.Citation8 The increasing relevance of the economic burden and the morbidity of BCC render primary interest in a better understanding of the molecular biology of BCC and the development of targeted treatments for this disease.Citation9
Current therapeutic approaches
Surgery is still the elective choice for most BCCs, especially the early-stage and low-risk ones, which can be removed by surgical excision, electrodessication, curettage (tissue destruction by electric current and removal by scraping with a curette), or cryosurgery (tissue destruction by freezing). In the presence of specific risk factors, Mohs micrographic surgery is the elective treatment.Citation10 Surgery is curative for most patients, although it leaves unaesthetic scars, but those that develop locally advanced or metastatic BCC require different therapeutic approaches.Citation10
Furthermore, patients with BCC present a high risk of developing additional tumors (up to 44% incidence within 3 years from the first tumor),Citation11 and some sort of preventive treatment on these patients may avoid the need for performing surgical resection again (which often causes disfigurement).
Until last year, the most common nonsurgical therapies for BCC involved radiotherapy, photodynamic therapy, or the topical administration of imiquimod and 5-fluorouracil, although the cure rates were low.Citation10,Citation12 Treatment of BCC with other agents, such as tazarotene, glycoalkaloid (BEC5) cream, cidofovir, and calcium dobesilate have also been reported,Citation13 although many of them did not look really promising, prompting the search for more targeted and preventive therapies.
Development of new BCC anticancer drugs: targeting the Hedgehog (Hh) pathway
Among the molecular signals involved in the development of BCC, the critical role of the morphogenetic Hh pathway has become evident.Citation14,Citation15 This pathway is found altered and activated in almost all BCCs, both sporadic and inherited,Citation16,Citation17 as well as in most tumor stem cells and in other tumors such as medulloblastoma and rhabdomyosarcoma.Citation17–Citation19
The Hh pathway is a key regulator of embryonic patterning and cell fate determination.Citation20 It plays a critical role in the development of various tissues in mammals as a morphogenetic, mitogen and differentiation factor.Citation21–Citation26
The Hh pathway () is activated by a family of secreted ligands (Sonic, Desert, and Indian Hh). These ligands are synthesized as pro-proteins, which undergo several posttranslational modifications: autocatalytic cleavage to generate the active n-terminal fragment followed by linking to a cholesterol molecule and to a palmitic acid.Citation27,Citation28 These modifications are essential for Hh proteins to cross the membrane bilayers and to interact physically with lipoproteins, allowing diffusion of the ligand towards the nearby or distant target cells where Hh interacts with the transmembrane receptor Patched (Ptch).Citation27,Citation28 This bond prevents the inhibition mediated by Ptch on the coreceptor Smoothened (Smo), allowing Smo derepression and translocation in the primary cilium and leading to the downstream activation of the Gli family of transcription factors (Gli1-3).Citation14,Citation15,Citation29
Figure 1 The Hh pathway critical components which could be targeted by potential antibasal cell carcinoma drugs.
Abbreviations: Ac, acetyl group; Ch, cholesterol; EGFR, epidermal growth factor receptor; ERK, extracellular signal-related kinase; GANT, Gli antagonist; Hdac, histone deacetylase; Hh, Hedgehog; HPI, Hedgehog pathway inhibitor; KCASH, KCTD (Potassium Channel Tetramerization Domain) containing cullin3 adaptor suppressor of hedgehog; MEK, mitogen-activated protein kinase kinase; P, palmitate; PI3K, phosphatidylinositol 3-kinase; PKB/akt, protein kinase B; Ptch, Patched; Quinaz, quinazolines; Robotk, robotnikinin; Smo, Smoothened; SuFu, suppressor of fused; TSA, trichostatin A; VitD3, Vitamine D3.
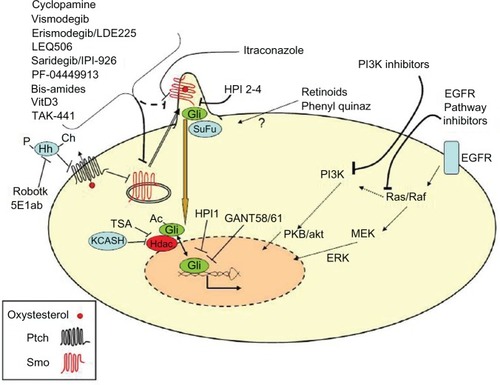
Recent studies suggest that Smo signaling activity can be modulated positively by natural and synthetic small molecules, including oxysterols (oxidized forms of cholesterol),Citation30 and Ptch regulates Smo by pumping away oxysterols from Smo.Citation31 Indeed, Ptch receptor contains a sterol sensing domain that has been linked to the regulation of the vesicular trafficking of Smo.Citation32
Specific oxysterols can drive differentiation of osteogenic cells in culture and in animals.Citation33,Citation34 The most potent activator so far identified is 20(S)-hydroxycholesterol, which binds Smo and leads to its accumulation in the primary cilium.Citation35,Citation36
Once activated, Gli transcription factors translocate into the nucleus and promote the expression of target genes. Recently, it has been demonstrated that Gli transcriptional activity and nuclear localization are also modulated negatively by acetylation. In fact, deacetylation by histone deacetylases is required for full transcriptional activity.Citation37 Gli proteins promote the expression of genes involved in the control of the most important cell functions such as proliferation, differentiation, and survival. Dysregulated Hh activity is for this reason frequently implicated in cancerogenesis.Citation38
The first hint of Hh involvement in BCC came from the analysis of the inherited forms of BCC, associated with BCNS, an autosomal dominant disorder characterized by skeletal abnormalities, endocrine disorders, and predisposition to the development of BCC and medulloblastoma,Citation39 which was found to be caused by a germline mutation of the Hh receptor Ptch1.Citation40,Citation41
Like the hereditary form of BCC, the most common genetic alterations found in sporadic BCC are mutations of the receptor Ptch1 (inactivating mutations or loss of heterozygosity),Citation42–Citation44 which prevent its inhibition on Smo receptor – allowing the activation of downstream signals even in the absence of ligands.Citation42 Less frequent genetic alterations are gain of function mutations on Smo or mutations of the Hh inhibitor suppressor of fused (SuFu).Citation43,Citation45–Citation47 Similarly, mouse models overexpressing Sonic Hh (Shh) ligand in the skin present BCC formation,Citation48 although human BCCs generally do not present this alteration.
Given the centrality of the Hh pathway in the pathophysiology of BCC, the primary efforts to identify molecular targets for the topical or systemic treatment of this cancer have focused on the Hh components (; ).
