Abstract
Objective
This study aims to identify independent risk factors for ultra-early recurrence in patients with early solitary hepatocellular carcinoma (HCC) and develop an individualized predictive nomogram for ultra-early recurrence.
Materials and Methods
A total of 332 patients with early solitary HCC who underwent curative liver resection at our hospital from January 2015 to May 2021 were included in this study. Based on the patients’ recurrence status at 6 months, they were divided into the non-ultra-early recurrence group and the ultra-early recurrence group. Univariate and multivariate Cox regression analyses were used to construct the nomogram, and internal validation of its performance was performed using calibration plots with bootstrapping.
Results
Among the 332 patients with early solitary HCC, 39 (11.7%) experienced ultra-early recurrence. Tumor morphology, age > 46 years, AFP > 332.4 ng/mL, GGT > 51.2 U/L, ALP > 126 U/L, PT > 12.8 s, and satellite nodules were identified as independent prognostic factors for ultra-early recurrence in patients with early solitary HCC and were incorporated into the final predictive nomogram. The C-index of the nomogram and bootstrap resampling were 0.842 and 0.815, respectively. The calibration plot demonstrated good agreement between the predicted and observed probabilities of ultra-early recurrence, and DCA indicated the favorable clinical utility of the nomogram. Additionally, AFP > 332.4 ng/mL, AST > 35 U/L, GGT > 51.2 U/L, ALP > 126 U/L, tumor morphology, tumor size, satellite nodules, and intratumoral hemorrhage were identified as risk factors for overall survival in patients with early solitary HCC.
Conclusion
Our study establishes a nomogram for predicting the postoperative ultra-early recurrence status in patients with early solitary HCC, which provides valuable supplementary decision-making information for clinical decision-makers and guides the selection of the most appropriate treatment strategy.
Introduction
Surgical treatment remains the preferred approach for early-stage solitary hepatocellular carcinoma (HCC) patients.Citation1 However, up to 70% of patients who undergo curative liver resection experience postoperative recurrence within 5 years,Citation2,Citation3 leading to a suboptimal long-term prognosis. Currently, most studies define early recurrence of HCC as recurrence within 2 years, but this cutoff is not universally accepted. Some studies have defined early recurrence as recurrence at any time between 6 months to 2 years after liver resection.Citation4–7 Especially, cases of early recurrence without any disease-free interval severely reduce patients’ overall survival time and increase the difficulty of retreatment and the risk of fatal complications.Citation11,Citation12 Therefore, a comprehensive understanding of the risk factors for early recurrence after liver resection in early-stage solitary HCC patients can help clinicians reevaluate the necessity of surgery for such patients. Additionally, accurate stratification of early-stage solitary HCC patients before surgery and personalized neoadjuvant and adjuvant therapies for patients with early recurrence may potentially extend overall survival.Citation13–15
Currently, we face some challenges in accurately assessing short-term recurrence in patients with HCC after surgery. Most clinical prediction models are based on postoperative pathological factors, such as tumor differentiation, microvascular invasion, etc., making it difficult to assess patients’ recurrence status before surgery.Citation5–7,Citation16,Citation17 However, these factors often need to be identified after surgery, which limits the accurate assessment of recurrence before surgery. Therefore, there is an urgent need for a non-invasive method to predict short-term recurrence in HCC patients after surgery, guiding the selection of appropriate candidates for liver resection. Previous studies have demonstrated that the imaging features defined by the Liver Imaging Reporting and Data System (LI-RADS) combined with clinical laboratory examinations can be used to evaluate long-term survival and adverse prognosis in HCC patients. Zhang et alCitation4 found that tumor size, tumor number, macrovascular invasion, unsmooth tumor margin, peritumoral enhancement, and low peritumoral signal on HBP images were related to the early recurrence of HCC, especially macrovascular invasion and unsmooth tumor margin were considered to be independent predictors. Li et alCitation8 also found that invasion of large blood vessels was a key factor in predicting early recurrence of HCC. In addition, satellite nodules and single nodule with extraterritorial growth were also associated with recurrence. Our previous studyCitation18 excluded large vessel invasion cases that were not suitable for surgery and added more complementary features defined by LI-RADS. We further found that patients with tumor size > 5cm, intratumoral hemorrhage, and confluent multinodular type were more prone to early recurrence. In addition, these studies also took into account lab indicators, such as preoperative AFP levels,Citation4,Citation8,Citation18 ALT or AST,Citation8 and ALP,Citation18 which also show great potential in predicting early postoperative HCC recurrence. Although these predictors are still being developed and refined, the presence of these factors may suggest that the tumor has a higher degree of malignancy and a worse prognosis. However, these predictors have been relatively rare in studies evaluating short-term recurrence after surgery in patients with solitary hepatocellular carcinoma, meaning that more research and validation are needed.
