Abstract
Background
Edmondson-Steiner (E-S) grade is a pathological indicator of the degree of hepatocellular carcinoma (HCC) differentiation, and E-S grade III–IV is a poor prognostic factor for HCC patients. Predicting poorly differentiated HCC has essential significance for clinical decision-making. Although some studies have developed predictive models based on magnetic resonance imaging (MRI) and radiomics, radiomic features that require specific software for analysis are impractical for clinical work. This study aims to develop a novel and user-friendly nomogram model to predict E-S grade III–IV.
Patients and Methods
Medical data on patients meeting the inclusion criteria were obtained from the Nanjing Drum Tower Hospital HCC database (January 2020 to December 2022). Univariate analysis was used to screen for risk factors associated with E-S grade III–IV. A novel nomogram was established based on the subsequent multivariate logistic regression analysis. The performance of the established model was evaluated through diagnostic ability, calibration, and clinical benefits.
Results
Overall, 240 HCC patients were included in this study. Among them, 103 were highly differentiated (E-S grade I–II) HCC and 137 were poorly differentiated (E-S grade III–IV) HCC. A nomogram model that integrated alpha-fetoprotein (AFP), des-γ-carboxy prothrombin (DCP), hepatitis B virus surface antigen (HBsAg), hepatitis C virus antibodies (HCVAb), aspartate aminotransferase to lymphocyte ratio index (ALRI), and macrovascular invasion was established. The novel model had a good diagnostic performance with an area under the curve (AUC) value of 0.763. Meanwhile, the model had a diagnostic accuracy of 72.5%, a sensitivity of 78.1%, and a specificity of 65.1%. The calibration curve showed good calibration of the nomogram model (mean absolute error = 0.043), and the decision curve analysis (DCA) demonstrated that the clinical benefit was provided.
Conclusion
Our developed nomogram model could successfully predict E-S grade III–IV in HCC patients, which may be helpful in clinical decision-making.
Introduction
While the incidence and mortality rates of most cancers are declining, the incidence and mortality rates of hepatocellular carcinoma (HCC) are increasing.Citation1,Citation2 It is the second leading cause of cancer-related deaths.Citation2 Localized HCC is asymptomatic for most of its course. Most HCC patients have advanced disease at first diagnosis and do not qualify for potentially curative treatment.Citation1 Therefore, early identification of poor prognostic factors and carefully evaluating patient treatment options are critical. The Edmondson-Steiner (E-S) grade has been identified as one of the poor prognostic factors for HCC.Citation3 However, studies using routine laboratory tests and imaging features to predict the E-S grade are scarce. Since a large sample cohort study demonstrated that HCC patients with E-S grade III–IV could account for 86.7% of all patients,Citation4 it makes sense to focus on the clinical use of the E-S grade.
At present, the E-S grade is commonly used worldwide to distinguish the degree of differentiation of HCC. It is a histological classification based on the shape and size of HCC cells.Citation5 Kim et al showed that E-S grade III–IV was significantly associated with early recurrence (<1 year) of HCC (HR = 3.456; 95% confidence internal [CI], 1.123–10.517).Citation6 Furthermore, a prospective study that included 369 HCC patients demonstrated that E-S grade III–IV was an independent risk factor for poor overall survival (HR = 2.781; 95% CI, 1.386–5.577).Citation7 With the development of sequencing technology, studies have revealed notable heterogeneity in HCC between the genome, transcriptome, and immune microenvironment.Citation8 E-S grade was found to be correlated with specific RNA expression patterns and immune infiltrating cells.Citation8,Citation9 Moreover, patients with E-S grade III had significantly higher programmed cell death-ligand 1 (PD-L1) expression than patients with E-S grade I–II.Citation10 In conclusion, the E-S grade may also be the basis for screening patients for targeted therapy and immunotherapy. Microvascular invasion (MVI) is also an important indicator for the pathological diagnosis of HCC. Qu et al reported that the risk of MVI in poorly differentiated HCC patients was about 2.97 times that of well-differentiated patients.Citation11 Since wide resection margins or anatomic hepatectomy could improve the prognosis of patients with MVI,Citation12,Citation13 preoperative prediction of E-S grade may be helpful for the selection of surgery type. Local therapy, such as transcatheter arterial chemoembolization (TACE), is a crucial treatment modality for unresectable HCC.Citation14 A propensity score matching study suggested that HCC patients with E-S grade I–II may benefit from postoperative prophylactic TACE.Citation15
Nomograms are user-friendly statistical predictive models that identify and classify patients and aid clinical decision-making.Citation16 Simultaneously, some predictive models have also been developed for E-S grade based on magnetic resonance imaging (MRI) and radiomics.Citation17,Citation18 However, not all HCC patients undergo MRI, and radiomic features that require specific software analysis are impractical for most clinicians. This study aims to review the importance of predicting E-S grade and to develop a novel and user-friendly nomogram.
