Abstract
Purpose
Invariant NKT cells (iNKT) are CD1d-restricted T cells with the capacity of antitumor immunity. The safety of autologous iNKT cell treatment in hepatocellular carcinoma (HCC) has been verified. This study aimed to investigate its efficacy in advanced HCC after transarterial chemoembolization (TACE) failure.
Patients and methods
This open-label, randomized, controlled, trial enrolled 60 patients with unresectable HCC after TACE failure at three centers. Transarterial embolization (TAE) was used instead of TACE to protect iNKT cell function. Patients were randomly assigned (1:1) to receive TAE therapy with (TAE–iNKT) or without (TAE) biweekly iNKT cell infusion. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate (ORR), disease control rate (DCR), quality of life (QoL), peripheral blood cell count, and safety.
Results
Fifty-four patients completed the study. Median PFS was significantly higher in TAE–iNKT patients (5.7 months [95% CI, 4.3–7.0 months]) compared with TAE patients (2.7 months [95% CI, 2.3–3.2 months]; hazard ratio 0.32 [95% CI, 0.16–0.63]; P<0.001). Higher ORR and DCR were observed in TAE–iNKT patients (52% and 85%, respectively) compared with TAE patients (11% and 33%; respectively). Five TAE–iNKT patients and 1 TAE patient achieved completed response. The median time to deterioration in QoL was longer in TAE–iNKT patients (9.2 months [95% CI, 6.0–13.3 months]) compared with TAE patients (3.0 months [95% CI, 2.9–3.0 months]). The mean lymphocytes were higher in the TAE-iNKT group than in the TAE group at 8 (1.48 vs 0.95×109/L, P = 0.007) and 12 (1.49 vs 0.89×109/L, P = 0.001) weeks. Grade 3 adverse events occurred in 1 TAE-iNKT patient (4%) and 5 TAE patients (19%). All the other adverse events were grade 1–2.
Conclusion
iNKT cell infusion significantly improved PFS, ORR, DCR, and QoL with manageable toxicity during TAE therapy in patients with HCC. Trial Registration ClinicalTrials.gov Identifier: NCT04011033.
Introduction
Hepatocellular carcinoma (HCC) ranks as the fifth most prevalent cancer and remains a major cause of cancer-related mortality across all age groups and genders.Citation1 Despite advancements in developed nations, the 5-year survival rate for HCC stands at approximately 18%.Citation2 Transarterial embolization (TAE) or transarterial chemoembolization (TACE) represents the standard therapy for intermediate-stage HCC and is widely employed in clinical practice worldwide.Citation3,Citation4 TACE, in particular, is more frequently utilized due to its additional drug intervention, which enhances the anti-tumor effects.Citation5
However, repeated TACE procedures often result in refractory responses among HCC patients, with a median tumor doubling time of 46 days following the initial response, and over 90% of the recurrent nodules exhibiting a doubling time of less than three months.Citation6 Furthermore, the recurrence rates for intrahepatic lesions after TACE are reported at 37% and 61% at 6 and 12 months, respectively.Citation7 TACE failure, defined as an inadequate response after two or more consecutive procedures, is commonplace.Citation8,Citation9 The repeated administration of TACE/TAE therapies exhibits diminishing efficacy and can potentially impair liver function, thereby posing challenges for subsequent treatment.
Invariant natural killer T (iNKT) cells are a subset of innate-like T lymphocytes expressing surface receptors characteristic of both T cells and NK cells. These cells play a pivotal role in bridging innate and adaptive immune responses. iNKT cells exert anti-tumor effects through direct cytolysis and the regulation of immunosuppressive cells within the tumor microenvironment.Citation10 Remarkably, iNKT cells naturally reside and proliferate in the liver, accounting for approximately 3–5% of intrahepatic T cells.Citation11 Intravital fluorescence microscopic imaging has demonstrated that iNKT cells crawl and patrol within hepatic sinusoids, enabling intravascular immune surveillance and rapid activation in response to inflammation.Citation12
Generally, TACE/TAE induces ischemic tumor necrosis and local inflammation in the tumor microenvironment by occluding targeted intrahepatic arteries and delivering cytotoxic chemotherapy drugs.Citation13 Consequently, iNKT cells are rapidly recruited to the inflamed site following TACE/TAE treatment, where they become activated and contribute to the anti-tumor and immune regulatory functions. Additionally, TACE/TAE treatment facilitates interactions between iNKT cells, hepatocytes, macrophages (Kupffer cells), T cells, and dendritic cells through direct cell-to-cell contact and cytokine secretion. This interaction eliminates tumor-associated macrophages (TAMs), counteracts the immunosuppressive activity of myeloid-derived suppressor cells (MDSCs), and shifts the tumor microenvironment from a tolerogenic to an immunogenic state.Citation14–16
Notably, numerous studies have demonstrated a positive correlation between overall survival and the frequency and function of iNKT cells across various cancers, including malignant multiple myeloma, myelodysplastic syndromes, neuroblastomas, primary colorectal carcinomas, lung cancer, and head and neck cancer.Citation17–23 In our previous Phase 1 trial,Citation24 we observed a partial clinical response and an objective immune response in recurrent or refractory Barcelona Clinic Liver Cancer (BCLC) B/C stage HCC patients following autologous iNKT cell infusion, with relatively mild side effects.
