Abstract
Introduction
Chronic hepatitis C virus (HCV) infection is one of the leading causes of hepatocellular carcinoma (HCC) worldwide. Antiviral therapy in patients with HCV infection reduces the risk of primary HCC development by 71%–75%. HCV-infected patients with different primary cancers are also at risk for HCC development as a second primary malignancy (HCC-SPM). Limited information is available on the occurrence and characteristics of HCC-SPM. Herein, we determine the prevalence and clinical features of HCV-associated HCC-SPM when compared to primary HCC.
Materials and methods
Patients with HCV-associated HCC seen at MD Anderson Cancer Center (2011–2017) were enrolled in a prospective observational study. Patients with multiple cancers diagnosed simultaneously or with hepatitis B virus or HIV coinfections were excluded. At enrollment, patients completed a questionnaire on medical history and HCC risk factors. Information on demographics, comorbidities, HCV treatment, tumor characteristics, treatment modalities, and virologic and oncologic outcomes were extracted from the medical records.
Results
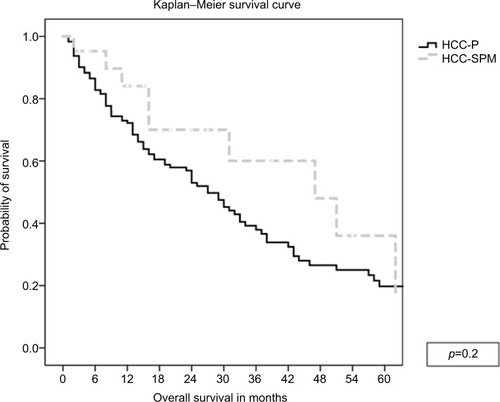
Among 171 consecutive patients with HCV-associated HCC enrolled, 26 (15%) had HCC-SPM. Most of the underlying primary cancers were solid tumors (85%). In 12 (46%) of these patients, the diagnosis was made incidentally while undergoing surveillance for primary malignancies, and the majority (81%) had their primary cancer in remission. Most patients (72%) with documented HCV viral load had chronic viremia due to lack of diagnosis, lack of treatment, or prior unsuccessful treatment of HCV infection and only 28% had undetectable viral load following successful antiviral therapy. The overall median survival for both groups was 29 months (95% CI: 23–35) without difference between groups (p=0.2).
Conclusion
Cancer patients with any malignancies must be screened for HCV as HCC-SPM can develop in 15% of infected patients. Early HCV diagnosis and treatment should be attempted to prevent the development of HCC-SPM, a condition associated with high mortality in cancer survivors.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Chronic hepatitis C virus (HCV) infection is a leading cause of hepatocellular carcinoma (HCC) worldwide. Relative to the uninfected population, patients with HCV infection have a ~10- to 20-fold greater risk of HCC.Citation1 Antiviral therapy for chronic HCV infection reduces the risk of primary HCC (HCC-P) development by 71%–75%.Citation2,Citation3 With an increasing number of cancer survivors and aging patient population, the number of patients with second primary malignancies (SPMs) is also increasing. HCV-infected patients with different primary cancers are at risk for HCC as an SPM (HCC-SPM).Citation4
In a retrospective analysis conducted by our group with 642 patients seen between 2008 and 2011 with chronic HCV infection and any type of primary cancer, HCC-SPMs developed in 7% of them.Citation5 In the first study to evaluate the predictors of HCC as an SPM in HCV-infected patients, we found that patients with HCC-SPMs were more likely to have solid tumors and progressive malignancy. The most common solid tumors were basal cell carcinoma (21%), prostate cancer (14%), and thyroid cancer (14%). Most common treatment modalities were surgery (69%), followed by chemotherapy (34%), most commonly with monoclonal antibodies (rituximab, bevacizumab, and trastuzumab). In addition, patients with HCC-SPMs were less likely to achieve sustained virologic response compared to patients in whom HCC did not develop.Citation6
In the study described herein, we prospectively analyzed the prevalence and clinical features of HCV-associated HCC-SPMs compared with HCC-P.
