Abstract
Objective
To investigate the survival and independent prognostic factors for single large hepatocellular carcinoma (SLHCC) after surgical resection.
Methods
Patients with SLHCC who underwent radical resection from January 2013 to December 2017 were retrospectively analyzed. The Kaplan-Meier method was used to analyze the overall survival (OS) rate and recurrence-free survival (RFS) rates. Cox forward stepwise regression was performed to analyze the independent prognostic factors.
Results
A total of 485 cases were included. The average age was 51.2±11.2 years, 88.9% had a history of hepatitis B virus infection, and most patients had normal liver function. The average tumor diameter was 8.8±3.0 cm. The 1-, 3-, and 5-year OS and RFS rates were 76.8%, 56.7%, and 45.7%, and 61.0%, 46.2%, and 34.7%, respectively. Multivariate analysis showed that liver cirrhosis (HR=1.456, P=0.004), total bilirubin (TB) ≥17.1 μmol/L (HR=1.437, P=0.011), glutamyl transferase (GGT) >60 U/L (HR=1.438, P=0.020), lactate dehydrogenase (LDH) >225 U/L (HR=1.442, P=0.007), blood loss ≥400 mL (HR=1.339, P=0.027), microvascular invasion (MVI) (HR=1.492, P=0.004), satellite lesions (HR=1.859, P<0.0001) and Edmondson-Steiner grade III+IV (HR=1.740, P=0.018) were independent risk factors for reduced OS in SLHCC patients. Sex (HR=1.763, P=0.003), liver cirrhosis (HR=1.382, P=0.007), GGT >60 U/L (HR=1.512, P=0.003), LDH >225 U/L (HR=1.480, P=0.002), MVI (HR=1.545, P=0.001), and satellite lesions (HR=1.564, P=0.001) were independent risk factors for reduced RFS. OS and RFS nomograms were constructed using risk factors with C-index values of 0.692 (95% CI: 0.659–0.724) and 0.659 (95% CI: 0.623–0.693), respectively. The Hosmer-Leme test demonstrated the good fit of both nomograms.
Conclusion
Surgical resection is the standard and effective treatment for SLHCC patients. Sex, liver cirrhosis, TB≥17.1 μmol/L, GGT>60 U/L, LDH>225 U/L, blood loss≥400 mL, MVI, Edmondson-Steiner grade III+IV, and satellite lesions were found to be independent prognostic factors in SLHCC patients following radical resection. The OS and RFS nomograms accurately predicted the prognosis of SLHCC patients.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and the third leading cause of cancer-related mortality.Citation1 HCC accounts for 70–85% of primary liver cancers, and chronic hepatitis virus infection and non-alcoholic fatty liver disease have been established as the main causes.Citation1,Citation2
In recent years, the surveillance and census of groups at high risk of HCC have significantly increased the early detection rate, significantly improving patient prognosis. However, it has been found that up to 32% of HCC patients have tumors larger than 5 cm in diameter at the time of the clinical diagnosis, and 10% to 20% of HCC patients have tumors larger than 10 cm.Citation3 There is debate over the suitability of surgical treatment for single large HCC tumors (>5 cm). The Barcelona Clinic Liver Cancer (BCLC) staging (2022 edition) classifies HCC as an early-stage liver cancer, with surgical resection the preferred treatment option.Citation4 However, the long-term efficacy and prognostic factors of surgically resected single large HCC (SLHCC) are not very clear.Citation5,Citation6 It has been reported that the 5-year OS rate following resection in SLHCC patients was similar to BCLC stage B tumors.Citation7 The larger the tumor, the greater the possibility of vascular invasion and the worse the histological differentiation.Citation8 Even after radical resection, the 5-year disease-free survival rate is still low.Citation9 Therefore, this study retrospectively analyzed the clinicopathological and prognostic data of patients with SLHCC who underwent radical surgical resection. The study aimed to provide a novel reference for the clinical treatment of SLHCC.
