Abstract
Background
Delayed gastric emptying is a common disorder with few effective therapeutic options. The goal of this study was to investigate whether ipamorelin, a synthetic peptidomimetic that acts on the ghrelin receptor, accelerates gastric emptying in a rodent model of gastroparesis induced by abdominal surgery and intestinal manipulation.
Methods
Fasted adult male rats were subjected to laparotomy and intestinal manipulation. Following the surgery rats received ipamorelin (0.014–0.14 µmol/kg) or vehicle control via intravenous administration. Gastric emptying was measured by the percent of total recovered radioactivity remaining in the stomach 15 minutes after intragastric gavage of 1.5 mL of 99mTc (technicium-99m) sulfur colloid in 0.5% methylcellulose. In a separate group of rats subjected to laparotomy and intestinal manipulation, the gastric fundus was isolated and tissue segments were suspended in an organ bath to assess the effect of ipamorelin (1 µM) on gastric smooth muscle contractility induced by acetylcholine and electrical field stimulation.
Results
Abdominal surgery caused a delay in gastric emptying with 78% ± 5% of the meal remaining in the stomach in vehicle controls. Ipamorelin (0.014 µmol/kg intravenous) resulted in a significant acceleration (P < 0.05 vs vehicle-treated rat) of gastric emptying with 52% ± 11% of the meal remaining in the stomach compared to nonsurgical control animals with 44% ± 6%. Following abdominal surgery and intestinal manipulation, isolated preparations of gastric smooth muscle exhibited a marked inhibition of acetylcholine and electrical field stimulation-induced contractile responses, which were reversed by ipamorelin and ghrelin.
Conclusion
These results suggest that ipamorelin accelerates gastric emptying in a rodent model of postoperative ileus through the stimulation of gastric contractility by activating a ghrelin receptor-mediated mechanism involving cholinergic excitatory neurons.
Keywords:
Introduction
Postoperative ileus (POI) is a transient loss of gastrointestinal (GI) motility that occurs following abdominal surgery or other major surgical procedures. Symptoms of POI include abdominal distention, nausea, vomiting, anorexia, and constipation, which can lead to prolonged hospitalization and potentially serious complications.Citation1 The mechanisms causing POI are complex, involving immune and neuronal responses resulting in delayed gastric emptying and impaired propulsive motility of the bowel.Citation2,Citation3 In addition, opioid drugs used for pain management contribute significantly to delayed GI transit following abdominal surgery.Citation4
Ghrelin is an important orexigenic peptide that exerts gastroprokinetic effects and represents a novel therapeutic approach for treating GI motility disorders.Citation5–Citation7 Recently, a number of agents that mimic the activity of endogenous ghrelin have been proposed as treatments of POI. For example, TZP-101, a small molecule with potent binding affinity and full agonist activity at the human recombinant ghrelin receptor (GRLN-R), is now in clinical development for the treatment of gastroparesis and associated symptoms.Citation8–Citation10 GRLN-R has been previously identified as an orphan receptor referred to as the growth hormone releasing peptide (GHRP)-receptor or growth hormone secretagogue receptor because its activation by synthetic small peptide compounds resulted in the release of growth hormone from the pituitary gland.Citation11 The GI prokinetic effects of ghrelin mimetics have been demonstrated in experimental models and humans.Citation12,Citation13 Additionally, in ex vivo models, ghrelin and other ghrelin mimetics have been shown to stimulate smooth muscle contractility in multiple isolated GI preparations.Citation14–Citation16
In a rodent model of POI, we previously found that ipamorelin, which selectively stimulates GRLN-R without significantly affecting plasma adrenocorticotropic hormone and cortisol levels,Citation17 caused a dose-dependent decrease in the time to the first bowel movement and increases in cumulative fecal pellet output following abdominal surgery.Citation18 Although ipamorelin was shown to have these effects in the lower GI tract, the effect of the compound on more proximal regions of the GI tract are unknown. Therefore, the goal of the current study was to investigate whether ipamorelin affects the upper GI tract. Specifically, we examined whether the compound accelerates gastric emptying in a rodent model of gastroparesis induced by abdominal surgery and intestinal manipulation. We also aimed to investigate potential mechanisms by which ipamorelin can normalize the contractility of GI smooth muscle. Currently, both central and peripheral mechanisms are thought to be involved in the stimulation of GI motility by ghrelin and ghrelin mimetics. Central vagovagal reflex pathways likely play an important role in the promotility effects of ghrelin;Citation19–Citation22 a recent study proposed that down-regulation of the ghrelin receptor may account for delayed intestinal transit following vagotomy.Citation23 The prokinetic effects of ghrelin may also involve activation of the enteric nervous system. To support a peripheral neural mechanism, studies have identified ghrelin receptors on enteric neurons.Citation24 Moreover, in isolated smooth muscle from the rat fundus, ghrelin agonists decreased the “on” relaxation and enhanced a cholinergically mediated “off” contraction that had been previously unmasked by the addition of a nitric oxide synthase blocker, L-N (G) Nitroarginine methyl ester (L-NAME).Citation14,Citation15 Taken together, these observations suggest that, at a peripheral level, ghrelin interacts in the stomach with cholinergic and nitrergic neurons to enhance gastric smooth muscle contractility and stimulate GI transit.