Materials and methods
Study design and patient population
Patients with HCV-associated HCC seen at The University of Texas MD Anderson Cancer Center from 2011 to 2017 were enrolled in a prospective observational study at the time of HCC diagnosis. HCC was diagnosed by evaluation of liver mass by abdominal imaging (ultrasound or computerized tomography scan) and/or biopsy. Patients with multiple cancers diagnosed simultaneously or with hepatitis B virus or HIV coinfections were excluded. HCV diagnosis was made based on self-reported medical history of HCV infection and/or treatment or current active infection based on HCV RNA level.
At enrollment, patients completed a questionnaire on their medical history and HCC risk factors. The HCC risk factors investigated included tobacco and alcohol abuse, obesity, diabetes mellitus, and chronic liver diseases. The following information was extracted from the medical records: demographics (age, sex, race/ethnicity), comorbidities, prior history of malignancy, laboratories values at baseline, tumor characteristics, staging and treatment modalities for HCC, death, and overall survival at 5 years. Available data on HCV infection (past or active infection), HCV genotype, and antiviral therapy at the time of HCC diagnosis was also recorded.
HCC was defined as SPM if patients did have a prior history of cancer before the HCC diagnosis. Variables compared between patients with HCC-SPM and HCC-P include demographics, risk factors for HCC, clinical presentation and therapeutic approaches for HCC, and virologic and oncologic outcome.
Ethics/institutional review board approval of research
The study protocol was approved by The MD Anderson Institutional Review Board. A written informed consent was obtained from patients. The procedures followed were in accordance with the ethical standards.
Statistical analysis
To compare the demographic and clinical characteristics of the HCC-SPM and HCC-P patients, descriptive analysis was used to calculate frequencies, a Pearson χ2 test was used for categorical variables, and an independent Student t-test was used for continuous variables. P-values were used to assess for statistical significance, with p-values <0.05 considered to indicate significance. All statistical tests were conducted using SPSS version 24 (IBM Corporation, Armonk, NY, USA).
Results
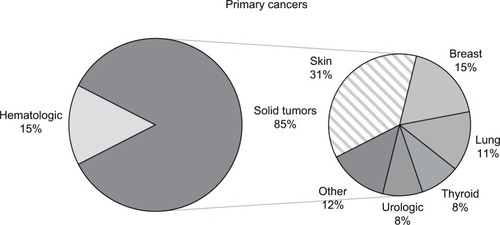
We identified 191 patients with HCV-associated HCC. Twenty patients had either hepatitis B virus or HIV coinfection, or had multiple cancers diagnosed simultaneously and were excluded. We included 171 consecutive patients in the final analysis. Most of them were more than 50 years old (97%), white (65%), and male (78%). Twenty-six (15%) patients had HCV-associated HCC-SPMs. Most of the underlying primary cancers were solid tumors (85%), predominantly skin (31%), breast (15%), and lung (12%) cancers (). The most common treatment modalities for the first primary cancers were surgery (78%) and chemotherapy (50%) ().
Table 1 Comparison of baseline clinical characteristics in the HCC-SPM and HCC-P patient groups (n=171)
At the time of diagnosis, 99% of the patients had liver cirrhosis. Twelve patients (46%) were asymptomatic and had HCC-SPMs incidentally diagnosed while undergoing surveillance for primary malignancies. When we compared HCC-SPMs and HCC-P, we found no differences between the 2 patient groups in smoking history, alcohol intake, body mass index, diabetes mellitus history, family history of liver cancer, or HCC pathology. Patients with HCC-SPMs had better liver function test results and tumor characteristics (), but we could not determine the adherence to routine medical care in the 2 groups. The majority of patients (50%) with HCC were treated with transarterial chemoembolization and transarterial radioembolization with yttrium-90, 7% had radiofrequency ablation, cryoablation, or ablation by ethanol, and 6% had surgical resection or transplant. However, 27% of the patients presented with advanced disease and received sorafenib systemic therapy and 8% received palliative supportive care. The difference in therapeutic approaches used for the treatment of HCC was not statistically significant (p=0.16) ().