Materials and Methods
Patients
A retrospective case-control study method was used to retrospectively analyze patients with SLHCC who underwent radical resection in our hospital from January 2013 to December 2017. The diagnostic criteria for SLHCC were as follows:Citation10 single tumor; maximum tumor diameter >5 cm; no macrovascular invasion; no intrahepatic and distant metastasis; postoperative pathologically confirmed HCC. The inclusion criteria were as follows: (1) patients met the diagnostic criteria for SLHCC; (2) R0 resection with negative margins; (3) patients had not received any adjuvant therapy or invasive therapy before surgery. The exclusion criteria were as follows: (1) patients who had undergone reoperation due to tumor recurrence; (2) patients with metastases in the surrounding organs, lymph nodes, or distant sites; (3) combined with a history of other malignant tumors; (4) incomplete or missing follow-up data. This study was approved by the ethics committee of Eastern Hepatobiliary Surgery Hospital, and all patients signed informed consent. This study complied with the principles of the Declaration of Helsinki (1964).
Data Collection
The data of patients before and after the surgery were collected for analysis, including general information such as sex, age, history of hepatitis B, and liver function classification. Preoperative laboratory examination data included the levels of total bilirubin (TB), albumin (ALB), alanine transaminase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), and carcino-embryonic antigen (CEA). Intraoperative data included surgical method, intraoperative blood loss, time of hepatic hilar occlusion, and tumor diameter. The postoperative histopathological analysis included liver cirrhosis, microvascular invasion (MVI), satellite lesions, and Edmondson-Steiner grading.Citation11
Surgical Procedures
All patients underwent routine examinations and imaging studies before surgery to exclude contraindications. Liver resection was performed by the right subcostal incision and extended to the left subcostal and xiphoid process if required. The surgical incision was usually about 20–25 cm long. The surgeon first checked the other organs in the abdominal cavity for metastasis, and then the primary focus, and this information combined with that from the preoperative imaging examination determined the surgical resection method. Anatomic resection was preferred for hemi-hepatic tumors or those located in liver lobes or segments. The hepatic parenchyma was separated by the clamp-crushing method and ultrasound knife, and the related hepatic pedicles and veins were ligated carefully. The hepatic portal vein was blocked by Pringle’s maneuver to control and restrict intraoperative bleeding, with clamping and unclamped cycles of 15 and 5 minutes respectively. Radical surgery was defined as the microscopic absence of remaining tumor around the margins.Citation12
Follow-Up
The follow-up methods mainly included outpatient review and telephone follow-up. For two years following the operation, the AFP, CEA, CA19-9, liver function, and abdominal B-ultrasound were reviewed every three months, whereas abdominal contrast-enhanced computed tomography or magnetic resonance imaging was performed every six months. In addition, 2 years after the surgery, the biochemical indices and abdominal B-ultrasound were reviewed every 6 months, and imaging examinations were performed every 12 months or if recurrence was suspected. The diagnostic criteria for recurrence were positive findings on imaging examinations and persistently elevated postoperative AFP. The follow-up deadline was February 2020.
Statistical Analysis
SPSS 26.0 statistical software (IBM Corp., NY, USA) and R software were used for the data processing and analysis, and Graph Pad Prism 9.0 (San Diego, CA, USA) was used for the graphical analysis. Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as frequency and proportion. The chi-squared test or Fisher’s exact test was used for analyzing differences between categorical variables. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Variables significantly associated with prognosis in the univariate analysis (P <0.05) were imported into the multivariate analysis. Survival analysis was performed using the Kaplan-Meier method and compared using the Log rank test. The rms package in R (version 4.0.3) was used to construct the prognostic nomograms based on the independent factors identified by the multivariate analysis. The calibration curve and C-index were used to test the performance of the nomograms. Bootstrapping with 1000 resamples was used for calibration curve construction. The calibration curves were depicted by the Kaplan–Meier method to evaluate the agreement between the nomogram prediction and actual observations, while the consistency of the model was determined by the Hosmer-Leme show test, and P > 0.05 was considered a good fit of the model. Version 3.6.1 of X-tile software from Yale University School of Medicine (Connecticut, United States)Citation13 was used to split patients into three risk groups based on the nomogram score. Overall survival (OS) was defined as the interval between the date of surgery and the date of death or the date of the last follow-up. Recurrence-free survival (RFS) was defined as the interval between the operation data and the diagnosis of tumor recurrence. P <0.05 was considered statistically significant.
