Abstract
Background
The diuretic activity of the Cucumis dipsaceus leaf, which is used in indigenous medicine, has been claimed but has not yet undergone scientific evaluation.
Objective
The objective of this study was to assess the diuretic activity of the aqueous and 80% methanol extracts derived from the leaves of Cucumis dipsaceus in rats.
Methods
For the extraction process, the maceration technique was employed to obtain the aqueous and 80% methanol extracts from the Cucumis dipsaceus leaves. Male rats were then divided randomly into eight groups, with six rats in each group. These groups consisted of a negative control group, a positive control group, and three different groups for each extract at varying doses. The urine output volumes, the concentrations of urinary electrolytes (sodium, potassium, and chloride) and urinary pH, were measured and analysed to compare the results among the different groups.
Results
Both the aqueous and 80% methanol extracts of Cucumis dipsaceus leaves demonstrated a significant increase in urinary output at doses of 200mg/kg body weight (p<0.01) and 400mg/kg body weight (p<0.001). When comparing the urinary electrolyte excretion with the negative control group, the groups treated with the 400mg/kg body weight dose of the aqueous extract showed significant differences in the urinary excretion of sodium (p<0.05), chloride (p<0.01), and K+ (p<0.01). Similarly, the urinary excretion of K+ and Cl- also exhibited significant differences at moderate doses (K+: p<0.01, Cl-: p<0.05) and the highest doses (both: p<0.01) of the 80% methanol extract. Furthermore, the highest doses of both the aqueous (p<0.01) and 80% methanol (p<0.01) extracts demonstrated significant differences in saluretic effect.
Conclusion
Both crude extracts of C. dipsaceus leaves have significant diuretic activity, providing support for the traditional use of the plant as a diuretic agent.
Introduction
Diuretics are medications that cause the kidney to excrete more sodium and water from the body. Their primary effect is to reduce the reabsorption of sodium and chloride from the filtrate, resulting in increased water loss due to salt excretion. This can be accomplished by either directly acting on nephron cells or indirectly modifying the filtrate content.Citation1 In technical terms, “natriuresis” refers to an increase in renal sodium excretion, whereas “diuresis” indicates an increase in urine volume. Important natriuretic medications are typically referred to as diuretics because they typically also increase water excretion; the increase in sodium excretion is presumed.Citation2
The primary action of most diuretics is to hinder the reabsorption of sodium in different segments of the renal tubular system or a combination of these locations.Citation3 The mechanisms of different types of conventionally used diuretics revolve around specific membrane transport proteins present in renal tubular epithelial cells. For instance, loop diuretics like furosemide, torsemide, azosemide, bumetanide, and ethacrynic acid block the Na+/K+/2Cl− co-transporter mainly in the medullary and thick ascending loop of Henle. This action leads to reduced reabsorption of Na+, K+, and Cl− from the urine, resulting in increased excretion of sodium and water.Citation4,Citation5 Thiazides, such as hydrochlorothiazide, chlorthalidone, indapamide, metolazone, and chlorothiazide, exert their diuretic effect by blocking the apical Na+/Cl− co-transporter (NCC) in the cortical thick ascending limb and early distal convoluted tubules (DCT). However, thiazides are generally less potent diuretics compared to loop diuretics.Citation2,Citation6 Potassium-sparing diuretics primarily act in the cortical part of the collecting duct and to a lesser extent in the late distal and collecting tubules. They achieve this by either blocking mineralocorticoid receptors, such as spironolactone and eplerenone, which are competitive antagonists of aldosterone or by blocking epithelial Na+ channels in the luminal/apical membrane of the late distal tubule and collecting duct, as seen with amiloride and triamterene. These agents have a limited ability to promote sodium excretion due to the small amount of sodium reabsorbed in these areas.Citation6,Citation7
Other diuretics, such as osmotic diuretics, including Mannitol, urea, glycerin, and isosorbide, are substances that freely pass through the glomerulus and are not easily reabsorbed. They have minimal pharmacological activity.Citation1 Their osmotic properties hinder the passive reabsorption of water and reduce sodium reabsorption by lowering the concentration of sodium in the tubular fluid.Citation8 Carbonic anhydrase inhibitors, such as acetazolamide and methazolamide, function by blocking the enzyme CA in the proximal convoluted tubule (PCT). This interference disrupts bicarbonate (HCO3−) reabsorption, leading to increased excretion of Na+, HCO3−, and water in the urine.Citation9 Naturally occurring diuretics like caffeine inhibit the reabsorption of Na+, and alcohol inhibits the secretion of anti-diuretic hormone (ADH).Citation10
Even, in the absence of overt oedema, patients with nephropathy or heart failure may have a 10 to 30% increase in extracellular and blood volume.Citation11 The use of a diuretic to prevent cardiovascular complications is critical in the treatment of arterial hypertension. Diuretics cause a decrease in blood pressure, a decrease in blood volume, and a decrease in resistance to blood flow by increasing the drainage of water and sodium into the urine.Citation12 In the vast majority of hypertensive patients, they lower both systolic and diastolic blood pressure. They are as effective as the majority of other antihypertensive medications.Citation13 Diuretics are used alone or in combination with other antihypertensive medications to treat hypertensive patients.Citation14 Thiazides are commonly prescribed in conjunction with other drugs for hypertension. Loop diuretics are primarily reserved for patients with renal failure, resistant hypertension, or heart failure.Citation15 In several poisoning conditions, high ceiling and osmotic diuretic agents are also used to increase poisoning agent excretion.Citation16,Citation17
Several countries use natural products derived from plant extracts/fractions as the best source of medicine/novel therapeutic agents for various infectious and degenerative diseases, and plants are the source of the most potent drugs.Citation18 Various plant parts (root, stem, flower, fruit, twig exudates, and modified plant organs) are used in herbal medicines. To use these plants, which are collected on a small scale by local communities and folk healers, numerous other plants are collected in large quantities as raw material for herbal industries.Citation19
Medicinal plants have long been used in the treatment of some renal diseases and have been widely used as diuretics since time immemorial.Citation20,Citation21 Several plants used in ethnomedicine have been shown to have diuretic activity in animal models.Citation22 The safety and efficacy of these plants for their claimed medicinal use, on the other hand, have not been thoroughly studied and will require further investigation. Traditional healers’ preparation techniques are generally not standardized and, in most cases, do not meet the requirements of good manufacturing practice.Citation23