Table 1 List of the anti-Hedgehog drugs currently undergoing clinical trials
Development of Smo inhibitors
Cyclopamine
The first Hh inhibitor that has been identified is cyclopamine, a natural molecule found in Veratrum californicum (corn lilies) and recognized as a teratogen in cattle since the second half of the 1900s.Citation49 The suppression of the Hh pathway through inhibition of Smo was later identified as the mechanism of the teratogenic malformation induced by cyclopamine.Citation50,Citation51
The feasibility of cyclopamine use in therapy was suggested by its Hh inhibition on tumor cells.Citation52,Citation53 Cyclopamine (and its more effective derivative KAAD-cyclopamine) was successfully used topically on BCC, although with an inconvenient treatment schedule,Citation54 verifying the rationale for the use of this class of molecules for cancer treatment. The availability of more potent and bioavailable derivatives (see below) has currently halted cyclopamine clinical development.
Vismodegib (GDC-0449)
Vismodegib (GDC-0449) belongs to the second class of Hh inhibitors, molecules capable of binding the target with higher affinity.Citation55,Citation56 Vismodegib was identified in a high-throughput screening of molecules optimized by means of targeted chemical modifications.Citation57 In 2009, Von Hoff et al published the promising results of a Phase I clinical trial of patients with locally advanced or metastatic BCC: of the 33 enrolled patients, 18 showed response to treatment (two complete and the other 16 partial) and the remaining 15 showed stable disease (n = 11) or progression (n = 4).Citation58 Following this trial, a 150 mg/day trial with 104 patients showed a 30% response rate per metastatic BCC and a 43% rate per locally advanced BCC. Adverse events have proved tolerable.Citation59 Given these results, vismodegib was approved by the Food and Drug Administration (FDA) in January 2012 for the treatment of locally advanced or metastatic BCC, ie, for those patients for whom it is impossible to resort to surgery or radiotherapy.Citation56,Citation60
Later on during 2012, results from one more clinical trial were published: Tang et al administered the drug to patients with BCNS and demonstrated its effectiveness in reducing preexisting lesions (mean −65% versus −11% placebo) and inhibiting new lesion formation (average two versus 29 per year).Citation61 Histological samples taken from patients who received the treatment for at least 1 month showed a reduction of 90% in Hh activity (measured by the levels of Gli1).
Although the efficacy of the therapy was striking and led to quick acceptance, given its low toxicity, questions still remain that prompt further research in the field:
– First of all, despite these positive results, trials have indicated that after discontinuation of therapy a resumption of growth may occur.Citation61 One hypothesis is that treatment is not able to kill a fraction of the cells that remain quiescent, and when treatment is suspended this population gives rise to full blown BCC.
– Second, a phenomenon observed during the use of vismodegib and potentially other inhibitors of Smo is drug resistance: it has been observed in medulloblastoma patients who have already undergone conventional treatments, so it may not occur with the same frequency in BCC patients – especially those who can be considered chemonaïve.Citation62 A potential mechanism for this resistance is the acquisition of a Smo mutation (D473H), which does not allow vismodegib binding but retains its ability to activate the pathway.Citation63
Other Smo inhibitors
In the last 10 years, several companies have invested in the search for good inhibitors of Hh, with variable results. Since the inhibition of Smo is the most well-known mechanism for interfering with the Hh pathway (as shown by the success of cyclopamine and vismodegib), several molecules have been developed for this purpose.Citation55
Cur-61414
One of the first attempts was made by researchers at Curis Inc (Lexington, MA) with Cur-61414, which – although successful in a mouse model of BCC – failed to provide positive results in human BCC, probably because of inadequate penetration in the skin.Citation64,Citation65
NVP-LDE225, erismodegib
A more promising inhibitor is LDE225 (NVP-LDE225, erismodegib) a new and specific inhibitor of Smo developed by Novartis (Basel, Switzerland), usable both locally and systemically (orally).Citation66
Skvara et al, after having shown that topical LDE225 is able to reverse the formation of basaloid tumors in skin punches taken from mice Ptch1+/−, enrolled eight patients suffering from BCNS: a total of 27 lesions were treated twice a day with cream LDE225 0.75% for 4 weeks.Citation67 Histopathology showed a reduction in Ki-67 staining and a reduction in nuclear Gli1 reactivity in the lesions treated compared to controls; accordingly, analysis by quantitative polymerase chain reaction showed a reduction in the levels of Gli1, Gli2, and Ptch1 messenger ribonucleic acid from −2 to −16 times in the treated lesions. Of the 13 lesions treated with the drug, twelve showed a response – three complete, understood as the disappearance of the lesions and nine partial, intended as a reduction of the lesions. None of the lesions showed evidence of complete histological clearance – Skvara et al suggest the existence of a subpopulation of resistant cells (cancer stem cells?), which may lead to tumor regrowth.Citation4,Citation67 Although other conclusive data have not yet been published, Novartis has suspended the development of the topical LDE225 cream since the clinical studies did not demonstrate tumor clearance rates sufficient enough to support further clinical investigation.Citation68
On the contrary, there are currently several ongoing clinical trials to test efficacy of oral administration of LDE225 in patients:
– a Phase I study of oral LDE225 in patients with advanced solid tumors (BCC, medulloblastoma, and others) (NCT00880308);
– a Phase II, randomized, double-blind study of efficacy and safety of two dose levels of LDE225 in patients with locally advanced or metastatic BCC (NCT01327053); and
– a Phase II trial evaluating the efficacy, safety, and pharmacokinetics of oral LDE225 in the treatment of adult patients with BCNS (NCT01350115).
A recently published paper indicates that NVP-LDE225 monotherapy in chemonaïve tumors had little effect on tumor growth.Citation69 Notably, however, NVP-LDE225 treatment was highly effective in preventing the recurrence of residual tumors following chemotherapy.Citation69
LEQ506
Another Novartis Smo inhibitor is LEQ506,Citation70,Citation71 which is currently undergoing a Phase I clinical trial on advanced solid tumors: recurrent or refractory medulloblastoma or locally advanced or metastatic BCC (NCT01106508), for which results are not currently available.