Results
Clinicopathological Features of SLHCC Patients
According to the inclusion and exclusion criteria, a total of 485 SLHCC patients () were included in this study with an average age of 51.2±11.2 years and including 406 males (83.7%) and 79 females (16.3%). Among them, 431 patients (88.9%) were HBsAg positive, almost all patients were classified as Child-Pugh A, and only 13 patients (2.7%) were classified as Child-Pugh B. The results of the tumor markers indicated that 213 patients (43.9%) had preoperative AFP>400 μg/L, 72 patients (14.8%) had elevated CA19-9, and 7 patients (1.4%) had CEA exceeding the normal level. Segmentectomy was performed in 293 cases (60.5%), lobectomy was conducted in 138 cases (28.4%), and hemihepatectomy in 54 cases (11.1%). The average time of the hepatic hilar occlusion was 20.2±9.8 min, 76 cases (15.7%) had more than 30 min, and 200 cases (41.2%) had intraoperative blood loss greater than 400 mL. The average diameter of the resected tumors was 8.8±3.0 cm, and 131 patients (27.0%) had tumors with a maximum diameter of more than 10 cm. Postoperative histopathological analysis showed significant microvascular invasion in 173 cases (35.7%) and satellite lesions in 147 cases (30.3%). There were 424 patients (87.4%) with Edmondson-Steiner grade III+IV and 229 patients (47.2%) with liver cirrhosis.
Table 1 Clinicopathologic Characteristics of All Patients and Univariate Analysis for OS and RFS
Analysis of OS and RFS in Patients with SLHCC
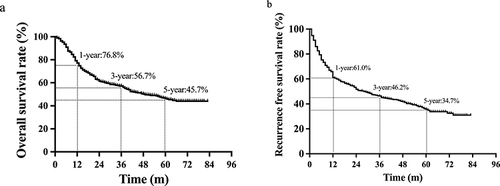
All patients were successfully followed up after surgery, and the median follow-up time was 41 months (1.0–84.0 months). The 1-, 3-, and 5-year OS rates of all patients were 76.8%, 56.7%, and 45.7%, respectively, and the median survival time was 48.1 months. Overall, 248 (51.1%) patients died during the follow-up period (). The 1-, 3-, and 5-year recurrence-free survival rates of all patients were 61.0%, 46.2%, and 34.7%, respectively. The median recurrence-free survival was 27.5 months. A total of 287 (59.2%) patients displayed tumor recurrence after the surgery, of which 224 (78.0%) recurred within 2 years of the surgery ().
Univariate and Multivariate Analyses of OS in Patients with SLHCC
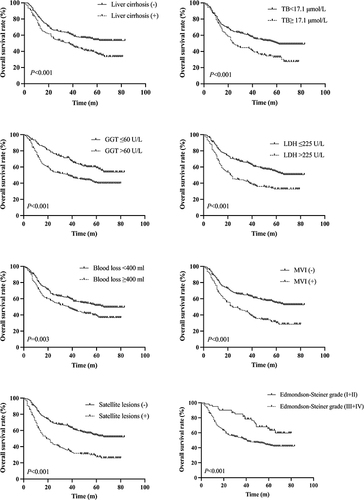
Univariate analysis found that AFP, TB, AST, GGT, LDH, ALP, intraoperative bleeding, tumor diameter, liver cirrhosis, microvascular invasion, satellite lesions, and Edmondson-Steiner grade were major risk factors for OS in patients with SLHCC (). Further multivariate analysis found that liver cirrhosis (HR=1.456, 95% CI:1.130–1.877, P=0.004), TB≥17.1 μmol/L (HR=1.437, 95% CI:1.086–1.903, P=0.011), GGT>60 U/L (HR=1.438, 95% CI:1.060–1.951, P=0.020), LDH>225 U/L (HR=1.442, 95% CI:1.108–1.879, P=0.007), intraoperative bleeding ≥400 mL (HR=1.339, 95% CI:1.033–1.735, P=0.027), MVI (HR=1.492, 95% CI:1.139–1.955, P=0.004), satellite lesions (HR=1.859, 95% CI: 1.418–2.436, P=0.000) and Edmondson-Steiner grade III+IV (HR=1.740, 95% CI: 1.100–2.753, P=0.018) were independent risk factors for patient OS (). The Kaplan-Meier curves of the above-mentioned risk factors for OS were shown in .