A growing number of studies claim diuretic effects from traditional medicines.Citation22 Pharmacological research on some traditional medicinal plants has supported their folkloric use as diuretics in Ethiopia. Some of the plants showing promise in this regard include: Carissa edulis,Citation24,Citation25 Rumex abyssinicus,Citation26 Ajuga remota,Citation27 Thymus serrulatusCitation28 and Moringa stenopetala.Citation29,Citation30
Cucumis dipsaceus C.G. Ehrenb ex Spach is an annual climbing and flowering plant in the genus Cucumis L., family Cucurbitaceae. It originated in Ethiopia and is cosmopolitan in distribution (Africa (Ethiopia, Kenya, Somalia, Tanzania, Uganda, Sudan, and Southern Egypt) and Asia: India, with approximately 52 species worldwide.Citation31,Citation32 It is known by several common names, including “teasel gourd”, “Arabian cucumber”, and “hedgehog”. Cucumis has traditionally been used to treat a variety of ailments. Cucumis dipsaceus leaves are typically consumed as a leafy vegetable;Citation33 its fruit juice is topically applied to prevent hair loss.Citation34 The poultice is prepared from leaves and tendrils for the treatment of wounds. In case of poisoning, fruit juice acts as an antidote.Citation35 Its stem decoction is used to treat nausea. Its fruit is used to treat gastrointestinal disorders such as diarrhea, stomach pain, constipation, and meningitis. Hepatitis, local application, snakebite, carnivore bite, and gallstones are all treated with the roots. Fresh leaf extract is used to treat hemorrhoids and rabies. The seeds are diuretic; the fruit juice is used as a nutritive and as a demulcent in anti-acne lotions.Citation36
Another study on the antioxidant activity of Cucumis dipsaceus revealed that both aqueous and methanol extracts of the plant exhibited significant reducing capacity. In a study assessing their impact on nitric oxide production, both extracts were examined for their ability to inhibit the release of nitric oxide. The methanolic extract displayed superior free radical scavenging activity compared to the aqueous extract when exposed to NO-induced free radical release.Citation37
Cucumis dipsaceus Ehrenb. C.G. Ex SpachCitation38 with a vernacular name of “Hare Goge” (Afan Oromo) or “Yewef Hareg” (Amaharic) is claimed to be for the treatment of gonorrhea, urinary retention and skin fungus in indigenous system of medicine. To achieve diuresis and treat urinary retention, traditional healers gather fresh leaves, crush them, and then take the resulting filtrate orally. There is no scientific study on the diuretic activity of the plant to date. It is necessary to investigate those plants scientifically which have been used in traditional medicine to improve the quality of natural medicines. Hence, the aim of present study was to evaluate the diuretic activity using the aqueous and 80% methanolic extract of Cucumis dipsaceus leaves.
In clinical practice, diuretics such as mannitol, thiazides, furosemide, and ethacrynic acid are used. However, the majority of them are associated with a wide range of undesirable effects, such as ototoxicity, electrolyte abnormalities (hypokalemia, hyperuricemia, hypercalcemia, hypomagnesemia, and hyponatremia), acid-base imbalance, metabolic abnormalities (hyperglycemia and hyperlipidemia), and acute hypovolemia,Citation39 Photosensitivity dermatitis and hypersensitivity.Citation40 Due to these reasons, still there is a search for more effective and less toxic diuretic agent. Many indigenous drugs have been claimed to have diuretic properties in the Ayurvedic medical system, but they have not been thoroughly investigated.Citation41 As a result, there is a need to investigate comparatively more effective and less toxic diuretics derived from local plant materials.
Materials and Methods
Materials
Chemicals and Drug
During the experiment, distilled water (Ethiopian pharmaceutical manufacturing, Ethiopia) and furosemide (Kawasan perindustrian Bandar Baru Bangi, 43000 Kajang, Selangor Darul Ehsan, Malaysia) were used as the positive and negative controls, respectively. The plant was extracted using absolute methanol (Carlo Erba, France) [which diluted to 80% using distilled water for extraction purpose], distilled water, and NS (Aculife Health Care, India).
Experimental Animals
Adult healthy Swiss albino rats, either male (for diuretic activity testing) or female (for acute toxicity testing), weighing 200–300g and aged 6–8 weeks, were used and obtained from the animal house unit of Addis Ababa University’s School of Pharmacy. The animals were kept in polypropylene cages (8–10 animals per cage) for one week to acclimate under standard environmental conditions on a 12h light-dark cycle. The animals had free access to a standard laboratory pellet diet as well as tap water. All animals were fasted for 15 hours prior to the experiment, with water available ad libitum. The animals were cared for and handled in accordance with internationally accepted guidelines for the use of experimental animals.Citation42,Citation43
Methods
Plant Material Collection and Authentication
In April 2018, fresh leaves of Cucumis dipsaceus (Cucurbitaceae) were collected in Dengego Valley, Dengego district, Dire Dawa. The area is 515 kilometers east of Addis Abeba. In December 2017, a small amount of plant leaves was collected for identification and submitted to taxonomists at Addis Ababa University’s National Herbarium, College of Natural Sciences and Computation. Plant identification and authentication were completed, and plant specimens were deposited with a voucher number of 001 for future reference.
Extraction and Preparation of the Plant
After collecting the fresh plant leaves, it was thoroughly washed with distilled water. The plant leaves were then dried for two weeks at room temperature in the shade. It was then finely powdered with a pestle and mortar before being used for extraction.
Preparation of 80% Methanol Extract
In an Erlenmeyer flask, a sample of 250g powdered Cucumis dipsaceus leaves was macerated with 2500 mL of 80% methanol for 72 hours at room temperature. The plant material and solvent were continuously shaken on a horizontal orbit shaker (Stuart, UK) to ensure proper mixing. The extract was filtered first with cotton gauze and then with Whatman filter paper, No. 1, and the marcs were re-macerated once with the same volume of solvent to extract the plant material completely. At 40°C, methanol was extracted from the extract using Rota vapour (Buchi, Switzerland) under reduced pressure. The extract was then dried using a lyophilizer (Operon, Korea) to remove the remaining water, the resulting dried 80% methanol extract of the plant was weighed, and the percentage yield was calculated before being stored in −20°C and reconstituted with distilled water for oral administration immediately before the experiment.
Preparation of Aqueous Extract
In an Erlenmeyer flask, 250 grams of dried powdered Cucumis dispaceus leaves were cold macerated with 2500mL of distilled water. The sample was then allowed to stand at room temperature for 72 hours. To ensure proper mixing, the plant material and solvent mixture were shaken continuously on a horizontal orbit shaker (Stuart, UK). After that, the mixture was filtered through gauze. Marcs from the plant were then re-macerated once more with the same volume of solvent to extract the material completely. To remove water, the filtrate was freeze-dried in a Lyophilizer (Operon, Korea). The plant’s freeze-dried extract was weighed, and the percentage yield was calculated. Finally, it was placed in a plastic vial and refrigerated at −20°C until it was used in the experiment. During the experiment, it was reconstituted with distilled water for oral administration immediately before administration.