In the present open-label, randomized, multicenter, phase 2 trial, we further assessed the efficacy of iNKT cell infusion combined with TAE therapy compared to TAE alone in BCLC B/C stage patients with HCC who experienced TACE failure. Considering the potential impact of chemical drugs on the biological characteristics of iNKT cells, TAE was employed instead of TACE in this study.Citation25
Methods
Preparation of the iNKT Cells
All procedures used for the preparation of iNKT cells were detailed in our previous phase 1 trial.Citation24 Approximately 6~9×107 cells/m2 of iNKT cells were infused in each patient, with > 95% purity and viability. Extensive details of iNKT cell preparation are provided in Supplement (Supplementary Method 1 in Supplement).
Study Design and Participants
This randomized, multicenter, open-label phase 2 trial was conducted at Beijing Youan (Lead unit), Beijing Shijitan (Participating unit), and Beijing Ditan Hospital of Capital Medical University (Participating unit) between March 2018 and March 2020. The study protocol and consent form were approved by the ethics committee of Beijing Youan Hospital (Jing You Ke Lun [2018] No. 016), and the other two participating units were subject to ethical examination and approval by the lead unit. The study complied with the standards of the Declaration of Helsinki, and written informed consent was obtained from each patient. Eligible patients were aged 18 to 80 years, Child-Pugh A/B, confirmed TACE failure/refractory BCLC B/C HCC. More details of the inclusion and exclusion criteria are provided in Supplement (Supplementary Method 2 in Supplement). The present study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Randomization and Masking
Simple randomization was performed at Youan Hospital using a computer-generated random allocation sequence. The successfully enrolled subjects were randomly assigned to either TAE treatment alone (TAE group) or a combination treatment that included TAE and iNKT cell infusion (TAE-iNKT group) in a 1:1 ratio, resulted in 27 subjects on TAE group and other 27 on TAE-iNKT group. The treatment assignment was unmasked to both patients and clinicians.
Procedures
Study treatment was initiated at randomization. The patients in the TAE and TAE-iNKT groups underwent TAE at weeks 0 and 4 after enrollment. TAE was performed at the local hospitals.Citation6,Citation8 Details of TAE are available in Supplement (Supplementary Method 3 in Supplement).
In the TAE-iNKT group, iNKT cells were administered at the 1st, 3rd, 5th, 7th, 9th, and 11th weeks after enrollment. After the iNKT cell infusion, a subcutaneous injection of interleukin (IL)-2 (25,000 IU/kg) was administered once every other day for 2 weeks (Supplementary Figure 1 in Supplement).
All target and non-target lesions were assessed by chest, abdomen, and pelvis CT or MRI at baseline and every 4 weeks until week 16, and then every 8 weeks until radiological progression. Routine blood tests and assessments of tumor biomarkers were conducted at the same time points.