Table 2 Multivariate Analysis of SLHCC Patients for OS
Univariate and Multivariate Analysis of RFS in Patients with SLHCC
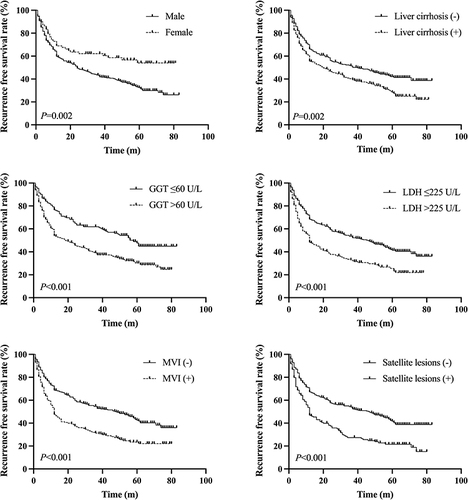
Univariate analysis found that the sex, AFP, TB, AST, GGT, LDH, intraoperative bleeding, liver cirrhosis, MVI, satellite lesions, and Edmondson-Steiner grade were risk factors for RFS in patients with SLHCC (). In addition, multivariate analysis found that the male sex (HR=1.763, 95% CI:1.220–2.547, P=0.003), liver cirrhosis (HR=1.382, 95% CI:1.092–1.747, P=0.007), GGT>60 U/L (HR=1.512, 95% CI:1.147–1.994, P=0.003), LDH>225 U/L (HR=1.480, 95% CI:1.160–1.888, P=0.002), MVI (HR=1.545, 95% CI: 1.201–1.987, P=0.001), and satellite lesions (HR=1.564, 95% CI: 1.211–2.019, P=0.001) were independent risk factors for RFS (). The Kaplan-Meier curves of the independent risk factors for RFS were shown in .
Table 3 Multivariate Analysis of SLHCC Patients for RFS
Construction of the OS and RFS Nomograms
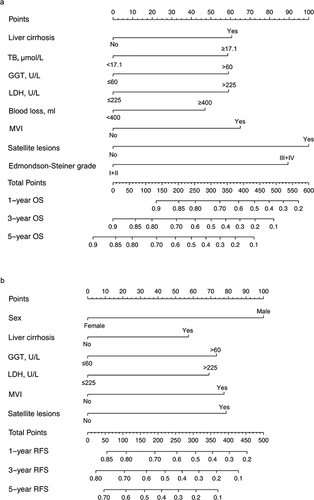
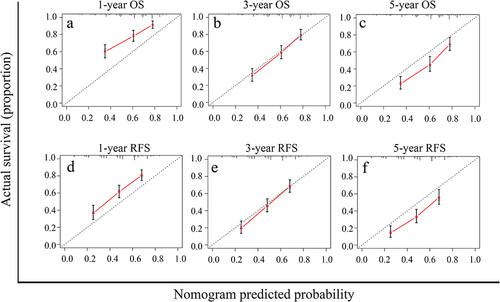
The OS and RFS nomograms were constructed using the independent risk factors identified by the multivariate analyses of OS and RFS (). Each risk factor was assigned a specific score, and the individualized grade for each included patient was defined by the sum of the scores of the factors. The total points on the scale indicate the probability of survival at 1, 3, and 5 years. The C-index values of the OS and RFS nomograms were 0.692 (95% CI: 0.659–0.724) and 0.659 (95% CI: 0.623–0.693), respectively. Moreover, the calibration curves showed good agreement between the actual observed and predicted values for OS () and RFS () at 1, 3, and 5 years. In addition, the Hosmer–Leme show test demonstrated that the two nomograms were a good fit (χ2=6.277, P = 0.616 for OS nomogram; χ2=4.391, P = 0.820 for RFS nomogram).
Risk of Mortality and Recurrence Based on the Nomograms
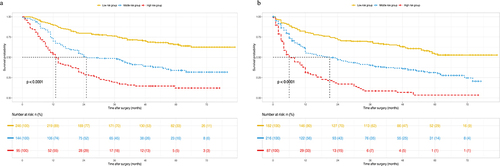
Each risk factor corresponded to a specific score based on the established nomograms. The total points of each patient were calculated, and the patients were then split into three groups using X-tile software. As per the nomogram of OS, all patients were split into low- (score ≤254), middle- (255–356), and high-risk (>356) groups, and the Kaplan-Meier curves of OS exhibited a clear and distinct prognosis rate in each risk group (). In the RFS nomogram, the patients were divided into low- (score ≤208), middle- (209–320) and high-risk (>320) groups, with each group representing a distinct prognosis ().