IPI-926 (saridegib)
Infinity Pharmaceuticals (Cambridge, MA) has developed a new molecule using the cyclopamine structure as starting material: making a series of chemical modifications to the ring system of the molecule has generated IPI-926 (saridegib) characterized by a greater chemical stability, solubility, potency, selectivity, and bioavailability compared to the natural compound.Citation72–Citation74
Recently, Lee et al demonstrated that saridegib lengthens the life of an Hh-dependent mouse model of medulloblastoma.Citation75 In this study, PtchC/C animals were treated for 6 weeks, with 100% survival in saridegib-treated mice versus 0% of controls and a reduction in clinical symptoms in treated animals.Citation75 Lee et al also suggested that saridegib reduces tumor-initiating capacity since they observed reduced tumor incidence, slower growth, and spontaneous tumor regression in allografts generated from previously treated autochthonous medulloblastomas compared with those from untreated donors. Lee et al did not observe the appearance of mutations at the level of Smo nor other resistance mechanisms such as amplification of Gli2 (actually, saridegib is active on cells transfected with a Smo mutant resistant to vismodegib, D473H). Despite this, a recovery in tumor growth accompanied by a progressive increase in the levels of Gli1 was observed after the 6 weeks of treatment.Citation75
The hypothesis presented is that saridegib would seem to interfere with the activity of adenosine triphosphate binding cassette transporters (ABC) such as P-glycoprotein. ABC transporters are membrane proteins that use adenosine triphosphate for a conformational change that transport a variety of substrates across the cell membranes to the exterior and are also known for their capability as drug transporters out of the cells, making it difficult for the drug to reach an effective concentration in the cells.Citation76,Citation77 In fact, an increase of P-glycoprotein expression was observed in saridegib-treated tumors while administration of verapamil (an inhibitor of P-glycoprotein) partially restored the reduction in Gli1 levels.Citation75
The effect of saridegib on P-glycoprotein casts some doubt on the feasibility of combined therapies, given the fact that stronger extracellular transport means reduced drug efficacy, and several authors are considering ABC transporters as potential targets for therapy.Citation78 Interestingly, on the contrary, Hhantag691 administration has been linked to P-glycoprotein inhibition,Citation79 while the activity of vismodegib on these molecules has been evaluated as modest.Citation80
Oral IPI-926 is currently undergoing clinical experimentation: a Phase I study on IPI-926 has been completed (NCT00761696) and preliminary results indicate that IPI-926 was well tolerated and resulted in clinical activity in patients with BCC.Citation81 Further studies (a Phase Ib/II study) are ongoing to address the potential of a combination with gemcitabine in patients with more aggressive cancers (ie, metastatic pancreatic cancer) (NCT01130142).
Pfizer PF-04449913
Pfizer, Inc (New York, NY) has developed its own Hh inhibitor, PF-04449913, an orally bioavailable Smo antagonist and benzimidazole derivative.Citation82 This molecule is currently being tested in Phase I clinical trials in hematologic malignancies (NCT00953758, NCT01546038) and selected advanced, metastatic solid tumors (NCT01286467) both as a single agent or in combination with standard chemotherapy agents. Recently presented preliminary data highlight the good tolerability of the drug, and early signs of efficacy have been observed in all hematologic diseases studied.Citation83 Treated patients presented downregulation of the antiapoptotic protein B-cell lymphoma-2 (Bcl2) and the transporter protein ABCA2.Citation84 Although not yet tested on BCC, the efficacy on BCC could be assessed using a topical formulation since the current formulation is in tablets.
TAK-441
Millennium Pharmaceuticals, Inc (Cambridge, MA) which is part of Takeda Pharmaceutical Company Ltd (Osaka, Japan) has launched a clinical trial on the oral administration of TAK-441, its Hh signaling pathway inhibitor (NCT01204073). This study involves adult patients with advanced nonhematological malignancies, but at the time of this review results were not yet available.
Bisamides
Dijkgraaf et al analyzed tumors with the Smo D473H mutation, resistant to vismodegib, and demonstrated an ability to overcome this resistance using a new type of inhibitor belonging to the class of bisamides.Citation85 Confirming the validity of this approach, a research group from AstraZeneca (London, UK) recently identified another bisamide with potent Smo-inhibiting activity and good pharmacokinetics in mice.Citation86 Both molecules, although promising, need further characterization in vitro and/or in vivo and to be validated in clinical trials.
Itraconazole
After a screening for inhibitors of Hh using about 2400 molecules previously tested for toxicity in humans or approved by the FDA, Kim et al identified itraconazole as a potent inhibitor of Hh.Citation87 Itraconazole is an antifungal medication commonly used in clinical practice, even for long periods of time; it inhibits the enzyme 14-α lanosterol demethylase, crucial for the biosynthesis of ergosterol in fungi and cholesterol in mammals.Citation9
Itraconazole gave positive results both in xenograft models of medulloblastoma and in the reduction of endogenous BCCs in murine models.Citation87 According to Kim et al, the inhibitory effect on Hh by itraconazole was not mediated by its action on the synthesis of sterols, since the drug acted downstream of Ptch; on the contrary, itraconazole prevented accumulation of Smo in the primary cilium. Furthermore, the drug was able to synergize with cyclopamine, suggesting that the two drugs recognized two different binding sites on Smo.Citation87
An exploratory Phase II proof of concept clinical trial with itraconazole on BCC patients has been performed by Stanford University (NCT01108094) with positive results, although additional studies with larger numbers of patients are needed to validate these findings.Citation88
An important aspect of itraconazole is that it has been shown to also inhibit angiogenesis through inhibition of vascular endothelial growth factor receptor trafficking and downstream signaling.Citation89 Therefore, its actions may be exerted simultaneously on both of these important therapeutic targets.
Following the discovery of itraconazole’s potential for Hh inhibition, an interesting attempt has been performed to search for analogs of itraconazole with greater potency: 25 itraconazole side chain analogs were synthesized obtaining molecules with greater inhibitory activity.Citation90 This work also helped identify the distinct regions critical for anti-Hh and antivascular endothelial growth factor receptor activity. Further preclinical and clinical work is now needed to validate these new compounds.