This study defines HCC recurrence within 6 months after curative liver resection as “ultra-early recurrence” and aims to predict the status of ultra-early recurrence and its impact on overall survival (OS) in early-stage solitary HCC patients using imaging features obtained from magnetic resonance imaging (MRI) combined with clinical laboratory indicators. The goal is to guide precise treatment decisions for clinical doctors and patients.
Materials and Methods
Patient Selection
This retrospective study was approved by the institutional review board and waived the requirement for informed consent from patients, following the ethical principles of the Helsinki Declaration. The study included 367 consecutive patients who underwent curative liver resection for solitary HCC between January 2015 and May 2021 at our hospital. Curative liver resection was defined as complete resection of the visible tumor with no residual tumor cells at the surgical margins. Early-stage solitary HCC was defined as a single tumor with good liver function and no clinically significant portal hypertension.Citation1,Citation19
Inclusion criteria: a. Patients diagnosed with primary liver cancer and underwent contrast-enhanced magnetic resonance imaging (MRI); b. MRI showed a solitary tumor with no vascular invasion or distant metastasis; c. Preoperative liver function was classified as Child-Pugh class A or good partial function of class B; d. Curative liver resection performed within 1 month after MRI without prior treatment; e. Postoperative pathology confirmed HCC.
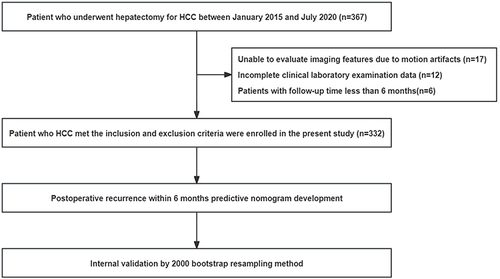
Exclusion criteria: a. Motion artifacts that hindered evaluation of imaging features; b. Incomplete clinical laboratory data; c. Patients with a follow-up duration of less than 6 months. The study flowchart is shown in , and a total of 332 patients were finally included in the study.
Clinical Laboratory Examinations
Patient clinical laboratory indicators, including gender, age, liver cirrhosis, hepatitis, alpha-fetoprotein (AFP), alanine transaminase (ALT), aspartate transaminase (AST), ALT/AST ratio, gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total bilirubin (TBIL), direct bilirubin (DBIL), platelet count (PLT), prothrombin time (PT), and Child-Pugh classification, were extracted from the electronic medical record system of our hospital.