Outcomes
The primary endpoint was the progression-free survival (PFS), ie, the time from enrollment to disease progression according to the mRECIST guidelines for immunotherapeutic trials,Citation26 or death from any cause, whichever occurred first. The secondary endpoints included overall survival (OS) (the time of randomization until the date of death from any cause), objective response rate (ORR, the sum of patients with a best overall response of complete response (CR) or partial response (PR)), and the disease control rate (DCR, the sum of patients with a best overall response of CR, PR, or stable disease (SD)). Patients without any post-baseline tumor assessment were considered non-responsive. Objective responses had to be confirmed at least 28 days after the initial documentation of the response. Quantification of lymphocytes was conducted once every 4 weeks. Adverse events were assessed and graded based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.3. QoL was assessed according to the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30,Citation27,Citation28 and the time to deterioration of QoL, as reported by the patient, with deterioration defined as a decrease from baseline of 10 points or more on the EORTC QLQ–C30 maintained for two consecutive assessments.Citation29
Statistical Analysis
The trial used a two-sided 5% type I error and had 80% power to detect an improvement in PFS from 1.5 months in the TAE group to 4.5 months in the TAE-iNKT group,Citation6,Citation8 corresponding to a hazard ratio (HR) of 0.33 in terms of median PFS. In consideration of these assumptions, the trial design was powered for 60 patients to be randomly assigned to each group as the intention-to-treat population (allocation ratio of 1:1, n = 30 patients in each group), and allowing for a drop-out rate of 20%.Citation30,Citation31 All efficacy analyses were conducted in the modified intention-to-treat population.
Continuous variables were expressed as the median and IQR and were compared using t-tests or Mann–Whitney tests. Categorical variables were presented as the frequency and percentage and were compared using Fisher’s exact tests. Survival and time to QoL deterioration were estimated using the Kaplan–Meier method, and their 95% CIs were calculated using the Brookmeyer and Crowley method. Survival differences were compared using Log rank tests. Peripheral blood cells were analyzed using Sidak’s multiple comparison test. The incidence rate of adverse events was compared using the chi-square test. The overall two-sided significance level of P < 0.05 was split into a two-sided significance level of P < 0.01 for tests of PFS, OS, ORR, DCR, and QoL according to the Bonferroni method (dividing the available total α (typically 0.05) equally among the chosen endpoints).Citation32 If the PFS and/or all secondary endpoints were statistically significant at a two-sided significance level of 0.01, the curative effect of TAE-iNKT was deemed better than that of TAE alone. All statistical analyses were conducted using SAS software version 9.4 (SAS Institute).
Results
Patients
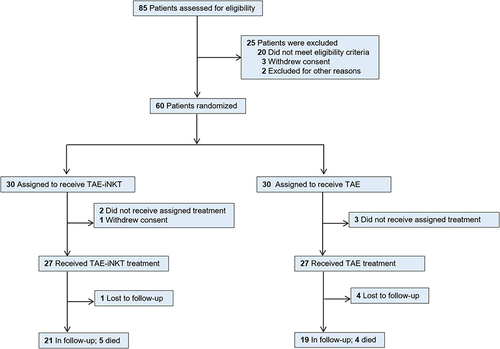
Between March 1, 2018, and March 1, 2020, 85 patients were assessed for eligibility; 20 did not meet the eligibility criteria, 3 refused to participate, and 2 patients with massive tumors at diagnosis were excluded due to the indications of hepatic failure after the first TACE. Therefore, a total of 60 patients were enrolled as the intention-to-treat population, signed the informed consent form, and were randomized into the TAE (n = 30) or TAE-iNKT (n = 30) groups ().
Among the intention-to-treat population, 3 patients refused to receive the assigned treatment in the TAE group, while 2 patients refused the assigned treatment and 1 patient withdrew informed consent in the TAE-iNKT group, resulting in a final total of 54 patients who completed the trial (n = 27 patients per group). The baseline characteristics of both groups are shown in (patient treatments prior to the trial can be found in Supplementary Table 1 in Supplement). Briefly, the median age of the patients was 64 years (range, 56–71 years); 47 patients (87%) were men, and 7 patients (13%) were women; all 54 patients (100%) were Chinese.
Table 1 Patient Demographic and Disease Characteristics
All 54 patients received either TAE therapy alone or iNKT cell infusion every 2 weeks, administered on weeks 1–11 plus TAE therapy after enrollment. At the time the data collection was finished, 4 patients (15%) in the TAE group and 1 patient (4%) in the TAE-iNKT group had completed treatment but were lost during follow-up. All of the lost patients were reported to the ethics committee of the Beijing Youan Hospital of Capital Medical University.
Efficacy
At the time of the data cut-off on July 20, 2020, the median follow-up time was 12.7 months (IQR, 11.2–16.5 months). Of the 27 patients in each group, 21 patients (82%) in the TAE-iNKT group experienced disease progression or died, compared with 23 patients (85%) in the TAE group.