Acute Toxicity
The study was conducted using a limit test in accordance with OECD 425 guideline.Citation43 The Limit test was used to determine whether the toxicity of a test substance is greater than or less than a specified dose. Female Swiss albino rats weighing 200–300 g and aged 6–8 weeks were divided into three groups of six animals each in this study. The animals were fasted for 15 hours prior to the experiment, and water was provided ad libitum. The animals were weighed on the day of the experiment, and the negative control group received distilled water, while the other two groups received a limit dose of 2g/kg BW aqueous and 80% methanol extract orally. The animals were carefully observed continuously for the first 4 hours after administration for any obvious signs of toxicity and death, and then for the next 24 hours. They were then kept under close observation for up to 14 days to look for signs of morbidity or mortality. The weight of each animal was then recorded on the seventh and fourteenth days of administration to confirm any weight changes that had occurred.
Screening and Dosing for Diuretic Activity
Using the method described by Kau,Citation44 each extract was tested for diuretic activity. Male Swiss albino rats weighing 200–300g were divided into eight groups of six rats each for the screening of each extract. The animals were housed in a typical metabolic cage. 15 hours before the experiment, food but not water was withheld. The test substance, distilled water, and standard drug were given to the animal based on its weight. Both extracts were first dissolved in distilled water to achieve the required concentrations before being administered orally. Before the experiment, all of the animals were given normal saline (2mL/100g, BW) orally.
As a negative control, Group 1 was given distilled water (20 mL/kg of BW), while Group 2 was given furosemide (10 mg/kg BW), a common diuretic medication. Aqueous extract was given to groups 3 through 5 and 80% methanol extract test substances were given to groups 6 through 8 at dose levels of 100, 200, and 400 mg/kg BW, respectively. After five hours, the total amount of urine excreted was calculated for each group, and the urine was stored at −20°C for later examination. According to MukherjeeCitation45 and Durairaj,Citation46 the parameters considered to compare the effects of the test doses of the extract with the negative control and standard drug, Furosemide, on diuresis were total urine volume, diuretic action, diuretic activity, urinary excretion, saluretic activity, natriuretic activity, and urinary electrolyte concentration of Na+, K+, and Cl−. The volume of urine excreted during the 5 hour study period is expressed as a percentage of the liquid (Distilled water) administered, yielding a measure of “urinary excretion” independent of animal weight, that is, urinary excretion was calculated as total urinary output divided by total liquid administered (Formula-1). The ratio of urinary excretion in the test group to urinary excretion in the control group was used to assess diuretic action (Formula-2). The diuretic action, also known as the “Diuretic Index”, Indices of 1.0 or higher are considered positive effects or potent diuretics. Because the diuretic index is prone to variation, the Lipschitz value, a less variable parameter, was calculated. To calculate the Lipschitz value, the urine volume of the treated rats was compared to that of the control group, which is known as diuretic activity. The diuretic activity was also calculated as the ratio of the test substances’ diuretic action to that of the standard drug (Formula-3).Citation45,Citation47
The ratio of urinary excretion in test group and control group was denoted “Diuretic action”, which will used as the measure of degree of diuresis.
Determination of Urine Excreted and Plant Extracts of Electrolyte Concentration
The experimental, negative control and standard drug-treated groups’ five-hour urine electrolyte concentrations (Na+, K+, and Cl−) were measured. Urinalysis was performed according to the user instruction manual of the Biochemical kits available in the laboratory of the Ethiopian Public Health Institute (EPHI) using the Ion Selective Electrode (ISE) analysis method for chloride and Flame photometry for sodium and potassium (Cobas6000, Roche, Germany). A digital automated pH meter (Sigma-Aldrich, USA) was used to measure the pH of the fresh urine sample.
Parameters considered in urinary Electrolyte excretion include:
Natriuretic activity (Na+/ K+ ratio)
Na+/K+:- Ratio of concentration of sodium ion in the urine of the test group to concentration of potassium ion in the urine of the same group. Estimate Natriuretic activity. Values greater than 2.0 indicate a favorable natriuretic effect and values greater than 10.0 indicate a potassium-sparing effect.
Saluretic index: Is the ratio of concentration of electrolyte in the urine of group to concentration of electrolyte in the urine of control group. The sum of Na+ and Cl− excretion was estimated for saluretic activity.
The ratio [Cl−] / [Na+] + [K+] (ion quotient) in the urine was derived to estimate carbonic anhydrase inhibition. Carbonic anhydrase inhibition can be excluded at ratios between 1.0 and 0.8, with decreasing ratios; slight-to-strong carbonic anhydrase inhibition can be assumed.Citation42
Statistical Analysis
The experimental results were analyzed using the Statistical Package for Social Sciences (SPSS), version 20 software. The study’s results were expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) and the Tukey post hoc test for multiple comparisons were used to determine statistical significance. Additionally, a significance threshold of P < 0.05 was applied.
Ethical Approval
The experimental protocol for the use of laboratory animals was approved by Institutional Review Committee of the College of Veterinary Medicine and Agriculture, Addis Ababa University with reference No.VM/ERC/01/05/16/2024; in accordance of Animal Research Ethics Guideline: based on Directive 2010/63/EU of the European Parliament and of the council on the protection of animals used for scientific purpose.
Result
Acute Toxicity Test
In the acute toxicity study, rats were observed for two weeks after oral administration of the test substances at a dose of 2g/kg body weight to see if the extracts had a toxic effect. All of the rats survived even after 14 days with progressive weight gain. This means that both extracts were found to be safe up to the highest dose tested. This was demonstrated by the absence of major toxicity signs such as tremors, weight loss, lethargy, paralysis, stress, or adverse behaviours, as well as the absence of diarrhoea. During this time period, no treated rats died. This indicates that the LD50 is greater than 2g/kg.
Diuretic Activity
Effect on Urine Volume
Aqueous Extract
All aqueous crude extract treated groups had increased urinary volume excretion beginning with the first hour and increasing significantly by the fifth hour, as shown in , for the lowest dose (AQ100) 77.66%, medium dose (AQ200) 97.72%, and highest dose (AQ400) 120.94%. However, when compared to NC, AQ100 had no significant effect on diuresis. When compared to NC, the AQ200 treated group had a significant difference in urinary excretion at the third hour (83.80%, p<0.05) and fifth hour (84.30%, p<0.01), but increased insignificantly by 80.23% at the fourth hour. Furthermore, as shown in , rats treated with the maximum dose (AQ400) had an increased diuresis beginning with the first hour of urine collection, but a significant diuresis began with the second hour (110.71%, p <0.01) and continued to the fifth hour (132.26%, p<0.001) when compared to NC.