MRI Acquisition
All patients underwent liver multi-parametric MRI using a 3.0-T MRI system and a 32-channel phased-array coil. Patients were asked to fast before the examination and undergo routine breath-holding training to minimize respiratory motion artifacts. Image scanning started at end-expiration with patients in a supine position, and the scanning range extended from the diaphragm to the lower edge of the liver. The liver multi-parametric MRI protocol included axial dual-echo (in-phase and opposed-phase) T1-weighted imaging, fat-suppressed T2-weighted imaging, and dynamic contrast-enhanced imaging. Dynamic contrast-enhanced imaging involved injecting a liver-specific contrast agent at a high-pressure injector through the antecubital vein at a rate of 1 mL/s with gadoteric acid (Primovist, 0.1 mL/kg) or at a rate of 2 mL/s with gadobenic acid (0.2 mL/kg), followed by a 20 mL flush of saline at 2 mL/s. The contrast-enhanced images were obtained at six-time points: pre-contrast and five post-contrast time points: arterial phase (28 s), portal venous phase (55 s), equilibrium phase (90 s), transitional phase (180 s), and hepatobiliary phase (20 min). Standard dynamic enhancement included only the arterial phase (25 s), portal venous phase (55 s), and delayed phase (180 s). Post-processing was performed to subtract the pre-contrast images from the post-contrast images, producing a clearer and more intuitive tumor enhancement status.
Image Analysis
All images were retrieved from the Picture Archiving and Communication System (PACS) in Digital Imaging and Communications in Medicine (DICOM) format. Two independent radiologists with 4 and 6 years of abdominal imaging experience, respectively, performed qualitative assessments of the imaging features of liver lesions and were blinded to all clinical pathology results and image interpretations. The two radiologists referred to LI-RADS Version 2018Citation20 and Chernyak’s reviewCitation21 and primarily evaluated the following imaging features: 1) tumor morphology:Citation18 a. nodular, b. nodular with extrahepatic growth, and c. confluent nodular; 2) tumor size; 3) arterial phase hyperenhancement: a. non-rim arterial phase hyperenhancement, b. no enhancement, or c. rim arterial phase hyperenhancement; 4) washout: a. non-washout, b. non-rim washout, and c. rim washout; 5) radiologic capsule: a. no capsule, b. incomplete capsule, and c. complete capsule; 6) peritumoral enhancement; 7) mosaic appearance; 8) intratumoral necrosisCitation22,Citation23; 9) intratumoral ischemiaCitation23; 10) intratumoral hemorrhageCitation24; 11) intratumoral fat; 12) satellite nodulesCitation24; 13) LI-RADS classification: a. LR-4, b. LR-5, and c. LR-M. All discrepancies in qualitative imaging evaluations were resolved through consensus or consultation with a third radiologist with 18 years of abdominal imaging experience. Specific feature definitions are shown in Supplementary Table 1.
Follow-Up of Patients After Surgical Resection
All patients underwent the first follow-up 4–6 weeks after surgery, followed by follow-ups at 3 months and 6 months post-surgery, and then every 6 months thereafter. The follow-up included medical history, physical examination, AFP level, liver function tests, and abdominal ultrasound or contrast-enhanced CT/MRI. Patients who did not return for follow-up at our hospital were contacted by telephone. The last follow-up date was July 30, 2022.
Definition of Ultra-Early Recurrence
Ultra-early recurrence was defined as the occurrence of new tumors within the liver and/or outside the liver within 6 months after curative liver resection. When ultra-early recurrence was suspected, patients were recommended for hospitalization and further contrast-enhanced CT or MRI, bone scan, or positron emission tomography-computed tomography (PET-CT) examinations to confirm the diagnosis. The date of the first HCC recurrence, the date of follow-up termination, and the date of death were recorded. The overall survival (OS) was defined as the period from the date of liver resection to death or the last follow-up date. Patients lost to follow-up were censored.