An additional follow-up period continued through March 1, 2022, and analysis of these data showed a median OS of 25.9 months (95% CI, 12.4 months–NA) in the TAE–iNKT group and 17.3 months in the TAE group (95% CI, 7.5–27.6 months; HR, 0.60; 95% CI, 0.32–1.13; P = 0.12) (Supplementary Figure 2 in Supplement). The median PFS in the TAE-iNKT group was 5.7 months (95% CI, 4.3–7.0 months), which was significantly longer than the median PFS in TAE patients (2.7 months [95% CI, 2.3–3.2 months]), (HR, 0.32 [95% CI, 0.16–0.63]; P < 0.001; ).
Figure 2 Kaplan-Meier Plot in the Modified Intention-to-Treat Population. (A) Progression-free survival since randomization. (B) Time from randomization to quality of life deterioration.
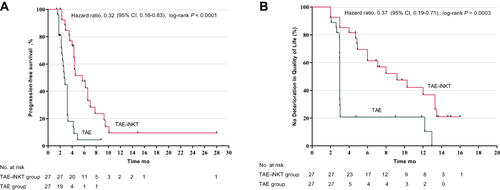
Treatment with iNKT cells delayed deterioration of QoL (median time to deterioration, 9.2 months in the TAE-iNKT group vs 3.0 months in the TAE group; HR, 0.37 [95% CI, 0.19 to 0.71]; P = 0.001) (), physical functioning (median time to deterioration, 9.6 months vs 3.2 months; HR, 0.45 [95% CI, 0.22 to 0.93]; P = 0.01) (Supplementary Figure 3 in Supplement), and role functioning (median time to deterioration, 9.6 months vs 3.1 months; HR, 0.42 [95% CI, 0.21 to 0.84]; P = 0.003) (Supplementary Figure 4 in Supplement).
Higher ORR and DCR were observed in the TAE-iNKT group (52%; 60.0 months) compared with the TAE group (ORR, 52% [n = 14] vs 11% [n = 3]; P = 0.003; and DCR, 85% [n = 23] vs 33% [n = 9]; P < 0.001) (Supplementary Table 2 in Supplement). Within the ORR of the 27 patients in each group, 5 (19%) in the TAE-iNKT group had CR compared with 1 patient (4%) in the TAE group; 9 patients (33%) in the TAE-iNKT group and 2 patients (7%) in the TAE group exhibited PR. Stable disease (SD) was reported in 9 of 27 patients (33%) in the TAE-iNKT group and 6 of 27 patients (22%) in the TAE group, 4 TAE-iNKT patients (15%) and 18 TAE patients (67%) had progressive disease (PD). shows the response durations for each responder.
Figure 3 Duration of Response and Quantification of Lymphocytes. (A) Duration of response among responders. (B) Quantification of lymphocytes (ns, p>0.05; **p<0.01).
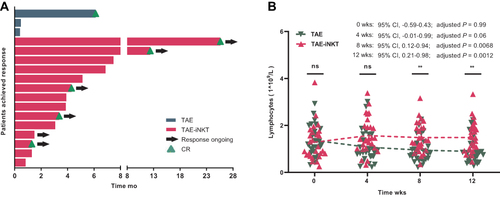
The mean lymphocyte absolute values were higher in the TAE-iNKT group than in the TAE group at 8 (1.48 vs 0.95 ×109/L, P = 0.007) and 12 (1.49 vs 0.89×109/L, P = 0.001) weeks, with no obvious differences at 0 or 4 weeks (P = 0.99 and P = 0.056) (). No differences were observed in white blood cell or platelet counts (all P > 0.05) (Supplementary Figures 5 and 6 in Supplement).
Safety
Adverse effects were recorded in all 54 patients who received the allocated treatment. The incidence of fatigue, fever, elevated alanine aminotransferase, elevated aspartate aminotransferase, increased blood bilirubin, and elevated alkaline phosphatase were lower in the TAE-iNKT group compared with the TAE group. The incidence rate of increased bilirubin and transaminase levels was higher in the TAE group than in the TAE-iNKT group (70.4% vs 29.6%, P = 0.003). Grade 3 adverse events were reported in 1 of 27 TAE-iNKT group patients (4%) and 5 of 27 TAE group patients (19%). The grade 3 adverse elevation in glutamyl transpeptidase detected in the TAE-iNKT group returned to normal without treatment or treatment interruption. No grade 4–5 adverse events were observed. All adverse events are shown in .