Table 1 Urinary Output at Different Time Intervals After Oral Administration of Aqueous Crude Extract of Cucumis Dipsaceus Leaves in Rats
Furosemide-treated rats produced significantly more diuresis and showed a significant difference beginning at the first hour (316.67%, p<0.05) and continuing until the end of the fifth hour (132.26%, p<0.001) when compared to NC. Starting from the third hour to the end of the fifth hour, the standard drug SF10 had a significantly greater diuretic effect than AQ100 (p<0.05), but a comparable significant diuretic effect with AQ400 (p <0.001).
The diuretic activity of AQ400 was 0.94 when compared to the standard drug SF10, as indicated in . This result also reveals that their diuretic effects are comparable. In contrast, AQ100 and AQ200 had lower diuretic activities of 0.60 and 0.76, respectively.
The highest dose, AQ400, produced greater diuresis than AQ100 and this difference was significant by the end of the fifth hour (p<0.01) when the three doses of the aqueous extracts treated groups were compared to each other. As compared to AQ100, the SF10 significantly increased (p<0.01) in diuresis starting in the second hour when the aqueous extract-treated group was compared to the standard.
80% Methanol Extract
demonstrates that, at the lowest dose (HM100), medium dose (HM200), and highest dose (HM400) at the fifth hour, the crude 80% methanol extract increased urinary volume excretion almost as effectively as the aqueous extract. However, the onset of significant diuresis was less pronounced than with the aqueous extract. While the diuresis for the medium dose (HM200) was statistically significant at the third hour (82.12%, p<0.05) and fifth hour (88.17%, p<0.01), it increased insignificantly by 74.29% at the fourth hour when compared to NC. Furthermore, compared to NC, the highest dose of HM400 caused a significant diuresis beginning at the end of the third hour (94.13%, p<0.01) and continuing until the end of the fifth hour (112.90%, p<0.001). In the fifth hour, the lowest dose, HM100, increased diuresis by 63.01% without significantly differentiating from NC. The lowest dose (HM100) treated group at the third hour produced a significantly (p<0.01) lower diuretic effect as compared to the standard, SF10, as a result of the 80% methanol extract treated groups. On the other hand, when the three doses were increased, the diuretic activity increased by 0.70, 0.82, and 0.90, respectively.
Table 2 Urinary Output at Different Time Intervals After Oral Administration of 80% Methanol Crude Extract of Cucumis Dipsaceus Leaves in Rats
Effect on Electrolyte
Aqueous Extract
Urine samples were taken over a period of five hours, and the electrolyte content of Na+, K+, and Cl− was determined, as indicated in . The medium dose (AQ200), despite being raised by 55.10%, did not result in a statistically significant difference when compared to the NC, while the lowest dose (AQ100) in sodium loss was equal to that of the NC. Urinary sodium loss was significantly different at the highest dose (AQ400) from NC (105.10%; p<0.05). Comparing SF10 to NC, the standard drug-treated group showed the greatest urinary Na+ loss (122.45%; p<0.01).
Table 3 Effect of Aqueous Crude Extract of Cucumis Dipsaceus Leaf on 5h Urinary Electrolyte Excretion and Other Parameters in Rats
Comparing the highest dose, AQ400, to NC, urinary K+ excretion revealed a statistically significant kaliuresis effect (210.10%; p<0.01). Comparing the lowest and medium treatment groups to NC, there was a slight increase in potassium loss of 59.58% and 76.50%, respectively. Along with having the lowest effect on potassium excretion when compared to groups treated with aqueous extract, the group treated with standard medication also demonstrated a 41.62% increase in potassium loss when compared to NC.
When urinary Cl− losses were present, AQ400 increased by 177.30% (p <0.01). When compared to NC, AQ100 (36.68%) and AQ200 (85.66%) had insignificantly higher urinary Cl− excretion. The chloride loss in the standard drug-treated group was 225.33% higher (p<0.001) than in the NC group, with the highest losses observed in the aqueous extract-treated groups.
displays the increasing saluretic activity with increasing dose for the lowest, medium, and highest doses relative to NC: 16.90%, 94.44%, and 138.36%, respectively. When compared to the NC, the groups treated with the standard drug (169.84%, p<0.001) and the aqueous extract at the highest dose (138.36%, p<0.01) had significantly higher urinary excretions of salt. For the extract at the lowest, medium, and highest doses, respectively, the saluretic index of Na+ (1, 00, 1.55, and 2.05), K+ (1.60, 1.77, and 3.10), and Cl− (1.37, 1.86, and 2.77) was discovered. In comparison to NC, the saluretic index of SF10 for Na+, K+, and Cl− was found to be 2.22, 1.42, and 3.25, respectively.
Urinary electrolyte excretion was compared between the aqueous extract and standard drug-treated groups; Na+ for AQ400 (p<0.05) and Na+ and Cl− for SF10 (p<0.001) were significantly higher than those of AQ100. Comparing the AQ400 treated group to the AQ100 and SF10 treated groups, there was a noteworthy increase (p<0.05) in K+ urinary excretion.
Furthermore, the Na+/K+ ratio (Natriuretic activity) of the extract-treated groups was found to be less than SF10 (1.29), with the lowest value (0.58) at the lowest dose (AQ100) and the highest value (0.77) at the medium dose (AQ200), across all doses. The Na+/K+ ratio did not differ significantly from the NC in either the aqueous extract-treated or standard medication-treated groups. All groups treated with varying doses of the extract were comparable to one another (0.40, 0.41, and 0.41 in increasing dose), but less than SF10 (0.69), according to the CI−/ (Na+ + K+) calculation.
The 80% Methanol Extract
illustrates that urinary Na+ excretion increased as extract dose increased, with the lowest, medium, and highest doses exhibiting 9.18%, 46.94%, and 57.14%, respectively, and no discernible difference when compared to NC. When comparing the K+ urine excretion to NC, it was found to be significantly higher at the highest dose (184.24%, p<0.01) and medium dose (179.07%, p<0.01), but not significantly higher at the lowest dose (88.37%). When CI− urinary excretion was compared to NC, HM200 and HM400 increased significantly by 158.66% (p<0.05) and 202.63% (p<0.01), respectively, while HM100 increased insignificantly by 85.78%.