Materials and methods
Animals
Experiments were performed using adult male Sprague-Dawley rats (225–275 g) obtained from Charles River (Wilmington, MA). The animals were purchased with indwelling catheters implanted in the right jugular vein for administration of drugs or vehicle. To prevent blockage, the catheters were gently flushed with 0.5 mL heparinized saline every 3–4 days. An acclimation period of at least 1 week was allowed prior to the experiments. All rats were single-housed with free access to food and water at 21°C–23°C and a 12-hour light/dark cycle. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Oklahoma Health Sciences Center (IACUC protocol # 09-046-H).
Abdominal surgery and intestinal manipulation
POI was induced by a surgical procedure described as “running of the bowel.”Citation25 Rats were fasted overnight (16–18 hours), anesthetized with isoflurane (2%–5% to effect), the abdomen was shaved, and the area was treated with alcohol followed by Betadine® (povidone-iodine ; Webster Vet Supplies Inc., Bessemer, AL) for disinfection, and a midline incision was made to expose the viscera. Running of the bowel was performed using two saline-soaked cotton-tipped applicators and gently massaging the bowel (stomach to distal colon) for 5 minutes. Upon completion of this procedure, the GI organs were covered with saline-soaked gauze for an additional 10 minutes. At the end of the surgery, the small intestine and the cecum were returned to the abdominal cavity and the incision was closed with running silk sutures. The surgical procedure lasted 25–30 minutes and was performed between 7 and 9 am.
Evaluation of gastric emptying and intestinal transit
Conscious rats received an intragastric gavage of 1.5 mL of 99mTc in 1.5% methylcellulose solution in distilled water. 99mTc radioactivity was adjusted to approximately 100,000 counts per minute (cpm). Having received the radioactive meal, the animals were placed in a wire-bottom cage without access to food or water. After 15 minutes the animals were euthanized by CO2 inhalation, the abdomen was opened, and the stomach was clamped using a single silk ligature at the esophageal junction and two parallel ligatures between the pyloric junction and the duodenum. The stomach was isolated and gastric emptying was assessed via the amount of radioactivity remaining in the stomach. Sections of the small intestine, each 10 cm in length, were separated, and transit was assessed by the geometric center calculated as a function of the amount of radioactivity in each intestinal segment. The geometric center = ∑ (% of total radioactivity per segment × number of segment)/100 with a geometric center of 1 representing the duodenum, 2–8 the jejunum, and >8 the ileum. The distance traveled by the head of the radiolabeled meal along the length of the small intestine was also determined as previously described.Citation25,Citation26
Evaluation of gastric muscle contractility
Circular muscle strips with the mucosa removed were isolated from the gastric fundus taken from normal controls and POI rats. Muscle strips were suspended vertically in organ baths (Radnoti Glass Technology Inc, Monrovia, CA) under optimal stretch (0.75 g), and isometric contractile activity was recorded using a force transducer attached to a MacLab Data Acquisition System (ADInstruments Inc, Colorado Spring, CO). The organ bath contained warmed and oxygenated (37°C) Krebs solution with the following composition: NaCl (120 mM), KCl (6 mM), MgCl2 (1.2 mM), NaH2PO4 (1.2 mM), CaCl2 (2.5 mM), NaHCO3 (14.4 mM), and glucose (11.5 mM). After 1 hour of equilibration, the contractile responses to acetylcholine (ACh; 100 µM) and electrical field stimulation (EFS: 0.5 ms pulse duration, 5–32 Hz, 1 ms pulse duration applied at 10s interval at 6 V) were assessed before and following ipamorelin (1 µM), ghrelin (1 µM), or the vehicle control administration. The concentration of 1 µM was selected based on a previous in vitro study with ipamorelinCitation17 and upon previous studies in which the efficacy of ipamorelin was found to be similar to that of GHRP.Citation17,Citation18 In separate experiments, atropine (1 µM) and tetrodoxin (TTX, 1 µM) were used to block the contractile effects of ACh and EFS, respectively.