Table 2 Adverse Events
Discussion
To our knowledge, this study represents the first phase 2 randomized clinical trial exploring the efficacy of autologous iNKT cell treatment in BCLC B/C stage patients with HCC after TACE failure. The findings in this study show that iNKT cell infusion plus TAE are associated with improved PFS, ORR, DCR, and QoL compared to the effects of TAE alone, and is accompanied by relatively low toxicity side effects.
For decades, TACE has been recommended as a primary intervention in unresectable HCC patients, especially those with BCLC-B stage, and is considered the most effective available option for improving prognosis in clinics around the world.Citation5,Citation9,Citation33–38 However, in the absence of further interventions, most patients with HCC will experience disease progression soon after an initial response to TACE,Citation6 and repeated TACE procedures commonly result in TACE refractoriness/failure, with the incidence ranges from 37% to 49%.Citation39,Citation40 The results of our phase 1 trial indicated that autologous iNKT cell therapy has good tolerability in HCC patients and could potentially further improve outcomes. In the present phase 2 trial, we prospectively confirmed the efficacy of this iNKT cell-based strategy in BCLC B/C stage patients with HCC after TACE failure.
The findings of the current study show that median PFS is significantly improved in TAE-iNKT group subjects compared with those in the TAE group (5.7 vs 2.7 months). Furthermore, patients administered TAE-iNKT exhibit higher ORR and DCR than those treated with TAE alone (ORR, 52% vs 11%; and DCR, 85% vs 33%). Two underlying mechanisms may explain the effect of autologous iNKT cell treatment on PFS, ORR, and DCR. First, the increase in lymphocyte numbers after iNKT cell infusion may be a contributing factor in the better outcomes for patients with TACE failure compared to those for patients given TAE alone. Note our finding that the absolute value of lymphocyte counts steadily decreased with time after TACE treatment, while the lymphocyte populations gradually recovered after iNKT cell infusion. In the phase 1 study, we also found that lymphocyte populations increase after iNKT cell infusion, as do levels of T-helper 1 (Th1) cytokines (eg, IFN-γ, perforin, and granzyme B) produced by iNKT cells.Citation24 The expanded autologous iNKT cells and increased lymphocyte populations are known to facilitate restoration of immune cells,Citation41,Citation42 thereby “resetting” the immune system from a tolerogenic to an immunogenic state-a primary goal of tumor immunotherapy.Citation14 Second, iNKT cells can exert strong, direct anti-tumor effects, which also likely contributed to the observed improvements in PFS and OS in the TAE-iNKT group.
It should be noted that iNKT cells specifically home-in on liver tissue rather than other tissues and can serve as a rich source of hepatic lymphocytes.Citation43,Citation44 CD1d is expressed on sinusoid-lining endothelial cells, Kupffer cells, hepatocytes, and other cells in the liver.Citation45–48 TAE induces ischemic tumor necrosis, and reperfusion can initiate a cascade of pathways that cause further cellular injury after prolonged ischemia.Citation49 To ameliorate this effect, iNKT cells are recruited to the damage site and rapidly activated by these cells in a CD1d-dependent manner after reperfusion. Activated iNKT cells release large amounts of granzyme B and perforin, which can directly target and kill tumor cells. These immune mechanisms may at least partially explain the efficacy of autologous iNKT cell treatment.
In terms of efficacy, QoL has been shown to be a clinical outcome as important as PFS or OS in the management of patients monitored for HCC, especially for patients with recurrent or refractory HCC (whose QoL is already diminished by the disease itself as well as previous therapies).Citation50 For these patients, the ideal therapy would both prolong survival and preserve (or improve) QoL. In the present study, we found that the median time to QoL deterioration was significantly longer in patients treated with TAE-iNKT compared to those given TAE (9.2 vs 3.0 months). It bears emphasis that this improvement to QoL under autologous iNKT cell treatment also promotes patient participation in further follow-up treatment opportunities and can eventually lead to improved oncological outcomes. Combined with efficacy and safety outcomes, these data support autologous iNKT cell treatment as a promising strategy for BCLC B/C stage patients with HCC.