Table 4 Effect of 80% Methanol Crude Extract of Cucumis Dipsaceus Leaf on 5h Urinary Electrolyte Excretion and Other Parameters in Rats
When 80% methanol extract and SF10 treated groups were compared for urinary electrolyte excretion, the SF10 treated group had a significantly higher urinary Na+ excretion than HM100 (p<0.01). Regarding K+ and CI−, no discernible variation was found between the reference value and the three distinct dosages of the 80% methanol extract. Additionally, there was no discernible difference in the urinary electrolyte loss between the three doses of 80% methanol extract treated groups.
demonstrates that, in comparison to NC, the saluretic activity was insignificantly increased by 44.47% and 98.40% for the lowest and medium doses, respectively, and significantly increased for the standard drug (SF10; 169.84%; p<0.001) and highest dose (HM400; 124.16%; p<0.01) treated groups. Additionally, saluretic indices were computed, yielding results for K+ (1.88, 2.79, and 2.84), Na+ (1.09, 1.47, and 1.56), and CI-(1.86, 2.57, and 3.03) for the lowest, medium, and highest doses, respectively, in comparison to NC. By increasing the extract doses, values of 0.54, 0.45, and 0.46 the quotient of Na+/K+, or natriuretic activity were obtained. The greater result was demonstrated for SF10 (1.29). Comparably, the value of [CI−] / [Na++K+] was also computed, yielding values for HM100, HM200, and HM400 of 0.44, 0.45, and 0.52, respectively. These findings were found to be lower than SF10’s (0.69).
When the two extracts were compared for urinary electrolyte excretion at the highest aqueous, AQ400, the treated group had significantly higher levels of Na+ and K+ (p<0.05) than the HM100 treated group. However, when compared to the AQ100 treated group, the highest dose, HM400, the treated group showed a significant (p<0.05) increase in urinary excretion of Cl− and saluretic activity.
Effects on pH
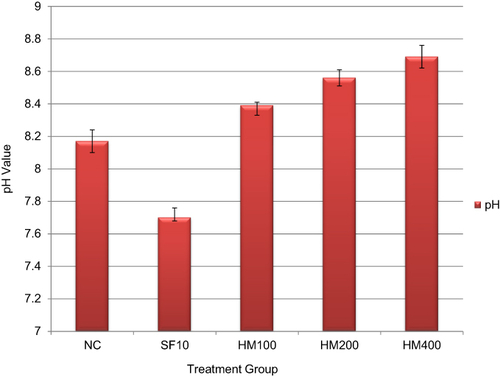
The various treatment groups of both aqueous and 80% methanol extracts produced comparatively alkaline urine, according to a urinary pH test ( and ). At doses of 100, 200, and 400 mg/kg BW, respectively, the five-hour mean ± SEM urinary pH was found to be 8.48 ± 0.06, 8.82 ± 0.05, and 8.64 ±0.10 for the aqueous extract and 8.39±0.06, 8.56±0.05, and 8.69±0.07 for the 80% methanol extract of urine sample. Furthermore, during the course of the five-hour urine collection, the results for the standard drug-treated group and the negative control group were 8.17±0.08 and 7.70 ± 0.02, respectively. When the aqueous extract, 80% methanol extract, and standard drug-treated groups were compared to the NC, no statistically significant differences were found. The highest pH value was produced by a 200 mg/kg dose of aqueous extract, and the lowest pH value was produced by SF10, which made the urine less alkaline ( and ).
Figure 1 Urinary pH of rats treated with aqueous crude extract of C. dipsaceus leaves.
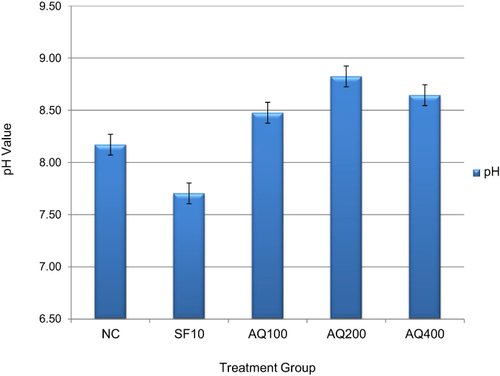
Figure 2 Urinary pH of rats treated with 80% methanol crude extract of C. dipsaceus leaves.
Electrolyte Content of the Extracts
The electrolyte content of aqueous and 80% methanol extracts was studied. The aqueous extract yielded the following results: Na+ (2.06, 4.11, and 8.10mmol/lit), K+ (14.41, 28.71, and 56.37mmol/lit), and Cl− (6.46, 12.87, and 25.26mmol/lit) contents for the lowest, medium, and highest doses, respectively. The lowest, medium, and highest doses of the 80% methanol extract revealed Na+ (2.15, 4.37, and 8.42mmol/lit), K+ (15.86, 32.29, and 62.20mmol/lit), and Cl− (10.69, 21.76, and 41.92mmol/lit) content, respectively. The Na+ content of both extracts was nearly identical, but the K+ and Cl− contents of the 80% methanol extract were higher.
Phytochemical Test
The leaf of Cucumis dipsaceus was subjected to qualitative phytochemical analyses using standardized techniques. The outcomes were confirmed based on the intensity of the characteristic color, indicating the presence of various chemical compounds. Saponins, Tannins, flavonoids, Terpenoids, and Cardiac glycosides were detected at varying levels ranging from (+) to (+++) ().
Table 5 Phytochemical Screenings of Aqueous and Hydro methanol Extract of Cucumis Dipsaceus Leaf
Discussion
Medicinal plants provide a natural defense against disease and are an important treatment for some diseases. As diuretics, a variety of mono- and poly-herbal preparations are used. Many studies have also been conducted to support the diuretic effects of many traditional herbal medicines.Citation48 Diuresis is characterized by two components: an increase in urine volume and a net loss of electrolytes in the urine.Citation1 Therefore, to assess the diuretic activity of the plant extracts, measurements of urine volume and electrolyte excretion were made in the current study. Comparing the aqueous and 80% methanol extracts of C. dipsaceus leaf to the negative control, it was found that both extracts increased urine volume output and electrolyte loss. The groups treated with AQ200, HM200, and HM400 extracts were able to produce significant diuresis as early as the third hour, as indicated in and . When compared to the negative control, the highest dose of aqueous extract AQ400 was able to cause a noticeable diuresis as early as the second hour. Beginning in the third hour, the AQ400 showed similar significant differences with the standard medication. When comparing groups treated with extract and standard drugs, the group treated with standard drug SF10 experienced a rapid and notable diuresis within the first hour of oral administration.