Phenyl quinazolines
Another promising class of Hh inhibitors is a small family of phenyl quinazolinone ureas, which has been reported as potent modulators of Hh protein function. Preliminary structure–activity relationship studies of the urea substituent led to a nanomolar Hh antagonist (compound 7d), which will need further testing for specificity, toxicity, and bioavalaibility.Citation91
Vitamin D3 (calcitriol)
The literature provides wide evidence for the antitumoral activity of vitamin D3, showing its capabilities of blocking the cell cycle, inducing differentiation and apoptosis, modulating inflammation, and inhibiting neoangiogenesis.Citation92,Citation93
Vitamin D3 was shown to act both by binding to its nuclear receptor (VDR) and activating transcription (genomic effects), and by nongenomic effects, such as increasing the influx of calcium (Ca2+).Citation93 A relationship between vitamin D3 and the Hh pathway came about by the observation of a Ptch-dependent secretion of provitamin D3 and by Smo inhibition by provitamin D3 or vitamin D3 itself.Citation94,Citation95 Furthermore, mice lacking VDR present higher levels of Hh signaling components in the epidermis and epidermal portion of the hair follicles.Citation96
The relevance of vitamin D3 and VDR in BCC tumorigenesis has been suggested by Kamradt et al, while the involvement of Hh in this mechanism was highlighted by an analysis of BCC formation in VDR null mice.Citation96,Citation97
The relevance of exploiting vitamin D3 in BCC chemotherapy has been demonstrated on mice overexpressing the Hh pathway in the skin, in which vitamin D3 leads to VDR activation and induces Hh repression, inhibiting BCC proliferation and inducing differentiation.Citation98
Since vitamin D3 does not seem to affect basal levels of Gli1 in Smo−/− cells, it may control the Hh signaling downstream of Ptch1. Interestingly, although the promoters of several Hh pathway components have putative consensus sequences for VDR binding,Citation93 the inhibitory effect on Hh seems to be independent of VDR binding by vitamin D3, because in VDR−/− cells the administration of calcitriol continues to repress Gli1 levels.Citation98 Similar results have been also obtained using murine BCC cell lines.Citation95
These data are in apparent contrast with what has been shown when using skin explants from VDR null mice, which did not present a reduction in Hh signaling following culture in the presence of vitamin D3,Citation96 arguing in favor of a VDR requirement. One hypothesis to reconcile these different results is that vitamin D3 may exert both genomic (VDR dependent) and nongenomic (a direct action on Smo)Citation94 effects, which may prevail alternatively in different cellular and physiological settings.
To evaluate the effectiveness of topical vitamin D3 treatment in BCC patients following the important preclinical data, a Phase III clinical trial has been initiated (NCT01358045) to clarify the feasibility and side effects of such treatments. In fact, one of the reasons why such treatment has not been extensively tested so far in different tumor types is because vitamin D3 through VDR acts on numerous physiological systems and its activation can lead to negative side effects. To facilitate clinical application, avoiding the side effects that cause prolonged administration of vitamin D3, researchers have recently produced semisynthetic derivatives that retain the ability to repress Hh and do not bind to VDR, which may be tested in future preclinical and clinical trials.Citation99
Blocking the Hh pathway upstream of Smo
Hh ligand antagonists
A few years ago, a search for small molecules acting at the level of the Hh ligands was performed, identifying robotnikinin, a macrocyclic Hh inhibitor that is able to bind the extracellular Shh protein and block Shh signaling.Citation100,Citation101
More recently, starting from the structure of robotnikinin, several new macrocyclic analogs with increased Hh inhibitory activity have been generated. Interestingly, the most promising molecule (BRD-6851), in contrast to robotnikinin, is again a Smo antagonist.Citation102
Another approach used to block the Hh pathway has been the generation of antibodies capable of binding Shh and to abolish its interaction with the Ptch receptor. The most effective and widely used antibody so far developed is called 5E1, which targets an epitope close to the region of Ptch/Shh interactions.Citation103,Citation104 This antibody has not so far been entered into any clinical trial.
Oxysterol modulation
Given the potential role of oxysterols in regulating the Hh pathway and more interestingly the fact that at least some oxysterols seem to be able to bind Smo on a site different to that which cyclopamine or Sag (Smo agonist) bind,Citation36 they represent a potential alternative target. It is currently unclear how the oxysterols involved in Hh signaling regulation are synthesized, transported, and degraded such that their levels can be perturbed, but regulation of signaling sterol turnover might be another potential control point of the Hh pathway.
Blocking the Hh pathway downstream of Smo
Hh pathway inhibitors (HPI)
Hyman et al, in a large scale high-throughput screening for compounds that can inhibit Hh target gene expression induced by Smo and/or Sag, identified four molecules (HPI1-4) able to suppress Hh activity.Citation105 They all act downstream of SuFu, so they are not able to directly bind Smo, but they have a different mechanism of action: HPI1 acts on Gli1 (both exogenous and endogenous), HPI2 and HPI3 act on the ciliary process required for Gli2 function, and HPI4 interferes with ciliogenesis. The capacity of these inhibitors to act downstream of Smo is very promising in overcoming the resistance against Smo inhibitors. For this reason, Chenna et al generated a polymer nanoparticle-encapsulated formulation of HPI1 (NanoHHI) that is able to arrest proliferation in cells which ectopically express the Smo mutant resistant to vismodegib (D473H).Citation106 Furthermore, NanoHHI reduced growth in a medulloblastoma xenograft model and, in combination with gemcitabine, significantly impeded growth in a pancreatic cancer xenograft and in orthotopic experiments with hepatocellular carcinoma cells.Citation106,Citation107
Gli antagonists (GANT)
Almost all the inhibitors of the Hh pathway that have been developed so far inhibit Smo activity. However, the development of molecules able to act further downstream would allow a direct hit on the final effectors of the signaling pathway (ie, Gli factors) and would provide a valid alternative in case of drug resistance.
Unfortunately, although searches for molecules acting on Gli factors have been performed for some years, the successful evidence provided is still limited to preclinical studies.
Lauth et al in 2007 identified two GANTs (GANT58 and GANT61) capable of inhibiting the transcriptional activity of Gli1 and Gli2: GANT61 is a hexahydropyrimidine while GANT58 has a thiophene core with four pyridine rings.Citation77 Despite these structural differences, they both inhibit Gli1 and Gli2. The two drugs showed a strong inhibitory activity on pancreatic cancer cells and have worked mainly in reducing tumor growth in a xenograft model.Citation77
Screens of 1990 synthetic chemicals and 94 natural products have identified a few compounds that can antagonize Hh target gene expression induced by Gli1 or Gli2 overexpression, including GANT58, GANT61, zerumbone, arcyriaflavin C, and physalin.Citation105 How these compounds antagonize Gli function has not been determined, although GANT61 appears to attenuate the DNA-binding activity of Gli1 in vivo and it has been suggested that arcyriaflavin C and physalin F indirectly antagonize Gli function through protein kinase C/mitogen-activated protein kinase pathway blockade. Similarly, the natural product forskolin can nonselectively inhibit Hh signaling by activating adenylate cyclase and consequently protein kinase A.
The study of these inhibitors has been limited to preclinical models, and clinical trials are not currently under way. Recently it was shown that GANT61 causes apoptosis in myeloid leukemia cells and is able to synergize with rapamycin,Citation108 and this raises hopes for further development of this type of antitumor drug.