Patients and Methods
Patients and Data Collection
Patient medical data were obtained from the Nanjing Drum Tower Hospital HCC database (January 2020 to December 2022). Patients undergoing hepatectomy were retrospectively reviewed for this study with the following inclusion criteria: first diagnosis of HCC (non-recurrent patients); preoperative testing for tumor markers alpha-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP); no receiving targeted, immune, or local anti-tumor therapy before the surgery; and no primary tumor at another site. Laboratory test data for all patients were obtained within 1 week before the liver resection, which included hepatitis B virus surface antigen (HBsAg), hepatitis C virus antibodies (HCVAb), red blood cell distribution width, neutrophil (NE), lymphocyte (LYM), monocyte, platelet (PLT), alanine aminotransferase, aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), total bilirubin (TB), albumin (ALB), prothrombin time, and international normalized ratio. The patient’s liver reserve function was assessed with the albumin-bilirubin (ALBI) score, an objective, evidence-based, and simple method. The ALBI score = 0.66 × log[TB (µmol/L)] − 0.085 × ALB (g/L), including three levels (grade 1: ≤−2.60; grade 2: greater than −2.60 to ≤ −1.39; grade 3: > −1.39) according to the calculation results.Citation19 The calculation formulas of inflammatory markers are as follows: platelet to lymphocyte ratio (PLR) = PLT count (109/L)/LYM count (109/L), neutrophil to lymphocyte ratio (NLR) = NE count (109/L)/LYM count (109/L), systemic immune-inflammation index (SII) = PLT count (109/L) × NE count (109/L)/LYM count (109/L), aspartate aminotransferase to neutrophil ratio index (ANRI) = AST (U/L)/NE count (109/L), aspartate aminotransferase to lymphocyte ratio index (ALRI) = AST (U/L)/LYM count (109/L), γ-glutamyl transferase to lymphocyte ratio (GLR) = GGT (U/L)/LYM count (109/L).Citation20
Pathological Examination
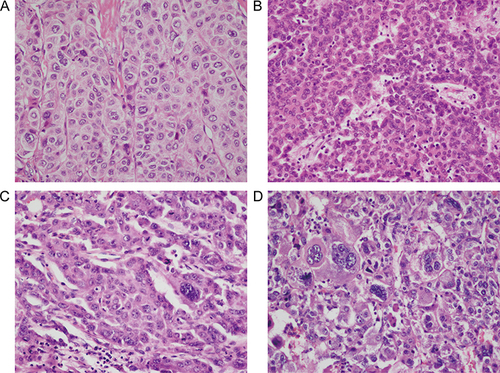
Pathological examination of all liver resection specimens was performed independently by two experienced pathologists. Any disagreements were negotiated and eventually reached a consensus. Tumor differentiation was determined according to the E-S grade,Citation5 typical pathological section images of E-S grade I to grade IV are shown in . MVI was defined as HCC cells that were microscopically visible in the lumen of the endothelium-lined vessel.Citation21
Imaging Examination
All included patients underwent MRI or contrast-enhanced computed tomography (CT) within 2 weeks before the hepatectomy. Supplementary Figure 1 presents typical imaging images of macrovascular invasion. The number of tumors was recorded as solitary or multiple. Tumor diameter was the average of the maximum diameter recorded by two radiologists. Besides, the presence of liver cirrhosis was considered when there were signs such as splenomegaly, ascites, esophageal and gastric varices, disproportionate liver lobes, and uneven liver contours.Citation22 Any disagreements were resolved by their discussion.