During the collection period, the urinary excretion was measured. Later, the diuretic action and urinary excretion were computed and used to create the final diuretic activity equation. When the two C. dipsaceus leaf extracts were compared, it was found that both extracts increased diuresis, with the effect being greatest at the highest dosages. For instance: as and demonstrate, AQ400’s (2.06) diuretic action was greater than HM400’s (1.97). In a similar vein, AQ400’s diuretic activity (0.94) exceeded HM400’s (0.90), which was still below the maximum aqueous extract doses. The diuretic effect of the aqueous extract was superior to that of the 80% methanol extract. Therefore, it is plausible to propose that the plant’s active ingredient or substances, which provide the diuretic effect, maybe more polar and thus more readily extracted in water than in 80% methanol. In particular, at AQ400 (2.06), the diuretic action of the plant extracts was quantitatively equivalent to that of SF10 (2.20). Given that C. dipsaceus’s diuretic activities at their highest doses were 0.94 and 0.90 for AQ400 and HM400, respectively, they were comparable to SF10.
The diuretic activity numerical results are categorized as follows, per Pérez:Citation49 If diuretic activity is less than 0.50, it is deemed null, moderated if it is between 0.89 and 0.70, low if it is between 0.69 and 0.50, and high if it is greater than or equal to 0.90. The diuretic activity of C. dipsaceus leaf extracts at both higher doses was therefore deemed to be high for AQ400 (0.94) and HM400 (0.90); moderate for AQ200 (0.76), HM100 (0.70), and HM200 (0.82); and low for AQ100 (0.60), as indicated by the results displayed in and . Consequently, these findings utilizing the plant C. dipsaceus’s crude aqueous and 80% methanol leaf extract indicate that the plant has a diuretic effect on rats.
The five hours of urine collection were used to measure the levels of urinary sodium, potassium, and chloride, as indicated in and . Urinary ionic excretion mirrored the increases in diuresis brought on by AQ and HM extract in comparable ways. When compared to NC, there was a significant increase in Na+, K+, and Cl− urinary loss in the AQ400, and a significant increase in K+ and Cl− urinary loss in the HM200 and HM400. Excretion of Cl− and Na+ were significantly higher in the positive control group than in the NC and AQ100, and urinary excretion of Na+ was also significantly higher than in the HM100. When compared to the NC, the extracts’ effect on water excretion appeared to be accompanied by an increase in the excretion of salt and electrolytes in the urine. This suggests that C. dipsaceus’s diuretic action was of the saluretic type, as opposed to the aquaretic type, which is characteristic of most diuretic agents.Citation50
The Na+/K+ ratio can predict the nature of the diuretic mechanism.Citation51 A Na+/K+ ratio of greater than one indicates a satisfactory diuresis without excessive urinary potassium loss.Citation52 Values greater than 2.0 indicate a favorable natriuretic effect, if the ratio exceeds 10.0, it would have potassium-sparing effect.Citation42 The ratio of Na+/K+ was calculated as an indicator of natriuretic activity and this observation suggests that the plant material has no potassium sparing activity.
The Cl−/ (Na+ + K+) ratio was calculated and showed the extent of CA inhibitory effect. CA inhibition can be excluded at ratios between 1.0 and 0.8. With decreasing ratios slight to strong CAI can be assumed.Citation42 Thus, the current study suggests that AQ may have the strongest CA inhibitory effect, with values of 0.40, 0.41, and 0.41 for the lowest, medium, and highest aqueous extract-treated doses, respectively; however, these results were found to be greater in the case of 80% methanol, with values of 0.44, 0.45, and 0.52 for increasing doses, respectively. Even though the lowest dose had the lowest CI−/Na++ K+ ratio for aqueous and 80% methanol extracts, the highest doses produced the greatest diuresis, implying that there must be another mode of action that manifested at higher doses.
One possibility for the observed diuretic properties could be due to direct action of K+ content of C. dipsaceus leaves extracts caused by highest potassium ion content.Citation53 An increment of urinary output in rats might result from high potassium content in the plant infusion.Citation32 Potassium overloading, which occurs when the kidney tubules are incapable of absorbing it, produces urinary excretion of the osmotic type.Citation54 Quantitative determinations of the electrolytes present in the AQ and HM of Cucumis dipsaceus revealed the presence of high amount of K+. The leaves of C. dipsaceus were also analyzed and quantified, for the presence of important macro- and micronutrients, the results shows the leaf sample is found to have N, K, Na, Ca, P, and Fe in a well appreciable amount.Citation55 This suggests that diuretic activity of the extract might seem to be an osmotic type, as K+ content of the extract was high to account for the diuretic activity. It was confirmed that the diuretic action of most active fraction should not be attributed exclusively to the presence of their potassium content but also to other constituents.Citation54 Plant extracts may be inhibiting potassium absorption or stimulating potassium secretion, or both, leading, in either case, to more potassium retention in the lumen of the kidney tubules and osmotic water flow.Citation56
According to previous study, as the leaves of Cucumis dipsaceus extract were analyzed and quantified it possess carbohydrates, proteins, amino acids, alkaloids, saponins, phenolic compounds, tannins, flavonoids, cardiac glycosides, phytosterols and fixed oils and fats and confirmed that it has an antioxidant activity.Citation11,Citation55,Citation57 The beneficial medicinal effects of plant materials typically result from the secondary products present in the plant, although it is usually not attributed to a single compound but a combination of the metabolites. The results revealed that the plant has potential phytochemicals with important biological activities. These phytochemicals also indicate the richness medicinal value in leaf.Citation55 The information of our study do not show specific mechanisms involved in the observed effects. The AQ and HM appears to have multiple mode of diuretic action, which could be due to the presence of several secondary metabolites in the plant extract that act synergistically or antagonistically to produce a resultant effect. Multiple mode of diuretic action is reported with some herbal medications.Citation22
Previous studies confirmed that there are several compounds which could be responsible for the plants diuretic effects in different plant extracts such as flavonoids, saponins, organic acids/ ascorbic acid, carbohydrates, phenolic compounds, terpenoids/triterpenes, alkaloids, glycosides, sterols, sesquiterpenes/or lactones, amino acids and carotinoidsCitation58 have diuretic activities. So, one can suppose that the identified natural compounds in the most active extract of Cucumis dipsaceus might be responsible for the observed diuretic activity and that they may act individually or synergistically promoting an initial vasodilatationCitation59 thereby increasing renal blood flow.Citation50
Conclusions
Observing the data from both aqueous and 80% methanol crude extracts of C. dipsaceus leaves revealed a significant diuresis and saluretic effect in rats. Both extracts had high diuretic activity at maximum doses because their values were greater than or equal to 0.90. Furthermore, as the dose was increased, the values of diuretic activity increased. Based on the electrolytes and urinary pH, it was possible to conclude that the plant could have multiple modes of action, one of which was the CAI mechanism. Finally, the data appear to indicate that the presence of active principles of a more polar nature in the plant extract, where flavonoids, saponins, steroids, and so on, may be the main chemical character for this activity. The plant’s safety, combined with the evidenced diuretic effect from both extracts in the current study, provides additional support to conclude that the current studies support the ethno-medical use of C. dipsaceus as a diuretic agent.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank Addis Ababa University (AAU) School of Pharmacy for their use of laboratory space and equipment.