Regulation of Gli acetylation
Another potential approach could be the use of negative regulators of histone deacetylase, which would maintain Gli acetylation and inhibit its transcriptional activity, as demonstrated by pharmacological inhibition with trichostatin A or overexpression of the KCASH family of genes, which drive histone deacetylase ubiquitination, targeting it for proteasomal degradation.Citation37,Citation109
Retinoids: anticancer drugs with a hint of the Hh pathway?
Retinoids have an important physiological role in the regulation of phenomena such as differentiation and proliferation, both during development and in adult life.Citation110–Citation112 Experimental evidence exists that retinoids have antitumoral action, as in the case of acute promyelocytic leukemia, neuroblastoma, and other diseases.Citation113,Citation114
Several retinoids such as acitretin (NCT00020956)Citation115,Citation116 and tretinoin (NCT00007631)Citation117 or tazarotene (see below) have been tested on BCC, either in the form of topical cream or systemic therapies and both as treatment and chemopreventive therapies. Tazarotene was the first retinoid to arouse some interest as a potential drug for the treatment of BCC.Citation118–Citation120 In clinical settings, tazarotene use is approved for the treatment of psoriasis and acne and is well tolerated in patients undergoing an oral or topical regimen.Citation119,Citation121
In 2004, it was demonstrated (on Ptch1+/− mice exposed to ultraviolet or ionizing radiation) that tazarotene inhibited the formation of new microscopic lesions and reduced BCC size.Citation122 In the same year, Bianchi et al presented positive results of an experimental administration of tazarotene locally to BCC patients (gel 0.1%) once a day for 24 weeks. At the end of the study, 70% of lesions showed response to treatment and about 30% healed with no disease recurrence in 3 years of follow-up.Citation119
There are currently two Phase II clinical trials in patients with BCNS (NCT00783965; NCT00489086) with the purpose of evaluating the preventive effect of this highly tolerable topical drug on BCC formation.
The antitumor effects of retinoids are elicited mainly by inducing apoptosis and differentiation, although the mechanisms have not been fully assessed. It is not clear if and how tazarotene or other retinoids may act on the Hh pathway, although a link has been suggested by Goyette et al.Citation123
Whether Hh and retinoids crosstalk or not needs to be further verified, but combined therapies with Hh inhibitors may provide a useful and promising tool, given their relative tolerability.
Overcoming resistance to Hh/Smo inhibitors: phosphatidylinositol 3-kinase and epidermal growth factor receptor inhibition
Experimental evidence points to the potential acquisition of tumor resistance to Smo antagonist treatment, both by Smo receptor mutations or by amplification of Hh pathway downstream target genes such as CCND1 and Gli2.Citation66,Citation85,Citation124
Recently a few studies have shown the possibility of overcoming drug resistance through the use of inhibitors of other pathways, which are able to interplay or act in synergy with Hh to promote tumorigenesis or tumor proliferation:
– Researchers at Novartis have shown that mechanisms of LDE225 resistance can be overcome by the use of inhibitors of phosphatidylinositol 3-kinase.Citation66 Importantly, this information has been confirmed by another group on vismodegib-resistant tumors.Citation85
– Another pathway which may cooperate with Hh in BCC is epidermal growth factor receptor signaling.Citation125,Citation126 Recent data in vitro in human cell lines and in vivo in mouse models suggest the relevance of targeting this cooperation as a new therapeutic hypothesis to be further developed.Citation126
Conclusion and future directions
The identification of the Hh pathway as a strategic target for BCC treatment has prompted the search of Hh inhibitors for therapeutic purposes. Relatively quickly a first molecule has been recently approved for clinical use by the FDA, and other molecules are undergoing clinical trials. Several drugs already approved for other therapies have also been tested and proven to be capable of suppressing Hh activity (). At the moment, most of the molecules that have shown therapeutic efficacy target the Smo intramembrane receptor, either in its function or in its capacity to migrate into the cilium, indicating Smo as a critical component of the pathway. Unfortunately, the possibility of BCC developing resistance to Smo-targeting drugs (and in general to drugs targeting a single structure) is evident, and there have already been published papers documenting both mutations of the receptor or constitutive activation of the Hh pathway downstream of Smo. For this reason, other drugs which target other structures of the Smo receptor, or even better with different downstream targets in the Hh pathway (including GANTs), need to be developed.
One of the reasons for which it is so strategic to suppress the Hh pathway in BCC is not only the specific role of Hh on initiation and promotion in this type of tumor, but also the increasing evidence of the role of the Hh pathway in the maintenance of the stem cell and cancer stem cell compartments.Citation127,Citation128 Targeting Hh could reduce the pool of cancer stem cells remaining after chemotherapy and in this way reduce tumor recurrence.
Furthermore, since Hh is not the only mechanism involved in tumor formation and maintenance, the use of combinatorial treatment is recommended to increase efficiency of the cure.
Another issue to be dealt with is the molecular characterization of the patient’s BCC, to avoid unnecessary delay in the identification of the best therapeutic approach (ie, do not use target Smo if Smo is mutated). Molecular characterization may be very expensive and in order to reduce the costs it is also necessary to work on the identification of a few discriminating target molecules to be analyzed.
In summary, the approval of vismodegib has provided a new and important tool for BCC treatment, but further efforts will be needed to find alternative drugs for resistant tumors and may be a more economical solution (considering the potential cost of vismodegib is currently about USD7500 per month and the world market is evaluated at at least USD1-4 billion per year).Citation56 Although competition, which was strong until FDA’s approval of vismodegib, may fade in the future, a substantial number of clinical trials are currently ongoing and there is a good chance that other useful therapeutic instruments will be available shortly.