Statistical Analysis and Model Development
HCC patients were divided into two groups: E-S grade I–II and III–IV. The χ2 test and Mann–Whitney U-test were used to compare whether there were differences between the two groups for categorical and continuous variables, respectively. The subsequent multivariate logistic regression analysis included preoperative variables with p < 0.05 in the univariate analysis. R software (version 4.3.0) was used to establish a novel nomogram based on the multivariate logistic regression model. The receiver operating characteristic (ROC) curve was plotted using 1000 bootstrap samples to evaluate the diagnostic accuracy of the nomogram model,Citation16 and the area under the curve (AUC) value was calculated. The calibration curve with mean absolute error value was used to evaluate the calibration of the nomogram model. Finally, decision curve analysis (DCA) and clinical impact curve were used to demonstrate the net benefit at the different thresholds.
Results
Patients
Overall, 240 HCC patients who met the criteria were included in this study. Among them, 103 were highly differentiated (E-S grade I–II) HCC, and 137 were poorly differentiated (E-S grade III–IV) HCC (Supplementary Figure 2A). Our analysis proved once again that patients with E-S grade III–IV had a significantly higher probability of developing MVI (14.6%, 15 of 103 vs 35%, 48 of 137; p < 0.001) (Supplementary Figure 2B), which also indicated the clinical significance of preoperative prediction of E-S grade.
Risk Factors for E-S Grade III-IV
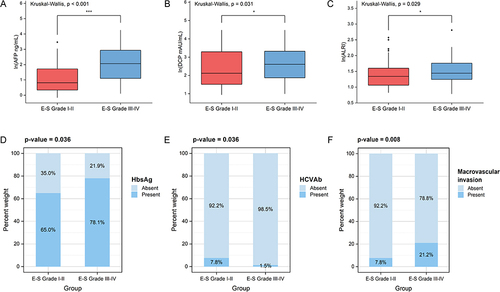
First, as shown in , the comparison of clinical characteristics was conducted between the two groups. Preoperative laboratory tests AFP, DCP, HBsAg, HCVAb, and ALRI and imaging-reported macrovascular invasion were associated with poor tumor differentiation (p < 0.05) (). Second, multivariate logistic regression was applied for the above six variables (). AFP, DCP, HCVAb, and imaging-reported macrovascular invasion were identified as independent predictors for E-S Grade III–IV.
Table 1 Baseline and Clinicopathological Participant Characteristics According to the Edmondson-Steiner Grade
Table 2 Multivariate Logistic Regression Analysis of Edmondson-Steiner Grade III–IV Based on Univariate Analysis
Figure 2 Six risk factors associated with Edmondson-Steiner grade III–IV in univariate analysis (p < 0.05). (A) Alpha-fetoprotein (AFP). (B) Des-γ-carboxy prothrombin (DCP). (C) Aspartate aminotransferase to lymphocyte ratio index (ALRI). (D) Hepatitis B virus surface antigen (HBsAg). (E) Hepatitis C virus antibodies (HCVAb). (F) Macrovascular invasion. *p < 0.05, ***p < 0.001. P-values less than 0.05 or 0.001 indicate significant difference.