Table 2 Comparison of HCC-SPM and HCC-P baseline pathology, staging, and treatment (n=171)
At the time of HCC diagnosis, most patients (72%) who had either HCC-SPMs or HCC-P with documented HCV viral load (n=88) had chronic viremia due to lack of diagnosis, lack of treatment, or prior unsuccessful treatment of HCV infection and only 28% had undetectable viral load following successful antiviral treatment. Genotype 1 was the predominant HCV genotype (70%), followed by geno-type 2 (14%). A total of 97 patients (57%) were treated for HCV infection. Those given direct-acting antivirals had a significantly higher sustained virologic response rate than did those who were given interferon-based therapy (81% vs 41%; p<0.01)
Among the 26 patients with HCC-SPMs, the majority (81%) had their primary cancer in remission, 11% had stable disease under surveillance, and 8% had progression or relapse at the time of HCC diagnosis. The overall median survival for both groups was 29 months (95% CI: 23–35) without statistically significant differences in the survival between groups ().
Discussion
To our knowledge, this is the first prospective analysis of the occurrence and characteristics of HCV-associated HCC-SPMs. In this prospective study, we found that chronic HCV infection can lead to HCC-SPMs in up to 15% of patients. This study is a validation of the findings reported in a retrospective series from our group, but with a higher rate of HCC-SPM occurrence as compared to 7% from our previous report,Citation6 likely due to better study design.
Many factors affect the natural progression of HCV infection. It is difficult to determine exactly the mechanism leading to the association between HCV and high incidence of hepatic and extrahepatic malignancies.Citation4 At present, most cancer centers do not screen patients for HCV routinely.Citation7 HCV testing in cancer patients is vital to identify chronically infected patients with any cancer.Citation4 The development of HCC in our patients potentially would have been very low with more effective screening and treatment of HCV infection, extrapolated from a significant reduction in the risk of HCC-P development following HCV eradication.Citation2
Baseline characteristics and clinical outcomes did not differ significantly between the HCC-SPM and HCC-P groups. Patients with HCC-SPMs had considerably smaller tumors and better liver function test results. HCC-P group of patients also had more advanced TNM staging at presentation probably related to earlier diagnosis and better linkage to care in patients undergoing routine cancer surveillance for their primary malignancies.
Our study has several limitations. First, it was a single-center study. Second, our patients were enrolled prospectively at the time of HCC diagnosis. Some of the details regarding HCV treatment and cancer treatment (eg, chemotherapy, steroids) of their non-HCC primary cancer were not available, which could have an impact on viral replication, HCC development, and progression.Citation8 Third, some patients completed their HCC treatment outside MD Anderson, which may have affected the outcomes.
Conclusion
As increased awareness of the occurrence of second primary HCC in HCV-infected cancer patients, a not uncommon but lethal condition, is needed. Early HCV infection diagnosis paired with prompt treatment is required to prevent the development of HCC-SPMs, a condition associated with high mortality in cancer survivors.
Acknowledgments
The authors thank Mr Donald Norwood of the Department of Scientific Publications at MD Anderson Cancer Center for editorial assistance. This study was presented in part at The International Liver Congress, April 11–15, 2018, Paris, France. The poster’s abstract was published in Hepatology, 2018 (68): S365–604 as a poster presentation. DOI: https://doi.org/10.1016/S0168-8278(18)31114-0.
Disclosure
The authors report no conflicts of interest in this work.
References
- MassarwehNNEl-SeragHBEpidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinomaCancer Control2017243 1073274817729245
- IoannouGNGreenPKBerryKHCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinomaJ Hepatol Epub201795
- RyersonABEhemanCRAltekruseSFAnnual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancerCancer201612291312133726959385
- TorresHAShigleTLHammoudiNThe oncologic burden of hepatitis C virus infection: a clinical perspectiveCA Cancer J Clin201767541143128683174
- TorresHAMahalePBlechaczBEffect of hepatitis C virus infection in patients with cancer: addressing a neglected populationJ Natl Compr Canc Netw2015131415025583768
- MahalePKasebAOHassanMMTorresHAHepatocellular carcinoma as a second primary cancer in patients with chronic hepatitis C virus infectionDig Liver Dis201547434834925563811
- AngelidakisGHwangJPDandachiDUniversal screening for hepatitis C: a needed approach in patients with hematologic malignanciesJ Viral Hepat Epub2018416
- TorresHAHosryJMahalePEconomidesMPJiangYLokASHepatitis C virus reactivation in patients receiving cancer treatment: a prospective observational studyHepatology2018671364728653760