Test and control articles
The test compound, ipamorelin free-base (lot #514356; Albany Molecular Research, Inc, Albany, NY) was provided by Helsinn Healthcare, Inc (Bridgewater, NJ). Since the solubility of ipamorelin is very low, 2 molar equivalents of glacial acetic acid are required to produce a soluble ipamorelin. Stock solutions of 0.5 mg/mL were prepared daily in sterile saline plus glacial acetic acid (0.1 µL/mL) to bring ipamorelin into solution (pH 3–4). The solution was titrated with NaOH to pH 7.0–7.2. Additional dilutions were made in saline. Sterile saline was used in the vehicle control experiments. The ghrelin agonist [D-LysCitation3]-GHRP-6, atropine, and TTX were purchased from Sigma-Aldrich (St Louis, MO) and dissolved in saline.
Experimental design and protocol
Following an overnight fast, the rats were anesthetized and subjected to a laporotomy and manipulation of the bowel surgically that was timed to last 30 minutes. Experiments to evaluate the ability of ipamorelin to reverse the effects of abdominal surgery and intestinal manipulation involved a single administration of the compound. The doses of ipamorelin (0.014 and 0.14 µmol/kg) were selected based upon data obtained in the same experimental model and to explore a wide dose range.Citation18 Both the test and vehicle control were administered as a bolus iv infusion at a volume of 0.2 mL/100 g body weight via the jugular catheter and immediately followed by the intragastric gavage of 1.5 mL 99mTc-labeled methylcellulose meal. The rats were then placed in clean wire-bottom cages (no food or water was supplied) and euthanized via CO2 inhalation 15 minutes after the oral gavage. For the in vivo studies, the stomach and small intestine were isolated for measurement of GI transit. For the in vitro studies, the gastric fundus was isolated and placed in organ baths to record smooth muscle contractility.
Data and statistical analysis
The data are expressed as the mean ± standard error of the mean for each group. Differences between groups were assessed for statistical significance by Student’s t-test, as well as by one-way or two-way analysis of variance followed by Bonferroni’s test for multiple comparisons where appropriate. A level of P < 0.05 was considered significant. The statistical procedures were performed using GraphPad Prism Version 4 software (GraphPad Software Inc, San Diego, CA).
Results
Effect of ipamorelin on delayed gastric emptying in vivo
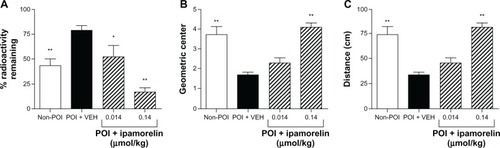
shows that, in a rodent model of POI, induced by abdominal surgery and manipulation of the bowel, gastric empting was significantly delayed. Specifically, we found that, compared to non-POI surgery controls (n = 10), “running of the bowel” induced a postsurgical delay in gastric emptying in vehicle-treated rats (n = 10), as measured by the increase in the amount of radioactivity remaining in the stomach. We then investigated the effect of ipamorelin administered intravenously at doses of 0.014 and 0.14 µmol/kg on the delay in gastric emptying produced by abdominal surgery and intestinal manipulation. As illustrated in , ipamorelin (0.014 µmol/kg iv, n = 5) decreased the amount of radioactivity remaining in the stomach to a level resembling the non-POI surgery control rats. The higher dose of ipamorelin (0.14 µmol/kg iv, n = 8) had a greater effect on the delay in gastric emptying induced by POI with less than 25% of the radioactivity remaining in the stomach after 15 minutes.
Figure 1 Effect of ipamorelin in a rat model of POI. Compared to non-POI control rats, abdominal surgery in VEH-treated rats increased the amount of radioactivity remaining in the stomach, decreased the geometric center, and decreased the distance traveled by the head of the radiolabeled meal. Ipamorelin administered intravenously decreased the amount of radioactivity remaining in the stomach (A), increased the geometric center (B), and increased distance traveled by the head of meal (C).
Abbreviations: POI, postoperative ileus; VEH, vehicle.