Discussion
The BCLC staging is based on the tumor burden and liver functional reserve, as well as the performance status, and can provide treatment strategies and prognostic information for each stage. Therefore, BCLC staging is the most widely used clinical staging system.Citation14 The latest BCLC staging points out that a single HCC tumor can exhibit better biological behavior, regardless of the size of the tumor, as it belongs to the early stage, and surgical treatment is the first choice.Citation15 However, some previous studies have considered the tumor diameter to be a risk factor for HCC recurrence, which could increase the potential risk of microvascular invasion and distant metastasis, and classified tumor sizes larger than 5 cm as stage B and recommended interventional therapy.Citation16,Citation17 Therefore, the choice of surgical treatment or interventional treatment for SLHCC remains the main issue of clinical controversy.
Jung et alCitation18 divided 1005 HCC patients into Stage A (613 cases), Stage B (268 cases), and SLHCC (124 cases) groups according to BCLC staging. These patients were compared in terms of tumor stage, treatment, and Child-Pugh grade. The results showed that the survival rate of patients in the SLHCC group was significantly lower than that in the Stage A group, but similar to that in the Stage B group, and they thus recommended that SLHCC be classified as stage B. However, analysis of the treatment methods used in the SLHCC group showed a median survival of 84.2 months after surgery, which was significantly better than the 28.9 months achieved with transarterial chemoembolization (TACE) treatment (P<0.001), thus suggesting that surgical resection leads to significantly better outcomes than TACE. Liu et alCitation3 studied 224 cases of SLHCC and found that the OS rates at 1, 3, and 5 years after surgical resection (88.0%, 76.0%, and 63.0%, respectively) were significantly better than those after TACE treatment (74.0%, 44.0%, and 35.0%, respectively), suggesting that surgical resection has a better curative effect. The 1-, 3-, and 5-year OS rates in the present study (76.8%, 56.7%, and 45.7%, respectively) were slightly lower than those described above but still substantially better than those in patients who received TACE. Therefore, regardless of the tumor stage, surgery is still the preferred treatment for SLHCC patients.
In this study, the Cox proportional hazards model was used to analyze the various prognostic factors of SLHCC, and it was found that factors associated with liver function (TB, GGT and LDH), surgical factor (intraoperative blood loss), tumor characteristics (Edmondson-Steiner grade, liver cirrhosis, MVI, satellite lesions), and sex were independent predictors of prognosis. A nomogram constructed from independent risk factors is an intuitive statistical model that maximizes predictive accuracy and evaluates individualized prognosis.Citation19 We used the above-mentioned independent risk factors and constructed OS and RFS nomograms, which could offer personalized survival predictions for SLHCC patients after surgery. The C-index values, calibration plots, and Hosmer-Leme test demonstrated the accuracy of the nomograms in predicting survival, indicating their potential for further clinical application. Although the C-index values were not as good as those described by Wang et al,Citation20 the nomograms also accurately divided patients into three risk groups for mortality or recurrence. Therefore, these nomograms would be helpful to guide clinicians in the monitoring and treatment of patients with a high risk of postoperative recurrence and to prescribe other adjuvant treatments or clinical trials for patients with a high risk of poor survival outcomes.
The liver function of HCC patients should be carefully considered in the choice between radical resection or interventional treatment. Preoperative liver function (measured by albumin and bilirubin levels) is closely related to the prognosis of patients after hepatectomy.Citation21 Our study demonstrated that total bilirubin has a clear impact on OS. LDH is a metabolic enzyme associated with anaerobic glycolysis that can monitor tumor initiation and progression.Citation22 In pathological conditions such as tumors, a large amount of LDH is released into the blood, resulting in significant increases in blood LDH levels. Therefore, the LDH level can reflect the tumor burden, which is of great significance for the evaluation of therapeutic effects and prognosis after tumor treatment. GGT is a key enzyme present in the cellular membrane and is responsible for counteracting oxidative stress and thus maintaining intracellular homeostasis by catalysis of glutathione.Citation23 Studies have shown that an elevated GGT level is an independent adverse risk factor for HCC patients.Citation24 The reason why GGT affects the prognosis may be related to increased DNA damage and mutation, genomic instability, the activation of reactive oxygen species (ROS), and the promotion of abnormal apoptosis. Our nomograms for OS and RFS also showed that higher levels of LDH and GGT indicated worse prognosis for HCC.