This study has some limitations. First, the overall survival data were not mature in this study. A longer follow-up is needed to assess whether the PFS benefits observed with autologous iNKT cell treatment indeed translate into OS benefits. Second, the sample size is small in the present study, and a larger cohort would be desirable for future explorations of this therapeutic approach. Another potential limitation was that this study only includes Chinese participants, in whom the etiology of HCC is largely from viral hepatitis or cirrhosis,Citation51 which is distinct from the etiology of Western populations. However, this etiological difference does not affect the objectivity of the results because patients in the present study have been treated with antiviral agents and have maintained the virus below the detection limit (HBV DNA < 10 IU/mL).
Conclusion
In the phase 2 randomized clinical trial, iNKT cell infusion plus TAE demonstrated superior PFS vs TAE alone in BCLC B/C stage patients with HCC after TACE failure. The patients with infused iNKT cells reported a better quality of life than patients without infusion. Treatment with iNKT cells was accompanied by manageable toxicity.
Data Sharing Statement
All data related to this study are included this article and its Supplementary Material Files. Further inquiries can be directed to the corresponding author on reasonable request ([email protected]).
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
- Chang PY, Huang CC, Hung CH, et al. Multidisciplinary Taiwan consensus recommendations for the use of DEBDOX-TACE in hepatocellular carcinoma treatment. Liver Cancer. 2018;7(4):312–322. doi:10.1159/000487608
- Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109–1113. doi:10.1111/hepr.13411
- Tsurusaki M, Murakami T. Surgical and locoregional therapy of HCC: TACE. Liver Cancer. 2015;4(3):165–175. doi:10.1159/000367739
- Woo HY, Jang JW, Choi JY, et al. Tumor doubling time after initial response to transarterial chemoembolization in patients with hepatocellular carcinoma. Scand J Gastroenterol. 2010;45(3):332–339. doi:10.3109/00365520903456573
- Terzi E, Golfieri R, Piscaglia F, et al. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57(6):1258–1267. doi:10.1016/j.jhep.2012.07.025
- Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(1):22–31. doi:10.1159/000368142
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461–469. doi:10.1053/j.gastro.2006.05.021
- Nair S, Dhodapkar MV. Natural killer T cells in cancer immunotherapy. Front Immunol. 2017;8:1178. doi:10.3389/fimmu.2017.01178
- Santodomingo-Garzon T, Swain MG. Role of NKT cells in autoimmune liver disease. Autoimmun Rev. 2011;10(12):793–800. doi:10.1016/j.autrev.2011.06.003
- Geissmann F, Cameron TO, Sidobre S, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3(4):e113. doi:10.1371/journal.pbio.0030113
- Jekarl DW, Lee S, Kwon JH, et al. Complex interaction networks of cytokines after transarterial chemotherapy in patients with hepatocellular carcinoma. PLoS One. 2019;14(11):e0224318. doi:10.1371/journal.pone.0224318
- Fujii SI, Shimizu K. Immune networks and therapeutic targeting of iNKT cells in cancer. Trends Immunol. 2019;40(11):984–997. doi:10.1016/j.it.2019.09.008
- Gu X, Chu Q, Ma X, et al. New insights into iNKT cells and their roles in liver diseases. Front Immunol. 2022;13:1035950. doi:10.3389/fimmu.2022.1035950
- Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182(4):1818–1828. doi:10.4049/jimmunol.0802430
- Dhodapkar MV, Geller MD, Chang DH, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197(12):1667–1676. doi:10.1084/jem.20021650
- Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122(4):617–622. doi:10.1046/j.1365-2141.2003.04465.x
- Metelitsa LS, Wu HW, Wang H, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199(9):1213–1221. doi:10.1084/jem.20031462
- Motohashi S, Okamoto Y, Yoshino I, Nakayama T. Anti-tumor immune responses induced by iNKT cell-based immunotherapy for lung cancer and head and neck cancer. Clin Immunol. 2011;140(2):167–176. doi:10.1016/j.clim.2011.01.009
- Schneiders FL, de Bruin RC, van den Eertwegh AJ, et al. Circulating invariant natural killer T-cell numbers predict outcome in head and neck squamous cell carcinoma: updated analysis with 10-year follow-up. J Clin Oncol. 2012;30(5):567–570. doi:10.1200/JCO.2011.38.8819
- Tachibana T, Onodera H, Tsuruyama T, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11(20):7322–7327. doi:10.1158/1078-0432.CCR-05-0877
- Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167(7):4046–4050. doi:10.4049/jimmunol.167.7.4046
- Gao Y, Guo J, Bao X, et al. Adoptive transfer of autologous invariant natural killer T cells as immunotherapy for advanced hepatocellular carcinoma: a phase I clinical trial. Oncologist. 2021;26(11):e1919–e1930. doi:10.1002/onco.13899
- Lu W, Li YH, He XF, Chen Y, Zeng QL, Qiu YR. Effect of dosage of anticancer agents during transcatheter arterial chemoembolization on T cell subsets in patients with hepatocellular carcinoma. Di Yi Jun Yi Da Xue Xue Bao. 2002;22(6):524–526.