Development and Evaluation of the Prediction Nomogram
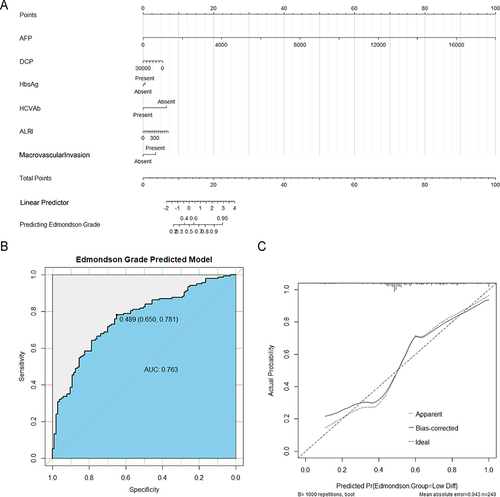
A novel nomogram was used to visualize the established multivariate logistic regression model (). The prediction model had a good diagnostic performance with an AUC value of 0.763 (95% CI: 0.702–0.823) (). Meanwhile, the model had a diagnostic accuracy of 72.5% (95% CI: 66.4–78.1%), a sensitivity of 78.1%, and a specificity of 65.1%. The calibration curve showed a good calibration of the nomogram model (mean absolute error = 0.043), indicating that the predicted E-S grade was basically consistent with the actual E-S grade (). DCA and clinical impact curve showed that the novel model increased the net clinical benefit ().
Figure 3 The development and evaluation of the nomogram model. (A) Established nomogram based on the multivariate logistic regression analysis. (B) The receiver operating characteristic (ROC) curve and area under the curve (AUC) value of the nomogram. (C) The calibration curve of the model (mean absolute error = 0.043).
Discussion
Although HCC patients who received curative treatment had a considerable improvement in survival, the overall 5-year survival rate for HCC was less than 15%.Citation23 This is partly because 70–80% of HCC patients can only receive local or systemic anti-tumor therapy after the first diagnosis.Citation14 However, micro- and macrovascular invasion, poor tumor differentiation, and the presence of satellite nodules are vital factors leading to postoperative recurrence.Citation24 Preoperative imaging allows careful evaluation of the patient’s macrovascular invasion, while MVI and E-S grade are pathologic diagnostic components. Nonetheless, not all hospital pathology departments can report the presence of MVI and the exact E-S grade. In fact, many models to predict MVI have been developed.Citation21,Citation25 Due to the important relationship between the two, focusing on the degree of tumor differentiation is necessary. This study found that patients with E-S grade III–IV had higher levels of AFP, DCP, and ALRI, higher positivity rates for HBsAg and macrovascular invasion, and a lower positivity rate for HCVAb. A user-friendly nomogram model integrating the six variables mentioned above was established.
AFP is a traditional biomarker for monitoring HCC, but using AFP alone had unacceptably low sensitivity.Citation23 Compared to several years ago, DCP is now routinely detected clinically. Both Wang et al and Zhou et al showed that the preoperative DCP positivity rate for resectable HCC was significantly higher than that of the AFP positivity rate.Citation26,Citation27 Besides, a notably higher proportion of patients with positive AFP and DCP had MVI and E-S grade III–IV.Citation27 Many previous studies have confirmed that elevated AFP and DCP are independent risk factors for MVI,Citation21,Citation25,Citation28 although the cutoff values differ. Our study revealed that higher levels of tumor markers were also linked to poor HCC differentiation. In other words, patients with double-positive biomarkers on admission should also be the focus of surveillance.
In China, hepatitis B virus (HBV) infection is the leading factor causing HCC. Serum HBsAg positive rate and HBV DNA load were significantly higher in the severe MVI group (M2) compared with the mild MVI group (M1) and the MVI-negative group.Citation29 HCC can be classified as early or late recurrence depending on the recurrence time after resection. Yoo et al first proved that HBsAg seroclearance after hepatectomy was independently associated with a reduced risk of late recurrence (≥2 years) of HCC.Citation30 Early recurrence is correlated with aggressive tumor characteristics (eg, presence of MVI and poor differentiation), whereas late recurrence is related to the severity of underlying liver disease (eg, uncontrolled chronic hepatitis B [CHB] and cirrhosis).Citation31,Citation32 We found that HBsAg positivity was a risk factor for poor HCC differentiation. Combined with previous studies, HBV infection status impacts both early and late HCC recurrences.Citation33 Hence, antiviral therapy should be administered throughout HCC treatment to achieve a functional cure (loss of HBsAg) for CHB.Citation34 Nevertheless, only 10 patients with hepatitis C virus (HCV)-associated HCC were included in this study, and the relationship between HCV infection and E-S grade needs to be confirmed by further studies.