Liver cirrhosis is a recognized precancerous lesion. Almost 90% of patients with HCC have cirrhosis due to hepatitis B or C infection.Citation25 The Edmondson-Steiner grading mainly considers the morphology and size of HCC cells,Citation26 and has been shown to be associated with HCC prognosis.Citation27 The findings presented here also demonstrate this. Intraoperative blood loss during hepatectomy has been reported to be a predictor of morbidity, mortality, and tumor recurrence after liver tumor resection.Citation28 Higher intraoperative blood loss promotes systemic inflammation and induces a cytokine milieu that reduces antitumor immunity,Citation29 resulting in increased morbidity and mortality in patients undergoing hepatectomy. Our study also confirmed that higher intraoperative blood loss often indicates poor prognosis after hepatectomy.
MVI refers to microscopic tumor infiltration in small intrahepatic blood vessels, including micro vessels and the small lymphatic vessels of the portal vein or hepatic artery.Citation30 MVI constitutes the main cause of intrahepatic metastasis, and increases in the tumor diameter or the tumor number can significantly increase the risk of MVI.Citation31 A study by Rodríguez-Perálvarez et alCitation32 reported an MVI incidence of 15.0–57.1%. The present study included 173 MVI-positive patients (35.7%), which was consistent with the above-mentioned results. Many previous studies have also shown MVI to be an independent risk factor for the poor prognosis of liver cancer. Even in patients with small HCC or those who have received liver transplantation, MVI could still increase the incidence of tumor recurrence and significantly shorten overall survival.Citation33,Citation34 The results of this study also showed that the prognosis of MVI-positive patients was significantly worse than that of MVI-negative patients. Since MVI is a postoperative pathological diagnosis, preoperative prediction of the risk of MVI and individualized treatment strategies can aid in further improving the prognosis of HCC patients. At present, there are many nomogram models for the preoperative prediction of the risk of MVI,Citation35–37 all of which possess good identification and prediction capabilities but are not uniform and require further research.
Satellite lesions refer to lesions within 2 cm of the main tumor that have similar histological properties to the main tumor. These are often separated by the normal liver parenchyma but within the same anatomical segment. These are diagnosed by postoperative pathology,Citation38 and the incidence rate is 7–30%.Citation39,Citation40 In this study, there were 147 cases (30.3%) of patients with microscopically identified positive sub-foci, which is consistent with the incidence rate described above. Pathologic analysis has established that these sub-foci are mostly the manifestation of intrahepatic metastases of the main tumor.Citation35 Moreover, several studies have shown that satellite lesions are the main cause of tumor recurrence and unfavorable long-term survival, which may be related to the multifaceted carcinogenic mechanisms underlying HCC and intrahepatic metastasis.Citation41,Citation42 The results of this study also indicated that the prognosis of SLHCC patients positive for satellite lesions was significantly worse than that for patients lacking these lesions.
We found that sex was an independent risk factor for the postoperative recurrence of SLHCC, and the postoperative recurrence rate of the male patients was 1.763 times higher than that of the female patients. A previous study has shown that men are 3–8 times more likely to develop HCC than women, which may be related to the secretion of various sex hormones.Citation43 Xu et alCitation44 also found in their study of HCC recurrence two years after liver resection that male patients were more likely to show recurrence after surgery than female patients. The authors suggested that this may have been related to closer and stricter surveillance for recurrence in the male patients later in the follow-up period. This finding also suggested that sex hormone therapy might effectively reduce the potential risk of liver cancer recurrence, although further research is needed on this topic.
Furthermore, the present study found that tumor diameter was not an independent risk factor for SLHCC prognosis, although tumor diameter was found to be associated with OS in the univariate analysis, which was consistent with the findings of Lim et al.Citation45 It has been reported that postoperative recurrence of liver cancer is closely related to the tumor diameter, especially for tumors with diameters > 5 cm.Citation44 However, the results of the present study found no significant difference in tumor recurrence between SLHCC patients with tumor diameters >10 cm and those with tumors ≤10 cm (P=0.396). Although the classification criteria of tumor diameter were not identical in the two studies, these findings also reflected that the biological behavior of the tumor itself might be more important than the tumor diameter for the prognosis of SLHCC.
There were three major limitations of this study. First, the study was a retrospective analysis and the selection of patients and treatment methods were primarily based on the judgment and suggestions of surgeons, thus selection bias could not be completely avoided. Second, all the SLHCC patients were from a single center. Different centers may have different treatment options for SLHCC patients. Thus, the results of this study need to be verified by multi-center and large-sample data. Third, our OS and RFS nomograms lacked other groups for further validation, which should be undertaken in further studies.