Statistical Analysis
X-tile software was used to determine the optimal cutoff values of clinical laboratory indicators for differentiating between the ultra-early recurrence group and the non-ultra-early recurrence group, and continuous variables were transformed into categorical variables for further analysis. Categorical variables were presented as proportions (%) and numbers (N), and comparisons were made using the chi-square test or Fisher’s exact test. Univariate and multivariate Cox proportional hazards models were used to determine the independent prognostic factors affecting ultra-early recurrence, and hazard ratios (HRs) were reported with 95% confidence intervals (CIs). The multivariate Cox regression analysis included potential predictive factors with P<0.1 in the univariate analysis, using a forward stepwise regression method to select appropriate variables for building the prediction model for ultra-early recurrence after HCC resection. The nomogram was established based on the prediction model and internally validated using 2000 bootstrap resamples. The concordance index (C-index) was used to measure the discriminative ability of the nomogram, with a larger C-index indicating more accurate recurrence prediction. Calibration curves were used to validate the consistency between observed outcomes and predicted probabilities, and decision curve analysis (DCA) was performed to quantify the net benefit probability to evaluate the clinical application value of the nomogram.
The Kaplan-Meier method with Log rank test was used to evaluate the impact of ultra-early recurrence status, clinical laboratory indicators, and qualitative imaging features on the overall survival of patients with early-stage solitary HCC. All statistical analyses were performed using SPSS version 26.0 (IBM, NY, USA) and R language version 3.3.0. A significance level of P<0.05 was considered statistically significant.
Results
Patient Characteristics
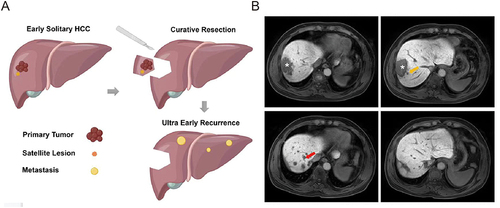
A total of 332 patients (268 males and 64 females) with a mean age of 53.9±9.2 years (range 18–73 years) were included in our study. Based on the time of recurrence, patients were divided into the ultra-early recurrence group (39 cases, 11.7%) and the non-ultra-early recurrence group (293 cases, 88.3%). Of these ultra-early recurrence patients, 24 cases (61.5%) had intrahepatic recurrence, 6 cases (15.4%) had intrahepatic combined recurrence, and 3 cases (7.7%) had only extrahepatic recurrence, 6 cases (15.4%) with recurrence was confirmed by telephone follow-up, but the location of recurrence could not be determined. Furthermore, out of these patients, 21 individuals (53.8%) decided to pursue further treatment. This included 2 opting for surgical interventions, 4 undergoing ablation treatments, 12 receiving TACE (Transhepatic Arterial Chemoembolization), and 3 choosing targeted immunotherapy. The median time to recurrence in the recurrence group was 3.80 months (95% CI, 3.07–4.00 months). The optimal cutoff values of clinical laboratory indicators for predicting ultra-early recurrence were determined, and they were transformed into categorical variables for analysis. summarizes the baseline characteristics of the patients, and illustrates examples of ultra-early recurrence status.
Table 1 Baseline Characteristics
Figure 2 (A) Schematic diagram; (B) Case: Male, 54 years old. On August 9, 2017, liver-specific contrast-enhanced MRI showed a solitary lesion (asterisk) in the right lobe of the liver, with a fusion nodular type of growth and multiple satellite nodules (yellow arrows) in the surrounding area. Preoperative AFP, GGT, ALP, and PT levels were 4881 ng/mL, 127 U/L, 87 U/L, and 13s, respectively. The postoperative pathological diagnosis was hepatocellular carcinoma. On January 3, 2018, liver-specific contrast-enhanced MRI showed multiple nodular lesions (red arrows) in the liver, but no obvious mass was observed in the surgical area.
Construction of the Nomogram to Predict Ultra-Early Recurrence
Among the 26 clinical laboratory and imaging features analyzed, 13 were found to have potential correlations with ultra-early recurrence in the univariate COX regression analysis (P<0.1, Supplementary Table 2). Among them, seven clinical laboratory indicators (age, AFP, AST, GGT, ALP, PLT, and PT) and five imaging features (tumor morphology, tumor size, peritumoral enhancement, intratumoral hemorrhage, and radiological capsule) were significantly associated with ultra-early recurrence (P<0.05) except for satellite nodules (P=0.068).