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
- The European Organisation for Research and Treatment of Cancer. EORTC QLQ-C30 Scoring Manual. Available from: https://www.eortc.org/app/uploads/sites/2/2018/02/SCmanual.pdf. Accessed January 15, 2018.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi:10.1093/jnci/85.5.365
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
- Lakatos E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics. 1988;44(1):229–241. doi:10.2307/2531910
- Lakatos E. Designing complex group sequential survival trials. Stat Med. 2002;21(14):1969–1989. doi:10.1002/sim.1193
- U.S. Food and Drug Administration. Multiple endpoints in clinical trials guidance for industry. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/multiple-endpoints-clinical-trials-guidance-industry. Accessed January 15, 2018.
- Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: approaching a personalized care. J Hepatol. 2015;62(1 Suppl):S144–56. doi:10.1016/j.jhep.2015.02.007
- Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47–54. doi:10.1148/radiol.2241011262
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi:10.1053/jhep.2002.33156
- Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015;4(1):39–50. doi:10.1159/000367727
- Kudo M. Clinical practice guidelines for hepatocellular carcinoma differ between Japan, United States, and Europe. Liver Cancer. 2015;4(2):85–95. doi:10.1159/000367730
- Kudo M. Locoregional therapy for hepatocellular carcinoma. Liver Cancer. 2015;4(3):163–164. doi:10.1159/000367741
- Byun J, Kim SY, Kim JH, et al. Prediction of transarterial chemoembolization refractoriness in patients with hepatocellular carcinoma using imaging features of gadoxetic acid-enhanced magnetic resonance imaging. Acta Radiol. 2021;62(12):1548–1558. doi:10.1177/0284185120971844
- Hu K, Lu S, Li M, et al. A novel pre-treatment model predicting risk of developing refractoriness to transarterial chemoembolization in unresectable hepatocellular carcinoma. J Cancer. 2020;11(15):4589–4596. doi:10.7150/jca.44847
- Feng H, Nakajima N, Wu L, et al. A glycolipid adjuvant, 7DW8-5, enhances the protective immune response to the current split influenza vaccine in mice. Front Microbiol. 2019;10:2157. doi:10.3389/fmicb.2019.02157
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195(5):625–636. doi:10.1084/jem.20011786
- Halder RC, Seki S, Weerasinghe A, Kawamura T, Watanabe H, Abo T. Characterization of NK cells and extrathymic T cells generated in the liver of irradiated mice with a liver shield. Clin Exp Immunol. 1998;114(3):434–447. doi:10.1046/j.1365-2249.1998.00726.x
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3(1):51–62. doi:10.1038/nri981
- Lei Z, Tang R, Qi Q, et al. Hepatocyte CD1d protects against liver immunopathology in mice with schistosomiasis japonica. Immunology. 2021;162(3):328–338. doi:10.1111/imm.13288
- Moody DB, Cotton RN. Four pathways of CD1 antigen presentation to T cells. Curr Opin Immunol. 2017;46:127–133. doi:10.1016/j.coi.2017.07.013
- Lynch L. Adipose invariant natural killer T cells. Immunology. 2014;142(3):337–346. doi:10.1111/imm.12269
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101–117. doi:10.1038/nri3369
- Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203(12):2639–2648. doi:10.1084/jem.20061097
- Muzellec L, Bourien H, Edeline J. Patients’ experience of systemic treatment of hepatocellular carcinoma: a review of the impact on quality of life. Cancers. 2021;14(1):179. doi:10.3390/cancers14010179
- Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30(6):571–579. doi:10.21147/j.issn.1000-9604.2018.06.01