In conclusion, radical surgical resection remains the preferred treatment for SLHCC patients regardless of the tumor stage. It is worth highlighting that sex, TB≥17.1 μmol/L, intraoperative blood loss ≥400 mL, Edmondson-Steiner grade III+IV, liver cirrhosis, GGT>60 U/L, LDH>225 U/L, MVI, and satellite lesions can act as independent risk factors affecting the prognosis of SLHCC patients. The OS and RFS nomograms based on the risk factors could accurately predict the prognosis of SLHCC patients and accurately distinguish patients with different risks of survival and recurrence. Thus, preoperative liver protection and enzyme reduction should be actively performed to improve liver function. Intraoperative bleeding should be controlled and R0 resection should be achieved. Postoperative follow-ups should be meticulously followed, with timely monitoring and treatment of recurring tumors. These recommendations can help to maximize the improvement of patient prognosis and facilitate better clinical management of SLHCC.
Abbreviations
SLHCC, Single large hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; PLT, platelets; TB, total bilirubin; ALB, albumin; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; AFP, alpha-fetoprotein; CA19-9, carbohydrate antigen 19-9; CEA, carcino-embryonic antigen; MVI, microvascular invasion; OS, overall survival; RFS, recurrence free survival.
Data Sharing Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the ethics committee of Eastern Hepatobiliary Surgery Hospital, and all patients signed informed documents.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thank all the staff authors for their contributions in this study.
Additional information
Funding
References
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538. doi:10.1016/j.jhep.2006.05.013
- Liu PH, Su CW, Hsu CY, et al. Solitary large hepatocellular carcinoma: staging and treatment strategy. PLoS One. 2016;11(5):e0155588. doi:10.1371/journal.pone.0155588
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Zhao HC, Wu RL, Liu FB, et al. A retrospective analysis of long term outcomes in patients undergoing hepatic resection for large (>5 cm) hepatocellular carcinoma. HPB. 2016;18(11):943–949. doi:10.1016/j.hpb.2016.08.005
- Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249(1):118–123. doi:10.1097/SLA.0b013e3181904988
- Tsilimigras DI, Bagante F, Sahara K, et al. Prognosis after resection of Barcelona Clinic Liver Cancer (BCLC) stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26(11):3693–3700. doi:10.1245/s10434-019-07580-9
- Kim J, Kim JY, Lee JH, et al. Long-term outcomes of transarterial radioembolization for large single hepatocellular carcinoma: a comparison to resection. J Nucl Med. 2022;63(8):1215–1222. doi:10.2967/jnumed.121.263147
- Hanazaki K, Kajikawa S, Shimozawa N, et al. Hepatic resection for large hepatocellular carcinoma. Am J Surg. 2001;181(4):347–353. doi:10.1016/S0002-9610(01)00584-0
- Fang KC, Kao WY, Su CW, et al. The prognosis of single large hepatocellular carcinoma was distinct from Barcelona clinic liver cancer stage A or B: the role of albumin-bilirubin grade. Liver Cancer. 2018;7(4):335–358. doi:10.1159/000487407
- Zhou L, Rui JA, Ye DX, Wang SB, Chen SG, Qu Q. Edmondson-Steiner grading increases the predictive efficiency of TNM staging for long-term survival of patients with hepatocellular carcinoma after curative resection. World J Surg. 2008;32(8):1748–1756. doi:10.1007/s00268-008-9615-8
- Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathol Res Pract. 1994;190(2):115–123. doi:10.1016/S0344-0338(11)80700-4
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
- Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi:10.1055/s-0030-1247133
- European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi:10.1016/j.jhep.2011.12.001
- Eguchi S, Takatsuki M, Hidaka M, et al. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34(5):1034–1038. doi:10.1007/s00268-010-0424-5
- Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–1092. doi:10.1002/lt.20472
- Jung YK, Jung CH, Seo YS, et al. BCLC stage B is a better designation for single large hepatocellular carcinoma than BCLC stage A. J Gastroenterol Hepatol. 2016;31(2):467–474. doi:10.1111/jgh.13152
- Huang J, Yang Y, Xia Y, et al. Prediction of patient survival following hepatic resection in early-stage hepatocellular carcinoma with indexed ratios of aspartate aminotransferase to platelets: a retrospective cohort study. Cancer Manag Res. 2021;13:1733–1746. doi:10.2147/CMAR.S284950