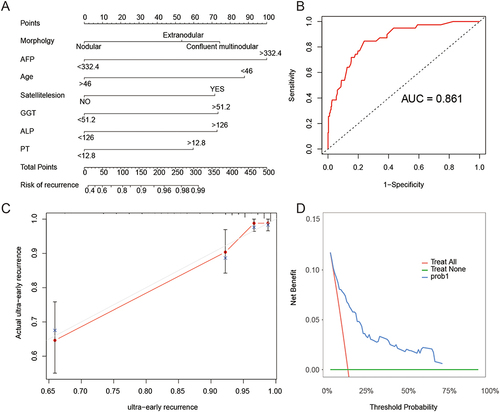
The potential predictive factors with P<0.1 in the univariate COX regression analysis were included in the multivariate COX regression analysis (). The results showed that tumor morphology, age > 46 years, AFP > 332.4 ng/mL, GGT > 51.2 U/L, ALP > 126 U/L, PT > 12.8 s, and satellite nodules were independent prognostic factors affecting ultra-early recurrence. Based on these seven independent prognostic factors, the nomogram for predicting ultra-early recurrence in patients with early-stage solitary HCC was constructed (), and it was found that AFP > 332.4 ng/mL (HR=4.432 [95% CI: 2.278–8.621]) and the confluent-type nodule (HR=3.033 [95% CI: 1.281–7.183]) had the greatest impact on ultra-early recurrence in patients with early-stage solitary HCC.
Table 2 Univariate and Multivariate Cox Regression Analysis Results for Ultra-Early Recurrence
Figure 3 (A) Nomogram for preoperative prediction of the risk of ultra-early recurrence after curative resection of early isolated hepatocellular carcinoma; (B) The area under receiver operating characteristic (ROC) curve (AUC) of the nomogram for ultra-early recurrence; (C) Calibration curves of the nomogram comparing and actual and predicted probabilities of ultra-early recurrence; (D) Decision curve analysis of the nomogram for ultra-early recurrence prediction.
Validation of the Nomogram for Predicting Ultra-Early Recurrence
The predictive performance of the nomogram was evaluated using the concordance index (C-index). The results showed that the nomogram had good predictive performance for ultra-early recurrence in patients with early-stage solitary HCC (C-index = 0.842, AUC = 0.861) (), and the internal validation using bootstrap analysis also demonstrated good predictive value (C-index = 0.815). The calibration curve showed good consistency between the predicted risk of ultra-early recurrence and the observed incidence of ultra-early recurrence, indicating the reliability of the nomogram. In addition, the DCA further confirmed the clinical utility of the nomogram ( and ).
Factors Affecting Overall Survival of Patients
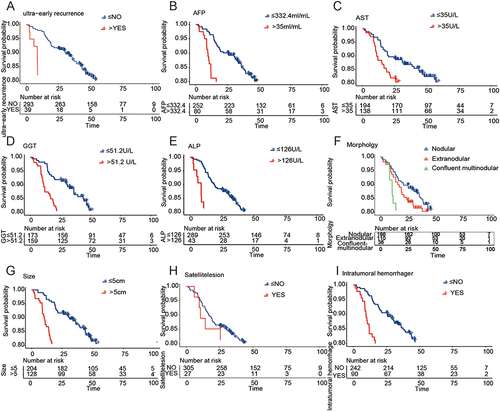
The results of our study showed that the overall survival of patients with ultra-early recurrence (mean 30.96 months, 95% CI 22.18–39.73 months) was significantly lower than that of patients without ultra-early recurrence (mean 80.11 months, 95% CI 76.18–84.04 months) (P<0.001, ). Moreover, AFP > 332.4 ng/mL, AST > 35 U/L, GGT > 51.2 U/L, ALP > 126 U/L, tumor morphology, tumor size, satellite nodules, and intratumoral hemorrhage were identified as risk factors affecting the overall survival of patients with early-stage solitary HCC (P<0.05, ).