It is known that chronic inflammation plays a central role in the development of HCC, and there is also a direct causal relationship between inflammatory tumor microenvironment and tumorigenesis.Citation35 The ALRI index allows assessment of the general inflammatory status of the patient. Hepatocellular damage due to chronic inflammation leads to elevated AST, and low LYM counts reflect the poor anti-cancer intensity of HCC patients. Casadei Gardini et al indicated that elevated ALRI was significantly associated with an increased risk of HCC in cirrhotic patients.Citation36 In addition, higher ALRI levels were related to higher TNM stage, larger tumor size, and shorter overall survival (OS) and disease-free survival (DFS) in HCC patients.Citation37 No previous studies have reported an association between ALRI and E-S grade, but Li et al discovered two other inflammatory markers, NLR and derived neutrophil to lymphocyte ratio (dNLR), could predict E-S grade III–IV.Citation38
Vascular invasion (VI) is a common phenomenon in HCC, with an incidence of 25%–50%.Citation39 Macrovascular invasion is tumor invasion of the hepatic or portal vein branches.Citation40 Krishnan et al first identified MYC proto-oncogene (MYC)-driven transcriptional, epigenetic, and proteomic changes as essential factors in the development of VI.Citation39 MYC is an upstream regulator of n-myc downstream-regulated gene 1 (NDRG1), which is also highly expressed in HCC.Citation41 Moreover, it was demonstrated that moderately and poorly differentiated HCC expressed higher levels of NDRG1 compared to well-differentiated HCC.Citation42 Our study revealed that macrovascular invasion was an independent predictor of E-S grade III–IV, reflecting the more aggressive nature of poorly differentiated tumor cells.
Three variables (NLR, dNLR, and tumor volume) were included in the user-friendly nomogram established by Li et al to predict E-S grade III–IV.Citation38 The model had an AUC value of 0.727 (95% CI: 0.690–0.761), a sensitivity of 51.3%, and a specificity of 81.1%. Similar to tumor markers, predictive models should have a higher sensitivity to screen high-risk patients. Our developed nomogram had a diagnostic sensitivity of 78.1% and a higher AUC value, which is superior to the existing model. This study may be helpful in clinical practice in the following three aspects. For surgeons, patients predicted to be E-S grade III–IV preoperatively may undergo anatomic or wide-margin (≥1cm) hepatectomy. For pathologists, pathologic specimens from high-risk patients should be examined more closely to determine the accurate E-S grade. For patients, they should receive more frequent follow-up visits and cooperate with the doctor’s treatment if predicted to have a poorly differentiated HCC.
Our research has several limitations. First, a single-center retrospective study had problems with insufficient sample size and selection bias. Second, external validation cohorts are lacking to assess the diagnostic accuracy of the model further. Lastly, the realities of HCC incidence in China led us to include mainly patients with HBV-related HCC.
Conclusion
In conclusion, we established a novel and user-friendly nomogram that integrated AFP, DCP, HBsAg, HCVAb, ALRI, and macrovascular invasion to predict E-S grade III–IV in HCC patients. The model performs well in diagnostic ability, calibration, and clinical benefits, which may be helpful in clinical decision-making. Predicted high-risk patients should be carefully evaluated for treatment options and be the focus of follow-up monitoring.
Data Sharing Statement
The dataset analyzed during the current study is available from corresponding authors upon reasonable request.
Ethics Approval
The institutional review board of The Affiliated Drum Tower Hospital of Nanjing University Medical School approved this retrospective study, and the requirement for written informed consent was waived due to its retrospective study nature. All included patients’ personal information is strictly confidential. This study followed the 1964 Declaration of Helsinki and its later amendments.