- Wang JC, Hou JY, Chen JC, et al. Development and validation of prognostic nomograms for single large and huge hepatocellular carcinoma after curative resection. Eur J Cancer. 2021;155:85–96. doi:10.1016/j.ejca.2021.07.009
- Wang H, Feng M, Jackson A, Ten Haken RK, Lawrence TS, Cao Y. Local and global function model of the liver. Int J Radiat Oncol Biol Phys. 2016;94(1):181–188. doi:10.1016/j.ijrobp.2015.09.044
- Zhang JP, Wang HB, Lin YH, et al. Lactate dehydrogenase is an important prognostic indicator for hepatocellular carcinoma after partial hepatectomy. Transl Oncol. 2015;8(6):497–503. doi:10.1016/j.tranon.2015.11.006
- Xia J, Song P, Sun Z, Sawakami T, Jia M, Wang Z. Advances of diagnostic and mechanistic studies of gamma-glutamyl transpeptidase in hepatocellular carcinoma. Drug Discov Ther. 2016;10(4):181–187. doi:10.5582/ddt.2016.01052
- Fukuda S, Itamoto T, Amano H, et al. Clinicopathologic features of hepatocellular carcinoma patients with compensated cirrhosis surviving more than 10 years after curative hepatectomy. World J Surg. 2007;31(2):345–352. doi:10.1007/s00268-006-0513-7
- Tanioka H, Omagari K, Kato Y, et al. Present status of hepatitis virus-associated hepatocellular carcinoma in Nagasaki Prefecture, Japan: a cross-sectional study of 1019 patients. J Infect Chemother. 2002;8(1):64–69. doi:10.1007/s101560200008
- Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi:10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E
- Kim SU, Jung KS, Lee S, et al. Histological subclassification of cirrhosis can predict recurrence after curative resection of hepatocellular carcinoma. Liver Int. 2014;34(7):1008–1017. doi:10.1111/liv.12475
- Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249(4):617–623. doi:10.1097/SLA.0b013e31819ed22f
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713–724. doi:10.1016/j.surg.2010.10.001
- Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17(Suppl 2):S72–S80. doi:10.1002/lt.22368
- Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143(2):293–303. doi:10.1007/s00432-016-2286-1
- Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–339. doi:10.1245/s10434-012-2513-1
- Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. doi:10.1186/1471-2407-14-38
- Kaibori M, Ishizaki M, Matsui K, Kwon AH. Predictors of microvascular invasion before hepatectomy for hepatocellular carcinoma. J Surg Oncol. 2010;102(5):462–468. doi:10.1002/jso.21631
- Shen J, Wen T, Chen W, Lu C, Yan L, Yang J. Model predicting the microvascular invasion and satellite lesions of hepatocellular carcinoma after hepatectomy. ANZ J Surg. 2018;88(11):E761–E766. doi:10.1111/ans.14473
- Li P, Huang W, Wang F, et al. Nomograms based on inflammatory biomarkers for predicting tumor grade and micro-vascular invasion in stage I/II hepatocellular carcinoma. Biosci Rep. 2018;38(6). doi:10.1042/BSR20180464
- Ma X, Wei J, Gu D, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29(7):3595–3605. doi:10.1007/s00330-018-5985-y
- Andreana L, Burroughs AK. Treatment of early hepatocellular carcinoma: how to predict and prevent recurrence. Dig Liver Dis. 2010;42(Suppl 3):S249–S257. doi:10.1016/S1590-8658(10)60513-0
- Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81(4):195–202. doi:10.1002/jso.10178
- An C, Kim DW, Park YN, Chung YE, Rhee H, Kim MJ. Single hepatocellular carcinoma: preoperative MR imaging to predict early recurrence after curative resection. Radiology. 2015;276(2):433–443. doi:10.1148/radiol.15142394
- Pandey D, Lee KH, Wai CT, Wagholikar G, Tan KC. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14(10):2817–2823. doi:10.1245/s10434-007-9518-1
- Plessier A, Codes L, Consigny Y, et al. Underestimation of the influence of satellite nodules as a risk factor for post-transplantation recurrence in patients with small hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S86–S90. doi:10.1002/lt.20039
- Hassan MM, Botrus G, Abdel-Wahab R, et al. Estrogen replacement reduces risk and increases survival times of women with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2017;15(11):1791–1799. doi:10.1016/j.cgh.2017.05.036
- Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi:10.1001/jamasurg.2018.4334
- Lim C, Mise Y, Sakamoto Y, et al. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World J Surg. 2014;38(11):2910–2918. doi:10.1007/s00268-014-2704-y