Effect of ipamorelin on intestinal transit in vivo
In the current study, we investigated the effect of ipamorelin on small intestinal transit in vivo. We found that in the non-POI controls the majority of the test meal traveled to the distal small intestine, whereas following abdominal surgery and intestinal manipulation, most of the test meal remained in the proximal small intestine. Specifically in vehicle-treated rats, POI caused a significant slowing of small intestinal transit as assessed as demonstrated by a decrease in the geometric center () and a decrease in the distance traveled along the small intestine by the head of the meal compared to nonsurgery controls (). In rats treated with the highest dose of ipamorelin (0.14 µmol/kg iv), there was a reversal of the POI-induced delay in upper GI transit to values that resembled non-POI surgery controls ().
Effects of ipamorelin on contractility in vitro
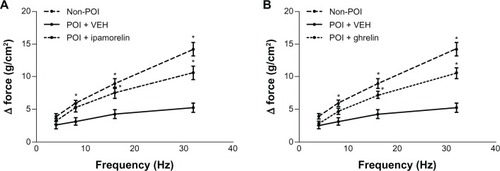
After demonstrating that ipamorelin reverses the delay in gastric emptying induced by abdominal surgery and intestinal manipulation in vivo, we designed a series of in vitro experiments using tissue isolated from the gastric fundus to investigate the potential mechanisms by which ipamorelin accelerates gastric emptying. As shown in , we found that following abdominal surgery and intestinal manipulation, there was a marked inhibition of the smooth muscle contractile responses to cholinergic stimulation with ACh at 100 µM compared to that observed in isolated gastric fundic smooth muscle preparations from nonsurgery control rats. The ACh-induced contractile response was completely antagonized by the muscarinic antagonist, 1 µM atropine (data not shown). shows that, in the gastric fundus isolated from rats with POI, both ipamorelin and ghrelin normalized the contractile response to ACh. We also found that the neurally mediated contractile responses induced by EFS of the enteric nerves within the gastric fundus were significantly inhibited in POI rats compared to nonsurgery controls (). These neurally induced contractile responses were completely blocked by the addition of TTX (1 µM), a potent neurotoxin that binds to voltage-gated fast Na+ channels in nerve membranes (data not shown). also shows that, in the gastric fundus isolated from rats with POI, both ipamorelin and ghrelin normalized the contractile response to EFS.
Figure 2 Effect of ipamorelin (1 µM) and ghrelin (1 µM) on gastric smooth muscle contractility induced by ACh at 100 µM isolated from a rat model of POI.
Abbreviations: ACh, acetylcholine; POI, postoperative ileus; VEH, vehicle.
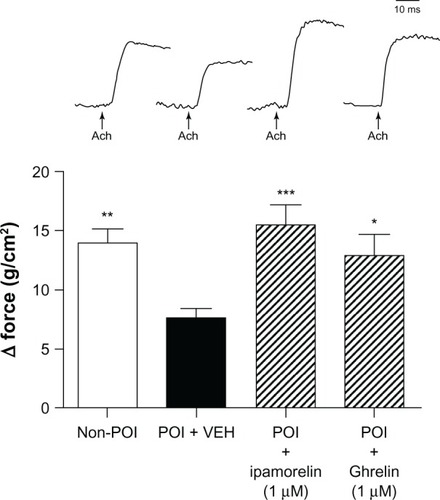
Figure 3 Effect of ipamorelin (1 µM) (A) and ghrelin (1 µM) (B) on gastric smooth muscle contractility induced by EFS in a rat model of POI.
Abbreviations: EFS, electrical field stimulation; POI, postoperative ileus; VEH, vehicle.
Discussion
Delayed gastric emptying, also known as gastroparesis or gastric stasis, occurs when the coordinated motility of the stomach is dysregulated and food is retained in the stomach, leading to symptoms of early satiety, nausea, vomiting, and abdominal pain. Based on the vast body of literature published on the prokinetic effects of ghrelin mimetics,Citation18,Citation26,Citation27 the aim of the current study was to investigate whether ipamorelin, a synthetic ghrelin mimetic that has specific growth hormone-releasing properties but does not increase plasma levels of adrenocorticotropic hormone or cortisol,Citation17,Citation28 has effects on upper GI tract dysmotility using a rodent model of POI transit induced by abdominal surgery and manipulation of the bowel.Citation25 The rat model of POI is commonly used to investigate the efficacy of new therapeutic treatments as well as the pathogenic mechanisms relevant to the postsurgical development of GI dysfunction in humans. Analysis of our data confirmed that abdominal surgery and manipulation of the bowel caused a marked delay in gastric emptying and upper GI transit in a rodent model. One of the major results in this study was the finding that administration of ipamorelin resulted in a significant acceleration of the test meal leaving the stomach, with the level of gastric emptying produced by ipamorelin virtually identical to that measured in non-surgical control animals. Similarly, prokinetic effects of ipamorelin were also observed in the small rat intestine. Following abdominal surgery and intestinal manipulation, intravenous administration of ipamorelin increased the geometric center and the distance traveled by the radiolabeled test meal along the length of the small intestine.