Acknowledgments
This work was supported, in part, by Associazione Italiana Ricerca Cancro MIUR (FIRB and PRIN projects), Ministry of Health, Fondazione Roma, Fondazione Mariani, EU Healing grant, Italian Institute of Technology, and Agenzia Spaziale Italiana.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Cancer SocietyCancer Facts and Figures 2012Atlanta, GAAmerican Cancer Society2012 Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdfAccessed November 5, 2012
- KimRHArmstrongAWNonmelanoma skin cancerDermatol Clin201230112513922117874
- RubinAIChenEHRatnerDBasal-cell carcinomaN Engl J Med2005353212262226916306523
- KasperMJaksVHohlDToftgardRBasal cell carcinoma – molecular biology and potential new therapiesJ Clin Invest2012122245546322293184
- KaragasMRWeinstockMANelsonHHKeratinocyte carcinomas (basal and squamous cell carcinomas of the skin)SchottenfeldDFraumeniJFJrCancer Epidemiology and Prevention3rd edOxfordOxford University Press200612301250
- WeissGJKornRLMetastatic basal cell carcinoma in the era of Hedgehog signaling pathway inhibitorsCancer2012118215310531922511370
- WallingHWFoskoSWGeraminejadPAWhitakerDCArpeyCJAggressive basal cell carcinoma: presentation, pathogenesis, and managementCancer Metastasis Rev2004233–438940215197337
- LomasALeonardi-BeeJBath-HextallFA systematic review of worldwide incidence of nonmelanoma skin cancerBr J Dermatol201216651069108022251204
- GuyGPEkwuemeDUYears of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: a systematic review of the literaturePharmacoeconomics2011291086387421846158
- WeinstockMAStillJMAssessing current treatment options for patients with severe/advanced basal cell carcinomaSemin Cutan Med Surg201130Suppl 4S10S1322177101
- MarcilISternRSRisk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysisArch Dermatol2000136121524153011115165
- DesaiTChenCLDesaiAKirbyWBasic pharmacology of topical imiquimod, 5-fluorouracil, and diclofenac for the dermatologic surgeonDermatol Surg20123819710322092926
- LeeSSelvaDHuilgolSCGoldbergRALeibovitchIPharmacological treatments for basal cell carcinomaDrugs200767691593417428108
- TeglundSToftgardRHedgehog beyond medulloblastoma and basal cell carcinomaBiochim Biophys Acta20101805218120820085802
- InghamPWNakanoYSegerCMechanisms and functions of Hedgehog signalling across the metazoaNat Rev Genet201112639340621502959
- CaroILowJAThe role of the Hedgehog signaling pathway in the development of basal cell carcinoma and opportunities for treatmentClin Cancer Res201016133335333920439455
- NgJMCurranTThe Hedgehog’s tale: developing strategies for targeting cancerNat Rev Cancer201111749350121614026
- PoAFerrettiEMieleEHedgehog controls neural stem cells through p53-independent regulation of NanogEMBO J201029152646265820581804
- TakebeNHarrisPJWarrenRQIvySPTargeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathwaysNat Rev Clin Oncol2011829710621151206
- VarjosaloMTaipaleJHedgehog signalingJ Cell Sci2007120Pt 13617182898
- InghamPWMcMahonAPHedgehog signaling in animal development: paradigms and principlesGenes Dev200115233059308711731473
- BarakatMTHumkeEWScottMPLearning from Jekyll to control Hyde: Hedgehog signaling in development and cancerTrends Mol Med201016833734820696410
- LumLBeachyPAThe Hedgehog response network: sensors, switches, and routersScience200430456781755175915205520
- HooperJEScottMPCommunicating with HedgehogsNat Rev Mol Cell Biol20056430631715803137
- InghamPWPlaczekMOrchestrating ontogenesis: variations on a theme by Sonic HedgehogNat Rev Genet200671184185017047684
- TowersMTickleCGrowing models of vertebrate limb developmentDevelopment2009136217919019103802
- MannRKBeachyPANovel lipid modifications of secreted protein signalsAnnu Rev Biochem20047389192315189162
- TherondPPRelease and transportation of Hedgehog moleculesCurr Opin Cell Biol201224217318022366329
- RohatgiRMilenkovicLScottMPPatched1 regulates Hedgehog signaling at the primary ciliumScience2007317583637237617641202
- WollamJAntebiASterol regulation of metabolism, homeostasis, and developmentAnnu Rev Biochem20118088591621495846
- CorcoranRBScottMPOxysterols stimulate Sonic Hedgehog signal transduction and proliferation of medulloblastoma cellsProc Natl Acad Sci U S A2006103228408841316707575
- StruttHThomasCNakanoYMutations in the sterol-sensing domain of Patched suggest a role for vesicular trafficking in Smoothened regulationCurr Biol200111860861311369206
- DwyerJRSeverNCarlsonMNelsonSFBeachyPAParhamiFOxysterols are novel activators of the Hedgehog signaling pathway in pluripotent mesenchymal cellsJ Biol Chem2007282128959896817200122
- JohnsonJSMelitonVKimWKNovel oxysterols have pro-osteogenic and anti-adipogenic effects in vitro and induce spinal fusion in vivoJ Cell Biochem201111261673168421503957
- SharpeHJde SauvageFJSignaling: an oxysterol ligand for SmoothenedNat Chem Biol20128213914022257852
- NachtergaeleSMydockLKKrishnanKOxysterols are allosteric activators of the oncoprotein SmoothenedNat Chem Biol20128221122022231273
- CanettieriGDi MarcotullioLGrecoAHistone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylationNat Cell Biol201012213214220081843
- Pasca di MaglianoMHebrokMHedgehog signalling in cancer formation and maintenanceNat Rev Cancer200331290391114737121
- GorlinRJNevoid basal-cell carcinoma syndromeMedicine (Baltimore)1987662981133547011
- HahnHWickingCZaphiropoulousPGMutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndromeCell19968568418518681379
- JohnsonRLRothmanALXieJHuman homolog of patched, a candidate gene for the basal cell nevus syndromeScience19962725268166816718658145
- AszterbaumMRothmanAJohnsonRLIdentification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndromeJ Invest Dermatol199811068858889620294
- ReifenbergerJWolterMKnobbeCBSomatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomasBr J Dermatol20051521435115656799
- WolterMReifenbergerJSommerCMutations in the human homologue of the Drosophila segment polarity gene patched (PTCH) in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous systemCancer Res19975713258125859205058
- LamCWXieJToKFA frequent activated smoothened mutation in sporadic basal cell carcinomasOncogene19991838338369989836
- XieJMuroneMLuohSMActivating Smoothened mutations in sporadic basal-cell carcinomaNature1998391666290929422511