This manuscript has been uploaded to Addis Ababa University web site as a thesis preprint:
References
- Jackson EK. Drugs affecting renal and cardiovascular function. Good Gilman’s Pharm basis Ther McGraw-Hill Prof New York. 2006:737.
- Ives HE, Warnock DG. Diuretic agents. Basic Clin Pharmacol. 2004;12:251–270.
- Mitch WE, Abras E, Walser M. Long-term effects of a new ketoacid–amino acid supplement in patients with chronic renal failure. Kidney Int. 1982;22(1):48–53. doi:10.1038/ki.1982.131
- Bevevino LH, Vieira FSA, Cassola AC, Sanioto SML. Effect of crude extract of roots of Bredemeyera floribunda Willd. I. Effect on arterial blood pressure and renal excretion in the rat. J Ethnopharmacol. 1994;43(3):197–201. doi:10.1016/0378-8741(94)90043-4
- Shchekochikhin D, Al Ammary F, Lindenfeld J, Schrier R. Role of diuretics and ultrafiltration in congestive heart failure. Pharmaceuticals. 2013;6(7):851–866. doi:10.3390/ph6070851
- Musini VM, Rezapour P, Wright JM, Bassett K, Jauca CD. Blood pressure‐lowering efficacy of loop diuretics for primary hypertension. Cochrane Database Syst Rev. 2015.
- Ernst ME, Gordon JA. Diuretic therapy: key aspects in hypertension and renal disease. J Nephrol. 2010;23(5):487–493.
- Ellison DH. Physiology and pathophysiology of diuretic action. In: Seldin and Geibisch’s the Kidney. Elsevier Inc; 2013:1353–1404.
- Snigdha M, Kumar SS, Jaya Y, Kasana B. Review Article a Review on “How Exactly Diuretic Drugs Are Working in Our Body”. J Drug Delivery Ther. 2013;3(5):115–120.
- Koti BC, Purnima A. Diuretic activity of extracts of Centratherum anthelminticum. Int J Green Pharm. 2008;2(4):2008.
- Lata S, Mittal SK. Pharmacognosy, phytochemistry and pharmacology of Cucumis dipsaceus Ehrenb. Int J Pharmacogn Phytochem Res. 2015;7(3):446–449.
- Cáceres A, Girón LM, Martínez AM. Diuretic activity of plants used for the treatment of urinary ailments in Guatemala. J Ethnopharmacol. 1987;19(3):233–245. doi:10.1016/0378-8741(87)90001-8
- Puschett JB. Diuretics and the therapy of hypertension. Am J Med Sci. 2000;319(1):1–9. doi:10.1016/S0002-9629(15)40675-5
- Materson BJ, Reda DJ, Cushman WC, of VACSG on A AD. Department of Veterans Affairs single-drug therapy of hypertension study: revised figures and new data. Am J Hypertens. 1995;8(2):189–192. doi:10.1016/0895-7061(94)00196-I
- Frishman WH, Bryzinski BS, Coulson LR, et al. A multifactorial trial design to assess combination therapy in hypertension: treatment with bisoprolol and hydrochlorothiazide. Arch Intern Med. 1994;154(13):1461–1468. doi:10.1001/archinte.1994.00420130048008
- Singh RG, Singh RP, Usha KP, Shukla KP, Singh P. Experimental evaluation of diuretic action of herbal drug (Tribulus terrestris Linn.) on albino rats. J Res Edu Ind Med. 1991;3:19–21.
- Weir MR, Flack JM, Applegate WB. Tolerability, safety, and quality of life and hypertensive therapy: the case for low-dose diuretics. Am J Med. 1996;101(3):83S–92S. doi:10.1016/S0002-9343(96)00271-9
- Srivastava J, Lambert J, Vietmeyer N. Medicinal Plants: An Expanding Role in Development. Vol. 320. World Bank Publications; 1996.
- Uniyal SK, Singh KN, Jamwal P, Lal B. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J Ethnobiol Ethnomed. 2006;2:1–8. doi:10.1186/1746-4269-2-14
- Pizzi RA. The Time Line: developing diuretics. Mod Drug Discov. 2003;6:19–22.
- Freitas PCM, Pucci LL, Vieira MS, et al. Diuretic activity and acute oral toxicity of Palicourea coriacea (Cham.) K Schum. J Ethnopharmacol. 2011;134(2):501–503. doi:10.1016/j.jep.2010.12.002
- Wright CI, Van-Buren L, Kroner CI, Koning MMG. Herbal medicines as diuretics: a review of the scientific evidence. J Ethnopharmacol. 2007;114(1):1–31. doi:10.1016/j.jep.2007.07.023
- Mosihuzzaman M, Choudhary MI. Protocols on safety, efficacy, standardization, and documentation of herbal medicine (IUPAC Technical Report). Pure Appl Chem. 2008;80(10):2195–2230. doi:10.1351/pac200880102195
- Nedi T, Mekonnen N, Urga K. Diuretic effect of the crude extracts of Carissa edulis in rats. J Ethnopharmacol. 2004;95(1):57–61. doi:10.1016/j.jep.2004.06.017
- Kebamo S, Makonnen E, Debella A, Geleta B. Evaluation of diuretic activity of different solvent fractions of methanol extract of Carissa edulis root bark in rats. Med Chem. 2015;5(11):472–478. doi:10.4172/2161-0444.1000304
- Mekonnen T, Urga K, Engidawork E. Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. J Ethnopharmacol. 2010;127(2):433–439. doi:10.1016/j.jep.2009.10.020
- Hailu W, Engidawork E. Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ajuga remota Benth (Lamiaceae) leaves in mice. BMC Complement Altern Med. 2014;14(1):1–8. doi:10.1186/1472-6882-14-135
- Melka AE, Makonnen E, Debella A, Fekadu N, Geleta B. Diuretic activity of the aqueous crude extract and solvent fractions of the leaves of Thymus serrulatus in mice. J Exp Pharmacol. 2016;61–67. doi:10.2147/JEP.S121133
- Geleta B, Eyasu M, Fekadu N, Debella A, Challa F. Evaluation of diuretic activity of hydro-ethanolic extract of Moringa stenopetala leaves in Swiss albino mice. Clin Exp Pharmacol. 2015;5(190):2161.