Our in vivo findings are consistent with those of previous studies in conscious rats where ghrelin, GHRP-6, and a series of small molecule synthetic selective ghrelin receptor agonists, accelerated gastric empting and enhanced small bowel transit in a rodent model of POI.Citation14,Citation26,Citation29 Moreover, these findings with ipamorelin extend the results of our previous studies, which demonstrated that ipamorelin after single and repeated administration reduces the time to first bowel movement in a rodent model of delayed GI transit.Citation18 Since our current results suggest that ipamorelin accelerates gastric emptying and promotes small intestinal function, our findings support the feasibility and potential benefits of using GRLN-R agonists as a new class of prokinetics accelerating gastric emptying. Furthermore, taken together with our previous observation that ipamorelin enhances colonic motility, our results suggest that this compound may possess a therapeutic advantage relevant to the pathophysiological heterogeneity of the patient population.
In the current study, we attempted to elucidate the mechanism(s) responsible for the prokinetic effects of ipamorelin. Specifically, we focused our investigation on the mechanisms by which ipamorelin accelerates gastric emptying in a rodent model of POI. In isolated preparations of gastric smooth muscle from rats that had undergone abdominal surgery and manipulation of the bowel, we examined the contractility of the muscle in response to cholinergic stimulation and electrical stimulation of the myenteric plexus. Following abdominal surgery and manipulation of the bowel, there was a marked inhibition of the smooth muscle contractile responses in tissue isolated from the gastric fundus in response to ACh and EFS compared to control tissue. The ACh-induced contractile response was found to be atropine-sensitive whereas the increase in contractility to EFS was sensitive to neuronal blockade with TTX, suggesting a neuronal origin for this response. In addition to demonstrating that POI leads to marked abnormalities not only in vivo but also in isolated smooth muscle contractility, another major finding of our study was that ipamorelin normalized the contractile response to ACh and EFS in the gastric fundus. Additionally, we found that the effects of ipamorelin on smooth muscle contractility were mimicked by ghrelin, suggesting that ipamorelin, a potent ghrelin agonist, is effective in an experimental model of delayed gastric emptying by stimulating motility in the stomach though GRLN-receptor mediated mechanisms located on cholinergic neurons.
Immune-mediated mechanisms have also been shown to play a pivotal role in the development of ileus,Citation2,Citation3 and a compound with prokinetic ability and anti-inflammatory effects may prove useful for the treatment of POI. Although not investigated in the current study, the possibility exists that ipamorelin, in addition to acting as a gastroproketic, may also possess anti-inflammatory effects. In support of this claim, recent studies in lipopolysaccharide-treated mice found that ghrelin inhibited lipopolysaccharide-induced inducible nitric oxide synthase expression in the GI tract and lowered overproduction of NO in the plasma, suggesting that ghrelin may downregulate the NO pathway in the GI tract to improve small intestinal transit.Citation31
In summary, we have shown for the first time in vivo that ipamorelin significantly accelerates gastric emptying in a rodent model of gastroparesis induced by abdominal surgery. In tissue isolated from the gastric fundus, ipamorelin normalized the contractile response to cholinergic neural stimulation. These results suggest that ipamorelin improves delayed gastric emptying by stimulating gastric contractility via GRLN receptor-mediated mechanisms located on cholinergic neurons. In conclusion, our study highlights the profound effects of ipamorelin on the upper GI tract and suggests that the compound may be of interest as a prokinetic agent to treat GI dysmotility, characterized by delayed gastric emptying and slow upper GI transit.
Disclosure
The authors report no conflicts of interest in this work.