Disclosure
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi:10.6004/jnccn.2021.0022
- Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
- Zhou L, Rui JA, Wang SB, et al. Prognostic factors of solitary large hepatocellular carcinoma: the importance of differentiation grade. Eur J Surg Oncol. 2011;37(6):521–525. doi:10.1016/j.ejso.2011.03.137
- Zeng J, Zeng J, Lin K, et al. Development of a machine learning model to predict early recurrence for hepatocellular carcinoma after curative resection. Hepatobiliary Surg Nutr. 2022;11(2):176–187. doi:10.21037/hbsn-20-466
- Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi:10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e
- Kim SU, Jung KS, Lee S, et al. Histological subclassification of cirrhosis can predict recurrence after curative resection of hepatocellular carcinoma. Liver Int. 2014;34(7):1008–1017. doi:10.1111/liv.12475
- Zhu P, Liao W, Zhang WG, et al. A prospective study using propensity score matching to compare long-term survival outcomes after robotic-assisted, laparoscopic, or open liver resection for patients with BCLC stage 0-A Hepatocellular carcinoma. Ann Surg. 2023;277(1):e103–e111. doi:10.1097/SLA.0000000000005380
- Xu LX, He MH, Dai ZH, et al. Genomic and transcriptional heterogeneity of multifocal hepatocellular carcinoma. Ann Oncol. 2019;30(6):990–997. doi:10.1093/annonc/mdz103
- Chang YS, Chou YP, Chung CC, et al. Molecular classification of Hepatocellular carcinoma using wnt-hippo signaling pathway-related genes. Cancers. 2022;14(19):4580. doi:10.3390/cancers14194580
- Mou H, Yang QA, Yu L, et al. Programmed cell death-ligand 1 expression in hepatocellular carcinoma and its correlation with clinicopathological characteristics. J Gastroenterol Hepatol. 2021;36(9):2601–2609. doi:10.1111/jgh.15475
- Qu C, Huang X, Liu K, et al. Effect of hepatitis B virus DNA replication level and anti-HBV therapy on microvascular invasion of hepatocellular carcinoma. Infect Agent Cancer. 2019;14:2. doi:10.1186/s13027-019-0219-8
- Yang P, Si A, Yang J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery. 2019;165(4):721–730. doi:10.1016/j.surg.2018.09.016
- Jiao S, Li G, Zhang D, et al. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int J Surg. 2020;80:243–255. doi:10.1016/j.ijsu.2020.05.008
- Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of Hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
- Wang L, Ke Q, Lin K, et al. Not all Hepatocellular carcinoma patients with microvascular invasion after R0 resection could be benefited from prophylactic transarterial chemoembolization: a propensity score matching study. Cancer Manag Res. 2020;12:3815–3825. doi:10.2147/CMAR.S251605
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
- Tang M, Zhou Q, Huang M, et al. Nomogram development and validation to predict hepatocellular carcinoma tumor behavior by preoperative gadoxetic acid-enhanced MRI. Eur Radiol. 2021;31(11):8615–8627. doi:10.1007/s00330-021-07941-7
- Mao Y, Wang J, Zhu Y, et al. Gd-EOB-DTPA-enhanced MRI radiomic features for predicting histological grade of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022;11(1):13–24. doi:10.21037/hbsn-19-870
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
- Mao S, Yu X, Shan Y, et al. Albumin-Bilirubin (ALBI) and Monocyte to Lymphocyte Ratio (MLR)-based nomogram model to predict tumor recurrence of AFP-negative hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:1355–1365. doi:10.2147/JHC.S339707
- Xu X, Zhang HL, Liu QP, et al. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol. 2019;70(6):1133–1144. doi:10.1016/j.jhep.2019.02.023
- Ji GW, Zhu FP, Xu Q, et al. Radiomic features at contrast-enhanced CT predict recurrence in early stage Hepatocellular carcinoma: a multi-institutional study. Radiology. 2020;294(3):568–579. doi:10.1148/radiol.2020191470