- ReifenbergerJWolterMWeberRGMissense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous systemCancer Res1998589179818039581815
- OroAEHigginsKMHuZBonifasJMEpsteinEHJrScottMPBasal cell carcinomas in mice overexpressing Sonic HedgehogScience199727653138178219115210
- KeelerRFTeratogenic compounds of Veratrum californicum (Durand) X. Cyclopia in rabbits produced by cyclopamineTeratology1970321751804986632
- IncardonaJPGaffieldWKapurRPRoelinkHThe teratogenic Veratrum alkaloid cyclopamine inhibits Sonic Hedgehog signal transductionDevelopment199812518355335629716521
- TaipaleJChenJKCooperMKEffects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamineNature200040667991005100910984056
- DahmaneNSanchezPGittonYThe Sonic Hedgehog–Gli pathway regulates dorsal brain growth and tumorigenesisDevelopment2001128245201521211748155
- ChenJKTaipaleJCooperMKBeachyPAInhibition of Hedgehog signaling by direct binding of cyclopamine to SmoothenedGenes Dev200216212743274812414725
- TabsSAvciOInduction of the differentiation and apoptosis of tumor cells in vivo with efficiency and selectivityEur J Dermatol20041429610215196999
- De SmaeleEFerrettiEGulinoAVismodegib, a small-molecule inhibitor of the Hedgehog pathway for the treatment of advanced cancersCurr Opin Investig Drugs2010116707718
- DlugoszAAgrawalSKirkpatrickPVismodegibNat Rev Drug Discov201211643743822653209
- RobargeKDBruntonSACastanedoGMGDC-0449 – a potent inhibitor of the Hedgehog pathwayBioorg Med Chem Lett200919195576558119716296
- Von HoffDDLoRussoPMRudinCMInhibition of the Hedgehog pathway in advanced basal-cell carcinomaN Engl J Med2009361121164117219726763
- SekulicAMigdenMROroAEEfficacy and safety of vismodegib in advanced basal-cell carcinomaN Engl J Med2012366232171217922670903
- US Food and Drug AdministrationFDA labeling information: Erivedge2012 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203388lbl.pdfAccessed November 5, 2012
- TangJYMackay-WigganJMAszterbaumMInhibiting the Hedgehog pathway in patients with the basal-cell nevus syndromeN Engl J Med2012366232180218822670904
- RudinCMHannCLLaterraJTreatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449N Engl J Med2009361121173117819726761
- YauchRLDijkgraafGJAlickeBSmoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastomaScience2009326595257257419726788
- WilliamsJAGuicheritOMZaharianBIIdentification of a small molecule inhibitor of the Hedgehog signaling pathway: effects on basal cell carcinoma-like lesionsProc Natl Acad Sci U S A200310084616462112679522
- Frank-KamenetskyMZhangXMBottegaSSmall-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonistsJ Biol2002121012437772
- BuonamiciSWilliamsJMorrisseyMInterfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastomaSci Transl Med201025151ra70
- SkvaraHKalthoffFMeingassnerJGTopical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitorJ Invest Dermatol201113181735174421430703
- NovartisInnovation Updates: Key Developments in the Fourth Quarter of 2011BaselNovartis2012 Available from: http://www.novartis.com/downloads/investors/financial-results/q4-2011-innovation-tables.pdfAccessed November 5, 2012
- ParkKSMartelottoLGPeiferMA crucial requirement for Hedgehog signaling in small cell lung cancerNat Med201117111504150821983857
- TangJYMarghoobAAEmerging treatments and signaling pathway inhibitorsSemin Cutan Med Surg201130Suppl 4S14S1822177102
- LappanoRMaggioliniMG protein-coupled receptors: novel targets for drug discovery in cancerNat Rev Drug Discov2011101476021193867
- TremblayMRNeslerMWeatherheadRCastroACRecent patents for Hedgehog pathway inhibitors for the treatment of malignancyExpert Opin Ther Pat20091981039105619505195
- TremblayMRLescarbeauAGroganMJDiscovery of a potent and orally active Hedgehog pathway antagonist (IPI-926)J Med Chem200952144400441819522463
- TremblayMRMcGovernKReadMACastroACNew developments in the discovery of small molecule Hedgehog pathway antagonistsCurr Opin Chem Biol201014342843520399136
- LeeMJHattonBAVillavicencioEHHedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma modelProc Natl Acad Sci U S A2012109207859786422550175
- DeanMRzhetskyAAllikmetsRThe human ATP-binding cassette (ABC) transporter superfamilyGenome Res20011171156116611435397
- LauthMBergstromAShimokawaTToftgardRInhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonistsProc Natl Acad Sci U S A2007104208455846017494766
- RobeyRWMasseyPRAmiri-KordestaniLBatesSEABC transporters: unvalidated therapeutic targets in cancer and the CNSAnticancer Agents Med Chem201010862563321189132
- ZhangYLaterraJPomperMGHedgehog pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP and ABCB1/PgpNeoplasia20091119610119107236
- WongHChenJZChouBPreclinical assessment of the absorption, distribution, metabolism and excretion of GDC-0449 (2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl) benzamide), an orally bioavailable systemic Hedgehog signalling pathway inhibitorXenobiotica2009391185086119845436
- RudinCMWeissGJChangAA phase 1 study of IPI-926, an inhibitor of the Hedgehog pathway, in patients with advanced or metastatic solid tumors [abstract]Ann Oncol201021Suppl 8viii164
- MunchhofMJLiQShavnyaADiscovery of PF-04449913, a potent and orally bioavailable inhibitor of SmoothenedACS Med Chem Lett20123210611124900436
- JamiesonCCortesJEOehlerVPhase 1 dose-escalation study of PF-04449913, an oral Hedgehog (Hh) inhibitor, in patients with select hematologic malignancies [abstract]Blood2011118424
- PapayannidisCGuadagnuoloVIacobucciIPF-04449913 reverts multi drug resistance (MDR) by a strong down-regulation of ABCA2 and BCL2 on leukemia stem cells in phase I acute myeloid leukemia and chronic myeloid leukemia treated patients [abstract]Cancer Res2012728 Suppl 14619
- DijkgraafGJAlickeBWeinmannLSmall molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistanceCancer Res201171243544421123452
- YangBHirdAWRussellDJDiscovery of novel Hedgehog antagonists from cell-based screening: isosteric modification of p38 bisamides as potent inhibitors of SMOBioorg Med Chem Lett201222144907491122704236
- KimJTangJYGongRItraconazole, a commonly used anti-fungal that inhibits Hedgehog pathway activity and cancer growthCancer Cell201017438839920385363
- KimDKimJSpaunhurstKAn open-label, exploratory phase II study of oral itraconazole for the treatment of basal cell carcinoma [abstract]Cancer Res201272Suppl 8LB-223