References
- SenagoreAJPathogenesis and clinical and economic consequences of postoperative ileusAm J Health Syst Pharm20076420 Suppl 13S3S717909274
- de JongeWJvan den WijngaardRMPostoperative ileus is maintained by intestinal immune infiltrates that activate inhibitory neural pathways in miceGastroenterology200312541137114714517797
- BauerAJBoeckxstaensGEMechanisms of postoperative ileusNeurogastroenterol Motil200416Suppl 2546015357852
- Greenwood-Van MeerveldBEmerging drugs for postoperative ileusExpert Opin Emerg Drugs200712461962617979603
- CharoenthongtrakulSGiulianaDLongoKAEnhanced gastrointestinal motility with orally active ghrelin receptor agonistsJ Pharmacol Exp Ther200932931178118619252061
- De SmetBMitselosADepoortereIMotilin and ghrelin as prokinetic drug targetsPharm Ther20091232207233
- Greenwood-Van MeerveldBKriegsmanMNelsonRGhrelin as a target for gastrointestinal motility disordersPeptides201132112352235621453735
- LasseterKCShaughnessyLCummingsDGhrelin agonist (TZP-101): safety, pharmacokinetics and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human studyJ Clin Pharmacol200848219320218199894
- EjskjaerNDimcevskiGWoJSafety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled studyNeurogastroenterol Motil201022101069e28120524987
- WoJMEjskjaerNHellstromPMRandomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting – randomized clinical study subset dataAliment Pharmacol Ther201133667968821214610
- DavenportAPBonnerTIFoordSMInternational Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and functionPharmacol Rev200557454154616382107
- ArigaHTsukamotoKChenCMantyhCPappasTNTakahashiTEndogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomachNeurogastroenterol Motil200719867568017640183
- TackJDepoortereIBisschopsRInfluence of ghrelin on interdigestive gastrointestinal motility in humansGut200655332733316216827
- DepoortereIDe WinterBThijsTDe ManJPelckmansPPeetersTComparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitroEur J Pharmacol20055151–316016815890336
- KitazawaTDe SmetBVerbekeKDepoortereIPeetersTLGastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitroGut20055481078108415843418
- XuLDepoortereITomasettoCEvidence for the presence of motilin, ghrelin, and the motilin and ghrelin receptor in neurons of the myenteric plexusRegul Pept20051241–311912515544849
- RaunKHansenBSJohansenNLIpamorelin, the first selective growth hormone secretagogueEur J Endocrinol199813955525619849822
- VenkovaKMannWNelsonRGreenwood-Van MeerveldBEfficacy of ipamorelin, a novel ghrelin mimetic, in a rodent model of postoperative ileusJ Pharmacol Exp Ther200932931110111619289567
- MasudaYTanakaTInomataNGhrelin stimulates gastric acid secretion and motility in ratsBiochem Biophys Res Commun2000276390590811027567
- FujinoKInuiAAsakawaAKiharaNFujimuraMFujimiyaMGhrelin induces fasted motor activity of the gastrointestinal tract in conscious fed ratsJ Physiol2003550Pt 122724012837928
- SakataIYamazakiMInoueKHayashiYKangawaKSakaiTGrowth hormone secretagogue receptor expression in the cells of the stomach-projected afferent nerve in the rat nodose ganglionNeurosci Lett2003342318318612757895
- NakamuraTOnageTKitizawaTGhrelin stimulates gastric motility of the guinea pig through activation of capsaicin-sensitive neural pathway: in vivo and in vitro functional studiesNeurogastroenterol Motil200922444645219840269
- YangCGQiuWCWangZGYuSYanJZhengODown regulation of ghrelin receptors in the small intestine delay small intestinal transit in vagotomized ratsMol Med Report20114610611065
- DassNBMunonyaraMBassilAKGrowth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelinNeuroscience2003120244345312890514
- KalffJCSchrautWHSimmonsRLBauerAJSurgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileusAnn Surg199822856526639833803
- VenkovaKFraserGHoveydaHRGreenwood-Van MeerveldBProkinetic effects of a new ghrelin receptor agonist TZP-101 in a rat model of postoperative ileusDig Dis Sci20075292241224817436082
- PeetersTLPotential of ghrelin as a therapeutic approach for gastrointestinal motility disordersCurr Opin Pharmacol20066655355817011824
- Jiménez-ReinaLCañeteRde la TorreMJBernalGInfluence of chronic treatment with the growth hormone secretagogue Ipamorelin, in young female rats: somatotroph response in vitroHistol Histopathol200217370771412168778
- TrudelLTomasettoCRioMCGhrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in ratAm J Physiol Gastrointest Liver Physiol20022826G948G95212016119
- PoitrasPPolvinoWJRocheleauBGastrokinetic effect of ghrelin analog RC-1139 in the rat. Effect on post-operative and on morphine induced ileusPeptides20052691598160116112398
- ChenYTTsaiSHSheuSYTsaiLHGhrelin improves LPS-induced gastrointestinal motility disturbances: role of NO and prostaglandin E2Shock201033220521219503023