- Tayob N, Kanwal F, Alsarraj A, et al. The performance of AFP, AFP-3, DCP as biomarkers for detection of Hepatocellular carcinoma (HCC): a Phase 3 biomarker study in the United States. Clin Gastroenterol Hepatol. 2023;21(2):415–423.e4. doi:10.1016/j.cgh.2022.01.047
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
- Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151(4):356–363. doi:10.1001/jamasurg.2015.4257
- Wang MD, Sun LY, Qian GJ, et al. Prothrombin induced by vitamin K Absence-II versus alpha-fetoprotein in detection of both resectable hepatocellular carcinoma and early recurrence after curative liver resection: a retrospective cohort study. Int J Surg. 2022;105:106843. doi:10.1016/j.ijsu.2022.106843
- Zhou Z, Liu J, Xu X. A commentary on ‘Prothrombin induced by vitamin K Absence-II versus alpha-fetoprotein in detection of both resectable hepatocellular carcinoma and early recurrence after curative liver resection: a retrospective cohort study’. Int J Surg. 2023;105:106843. doi:10.1097/JS9.0000000000000119
- He YZ, He K, Huang RQ, et al. Preoperative evaluation and prediction of clinical scores for hepatocellular carcinoma microvascular invasion: a single-center retrospective analysis. Ann Hepatol. 2020;19(6):654–661. doi:10.1016/j.aohep.2020.07.002
- Wei X, Li N, Li S, et al. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer. 2017;17(1):304. doi:10.1186/s12885-017-3293-6
- Yoo S, Kim JY, Lim YS, et al. Impact of HBsAg seroclearance on late recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. J Hepatol. 2022;77(4):939–946. doi:10.1016/j.jhep.2022.05.014
- Hoshida Y. Risk of recurrence in hepatitis B-related hepatocellular carcinoma: impact of viral load in late recurrence. J Hepatol. 2009;51(5):842–844. doi:10.1016/j.jhep.2009.08.003
- Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51(5):890–897. doi:10.1016/j.jhep.2009.07.009
- Li Z, Lei Z, Xia Y, et al. Association of preoperative antiviral treatment with incidences of microvascular invasion and early tumor recurrence in hepatitis B virus-related Hepatocellular carcinoma. JAMA Surg. 2018;153(10):e182721. doi:10.1001/jamasurg.2018.2721
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi:10.1002/hep.29800
- Zhang W, Zhangyuan G, Wang F, et al. The zinc finger protein Miz1 suppresses liver tumorigenesis by restricting hepatocyte-driven macrophage activation and inflammation. Immunity. 2021;54(6):1168–1185.e8. doi:10.1016/j.immuni.2021.04.027
- Casadei Gardini A, Foschi FG, Conti F, et al. Immune inflammation indicators and ALBI score to predict liver cancer in HCV-patients treated with direct-acting antivirals. Dig Liver Dis. 2019;51(5):681–688. doi:10.1016/j.dld.2018.09.016
- Liao M, Sun J, Zhang Q, et al. A novel post-operative ALRI model accurately predicts clinical outcomes of resected Hepatocellular carcinoma patients. Front Oncol. 2021;11:665497. doi:10.3389/fonc.2021.665497
- Li P, Huang W, Wang F, et al. Nomograms based on inflammatory biomarkers for predicting tumor grade and micro-vascular invasion in stage I/II hepatocellular carcinoma. Biosci Rep. 2018;38(6):BSR20180464. doi:10.1042/BSR20180464
- Krishnan MS, Rajan Kd A, Park J, et al. Genomic analysis of vascular invasion in HCC reveals molecular drivers and predictive biomarkers. Hepatology. 2021;73(6):2342–2360. doi:10.1002/hep.31614
- Costentin CE, Ferrone CR, Arellano RS, et al. Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy. Liver Cancer. 2017;6(4):360–374. doi:10.1159/000481315
- Chekmarev J, Azad MG, Richardson DR. The oncogenic signaling disruptor, NDRG1: molecular and cellular mechanisms of activity. Cells. 2021;10(9):2382. doi:10.3390/cells10092382
- Chua MS, Sun H, Cheung ST, et al. Overexpression of NDRG1 is an indicator of poor prognosis in hepatocellular carcinoma. Mod Pathol. 2007;20(1):76–83. doi:10.1038/modpathol.3800711