- NacevBAGrassiPDellAHaslamSMLiuJOThe antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cellsJ Biol Chem201128651440454405622025615
- ShiWNacevBAAftabBTHeadSRudinCMLiuJOItraconazole side chain analogues: structure–activity relationship studies for inhibition of endothelial cell proliferation, vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, and Hedgehog signalingJ Med Chem201154207363737421936514
- BruntonSAStibbardJHRubinLLPotent inhibitors of the Hedgehog signaling pathwayJ Med Chem20085151108111018275133
- DeebKKTrumpDLJohnsonCSVitamin D signalling pathways in cancer: potential for anticancer therapeuticsNat Rev Cancer20077968470017721433
- BikleDDVitamin D and the skin: physiology and pathophysiologyRev Endocr Metab Disord201213131921845365
- BijlsmaMFSpekCAZivkovicDvan de WaterSRezaeeFPeppelenboschMPRepression of smoothened by patched-dependent (pro-)vitamin D3 secretionPLoS Biol200648e23216895439
- TangJYXiaoTZOdaYVitamin D3 inhibits Hedgehog signaling and proliferation in murine basal cell carcinomasCancer Prev Res (Phila)20114574475121436386
- TeichertAEElaliehHEliasPMWelshJBikleDDOverexpression of Hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null miceJ Invest Dermatol2011131112289229721814234
- KamradtJRafiLMitscheleTAnalysis of the vitamin D system in cutaneous malignanciesRecent Results Cancer Res200316425926912899528
- UhmannANiemannHLammeringBAntitumoral effects of calcitriol in basal cell carcinomas involve inhibition of Hedgehog signaling and induction of vitamin D receptor signaling and differentiationMol Cancer Ther201110112179218821878656
- DeberardinisAMBanerjeeUMillerMLemieuxSHaddenMKProbing the structural requirements for vitamin D3 inhibition of the Hedgehog signaling pathwayBioorg Med Chem Lett201222144859486322687748
- StantonBZPengLFMaloofNA small molecule that binds Hedgehog and blocks its signaling in human cellsNat Chem Biol20095315415619151731
- PengLFStantonBZMaloofNWangXSchreiberSLSyntheses of aminoalcohol-derived macrocycles leading to a small-molecule binder to and inhibitor of Sonic HedgehogBioorg Med Chem Lett2009196319632519819139
- DockendorffCNagiecMMWeiwerMMacrocyclic Hedgehog pathway inhibitors: optimization of cellular activity and mode of action studiesACS Med Chem Lett201231080881323074541
- EricsonJMortonSKawakamiARoelinkHJessellTMTwo critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identityCell19968746616738929535
- PepinskyRBRayhornPDayESMapping Sonic Hedgehog-receptor interactions by steric interferenceJ Biol Chem200027515109951100110753901
- HymanJMFirestoneAJHeineVMSmall-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockadeProc Natl Acad Sci U S A200910633141321413719666565
- ChennaVHuCPramanikDA polymeric nanoparticle encapsulated small-molecule inhibitor of Hedgehog signaling (NanoHHI) bypasses secondary mutational resistance to Smoothened antagonistsMol Cancer Ther201211116517322027695
- XuYChennaVHuCPolymeric nanoparticle-encapsulated Hedgehog pathway inhibitor HPI-1 (NanoHHI) inhibits systemic metastases in an orthotopic model of human hepatocellular carcinomaClin Cancer Res20121851291130221868763
- PanDLiYLiZWangYWangPLiangYGli inhibitor GANT61 causes apoptosis in myeloid leukemia cells and acts in synergy with rapamycinLeuk Res201236674274822398221
- De SmaeleEDi MarcotullioLMorettiMIdentification and characterization of KCASH2 and KCASH3, 2 novel Cullin3 adaptors suppressing histone deacetylase and Hedgehog activity in medulloblastomaNeoplasia201113437438521472142
- Gutierrez-MazariegosJTheodosiouMCampo-PaysaaFSchubertMVitamin A: a multifunctional tool for developmentSemin Cell Dev Biol201122660361021693195
- GudasLJEmerging roles for retinoids in regeneration and differentiation in normal and disease statesBiochim Biophys Acta20121821121322121855651
- TangXHGudasLJRetinoids, retinoic acid receptors, and cancerAnnu Rev Pathol2011634536421073338
- HansenLASigmanCCAndreolaFRossSAKelloffGJDe LucaLMRetinoids in chemoprevention and differentiation therapyCarcinogenesis20002171271127910874003
- AltucciLLeibowitzMDOgilvieKMde LaraARGronemeyerHRAR and RXR modulation in cancer and metabolic diseaseNat Rev Drug Discov200761079381017906642
- IngvesCJemecGBCombined imiquimod and acitretin for nonsurgical treatment of basal cell carcinomaScand J Plast Reconstr Surg Hand Surg200337529329514649688
- LienMHFenskeNAGlassLFAdvances in the chemoprevention of non-melanoma skin cancer in high-risk organ transplant recipientsSemin Oncol201239213413822484184
- WeinstockMABinghamSFDigiovannaJJTretinoin and the prevention of keratinocyte carcinoma (basal and squamous cell carcinoma of the skin): a Veterans Affairs randomized chemoprevention trialJ Invest Dermatol201213261583159022318383
- ChandraratnaRATazarotene – first of a new generation of receptor-selective retinoidsBr J Dermatol1996135Suppl 4918259035701
- BianchiLOrlandiACampioneETopical treatment of basal cell carcinoma with tazarotene: a clinicopathological study on a large series of casesBr J Dermatol2004151114815615270884
- OrlandiABianchiLCostanzoACampioneEGiusto SpagnoliLChimentiSEvidence of increased apoptosis and reduced proliferation in basal cell carcinomas treated with tazaroteneJ Invest Dermatol200412241037104115102095
- JonesPHBurnettRDFainaruIA phase 1 study of tazarotene in adults with advanced cancerBr J Cancer200389580881512942109
- SoPLLeeKHebertJTopical tazarotene chemoprevention reduces basal cell carcinoma number and size in Ptch1+/− mice exposed to ultraviolet or ionizing radiationCancer Res200464134385438915231643
- GoyettePAllanDPeschardPChenCFWangWLohnesDRegulation of Gli activity by all-trans retinoic acid in mouse keratinocytesCancer Res200060195386538911034076
- MetcalfeCde SauvageFJHedgehog fights back: mechanisms of acquired resistance against Smoothened antagonistsCancer Res201171155057506121771911
- KasperMSchnidarHNeillGWSelective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytesMol Cell Biol200626166283629816880536
- EberlMKlingerSMangelbergerDHedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cellEMBO Mol Med20124321823322294553
- SellheyerKBasal cell carcinoma: cell of origin, cancer stem cell hypothesis and stem cell markersBr J Dermatol2011164469671121128907
- MerchantAAMatsuiWTargeting Hedgehog – a cancer stem cell pathwayClin Cancer Res201016123130314020530699