- Fekadu N, Basha H, Meresa A, Degu S, Girma B, Geleta B. Diuretic activity of the aqueous crude extract and hot tea infusion of Moringa stenopetala (Baker f.) Cufod. leaves in rats. J Exp Pharmacol. 2017;73–80. doi:10.2147/JEP.S133778
- Mabberley DJ. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses. Cambridge university press; 2017.
- Chandran R, Nivedhini V, Parimelazhagan T. Chemical composition and antioxidant activity of Cucumis dipsaceus Ehrenb. Ex Spach Fruit. 2014.
- Verdcourt B, Trump EC Common poisonous plants of East Africa. Comm Poisonous Plants East Africa. 1969.
- Bussmann RW, Glenn A. Medicinal plants used in Northern Peru for reproductive problems and female health. J Ethnobiol Ethnomed. 2010;6(1):1–12. doi:10.1186/1746-4269-6-30
- Christopher K, Ruffo ABT. Edible Wild Plants Tanzania Reg L Manag Unit (RELMA), Publ by Reg L Manag Unit. RELMA/Sida ICRAF House, Gigiri PO Box; 2002:63403.
- Kumar D, Kumar S, Singh J, Vashistha BD, Singh N. Free radical scavenging and analgesic activities of Cucumis sativus L. fruit extract. J Young Pharm. 2010;2(4):365–368. doi:10.4103/0975-1483.71627
- Urs SK, Kumar HN, Chandana E, Chauhan JB. Evaluation of the antioxidant activity of Cucumis dipsaceus. J Microbiol Biotech Res. 2013;3:32–40.
- Belayneh A, Bussa NF. Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):1–17. doi:10.1186/1746-4269-10-18
- Tamargo J, Segura J, Ruilope LM. Diuretics in the treatment of hypertension. Part 1: thiazide and thiazide-like diuretics. Expert Opin Pharmacother. 2014;15(4):527–547. doi:10.1517/14656566.2014.879118
- Howland RD, Mycek MJ, Harvey RA, Champe PC. Pharmacology, (Lippincott’s Illustrated Reviews Series); 2006.
- Samiulla DS, Harish MS. Effect of NR-AG-I and NR-AG-II (polyherbal formulations) on diuretic activity in rat. Indian J Pharmacol. 2001;33(2):112.
- Vogel HG, Vogel WH, Schölkens BA, et al. Guidelines for the care and use of laboratory animals. Drug Discov Eval. 2002;2023–2037.
- Development O for EC operation and. Acute Oral Toxicity: up-and-down Procedure. In: Test No. 425. OECD publishing; 2008.
- Kau ST, Keddie JR, Andrews D. A method for screening diuretic agents in the rat. J Pharmacol Meth. 1984;11(1):67–75. doi:10.1016/0160-5402(84)90054-8
- Mukherjee PK Evaluation of diuretic agents. Qual Cont Herb drug New Delhi Bus Horiz. 2002.
- Durairaj A, Mazumder UK, Gupta M. Effects of methanolic extract of Oxystelma esculentum on diuresis and urinary electrolytes excretion in rats. Iran J Pharmacol Ther. 2007;6(2):200–207.
- Somova LI, Shode FO, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharmacol. 2003;84(2–3):299–305. doi:10.1016/S0378-8741(02)00332-X
- Dutta KN, Chetia P, Lahkar S, Das S. Herbal plants used as diuretics: a comprehensive review. J Pharm Chem Biol Sci. 2014;2(1):27–32.
- Pérez M, Boffill Cárdenas MDLÁ, Morón F, Monteagudo E, Sueiro ML, Lorenzo Monteagudo G. Preliminary experimental diuretic activity of plants used by cuban population. Lat Am J Pharm. 2011;30.
- Martín-Herrera D, Abdala S, Benjumea D, Pérez-Paz P. Diuretic activity of Withania aristata: an endemic Canary Island species. J Ethnopharmacol. 2007;113(3):487–491. doi:10.1016/j.jep.2007.07.005
- Toma CC, Olah NK, Vlase L, Mogoșan C, Mocan A. Comparative studies on polyphenolic composition, antioxidant and diuretic effects of Nigella sativa L.(black cumin) and Nigella damascena L.(lady-in-a-mist) seeds. Molecules. 2015;20(6):9560–9574. doi:10.3390/molecules20069560
- Alexander WD, Branch RA, Levine DF, Hartog M. The urinary sodium: potassium ratio and response to diuretics in resistant oedema. Postgrad Med J. 1977;53(617):117–121. doi:10.1136/pgmj.53.617.117
- Jouad H, Lacaille-Dubois MA, Eddouks M. Chronic diuretic effect of the water extract of Spergularia purpurea in normal rats. J Ethnopharmacol. 2001;75(2–3):219–223. doi:10.1016/S0378-8741(01)00193-3
- Kanias GD, Loukis A, Philianos SM. Trace element pharmacognostical study on diuretic drugs by neutron activation analysis. J Radioanal Chem. 1979;54:103–112. doi:10.1007/BF02517766
- Chandran R, Nivedhini V, Parimelazhagan T, Kirk E, Tariq A, van den Heuvel E. Nutritional Composition and Antioxidant Properties of Cucumis dipsaceus Ehrenb. ex Spach Leaf. Sci World J. 2013;2013. doi:10.1155/2013/890451
- Kreydiyyeh SI, Usta J. Diuretic effect and mechanism of action of parsley. J Ethnopharmacol. 2002;79(3):353–357. doi:10.1016/S0378-8741(01)00408-1
- Anusuba V, Priya V. FTIR and GC-MS spectral analysis of Cucumis dipsaceous Ex. Spach. Ehreb leaves. J Pharmacogn Phytochem. 2018;7(2):2327–2333.
- Sayana SB, Christina C, Medabala T, Patil PS. Study of diuretic activity of ethonolic extract of leaves of Cissampelos pareira in rats. Asian J Pharm Clin Res. 2014;157–159.
- Stanic G, Samaržija I. Diuretic activity of Satureja Montana subsp. Montana extracts and oil in rats. Res. 1993;7(5):363–366. doi:10.1002/ptr.2650070508