Figure 4 Factors affecting the overall survival after liver resection in patients with early solitary hepatocellular carcinoma. (A) Survival curves for patients with super-early recurrence and those without super-early recurrence. (B-I) Survival curves for AFP, AST, GGT, ALP, tumor morphology, tumor size, satellite nodules, and intratumoral hemorrhage.
Discussion
Patients with early-stage solitary HCC and good liver function are recommended to undergo radical liver resection, regardless of tumor size.Citation1 However, our study found that approximately 11.7% of early-stage solitary HCC patients experience tumor recurrence within 6 months, which leads to the need for preoperative interventions or postoperative adjuvant therapies for this subgroup of patients. Therefore, developing a reliable diagnostic method to predict super early recurrence after radical liver resection in HCC patients is crucial. In our study, we established a nomogram consisting of five clinical laboratory indicators and two qualitative imaging features to predict the super early recurrence status of patients with early-stage solitary HCC. The nomogram demonstrated good discriminative performance and calibration ability, aiming to provide guidance for individualized diagnosis and treatment for clinicians and patients.
Previous studies have already demonstrated a significant correlation between tumor morphology and the 2-year recurrence-free survival rate and overall survival rate in HCC patients.Citation18 Our study further confirmed that tumor morphology is an independent risk factor influencing super-early recurrence in patients with early-stage solitary HCC. This finding is consistent with Xu et al’s conclusion that tumor morphology is independently related to the long-term prognosis of patients with solitary giant HCC (≥10 cm).Citation25 The analysis suggests that compared to nodular-type HCC, massive-type HCC with infiltrative or fused nodules has higher proliferative and invasive capabilities, leading to a higher tendency for vascular invasion and microvascular invasion.Citation26,Citation27 Additionally, the presence of satellite nodules has been reported to be independently associated with poor long-term survival and adverse prognosis in HCC patients.Citation5,Citation8 This may be due to satellite nodules representing micrometastases formed through the portal vein system from the main tumor. Thus, even with clear margins after radical liver resection, patients with satellite nodules still face a higher risk of super-early recurrence. It is noteworthy that in our study, the results of the univariate analysis showed a potential association between satellite nodules and super-early recurrence status, and in the multivariate analysis, satellite nodules emerged as another independent risk factor for predicting super-early recurrence in HCC patients. The reason behind this finding could be the interaction between satellite nodules and other factors such as tumor size, tumor capsule enhancement, intratumoral hemorrhage, and radiological capsule.Citation28 After removing the influence of other factors through multivariate analysis, the independent effect of satellite nodules was revealed.
In the current stage, the preoperative level of AFP has been proven to be useful for predicting early recurrence in HCC patientsCitation2,Citation4,Citation5,Citation8,Citation17,Citation24 However, the optimal cutoff value for AFP remains controversial. The widely used cutoff values in research are 20 ng/mL,Citation5 100 ng/mL,Citation24 and 400 ng/mL.Citation4,Citation5,Citation8 In our study, we selected 332.4 ng/mL as the optimal cutoff value for AFP based on the super early recurrence status of patients with early-stage solitary HCC. The results showed that AFP can serve as an independent risk factor for predicting super early recurrence after radical liver resection in early-stage solitary HCC patients. This may be because higher AFP levels can accelerate the proliferation and metastasis of HCC cells by inhibiting apoptosis and suppressing the anti-tumor immune response.Citation29 Currently, various AFP vaccines, such as DC vaccinesCitation30,Citation31 and peptide vaccines,Citation32 have been applied in HCC mouse models and clinical trials, showing potential as targeted therapies for high-risk patients in the adjuvant setting. Apart from AFP, our research also identified GGT > 51.2 U/L, ALP > 21 U/L, and PT > 12.8s as independent risk factors promoting super early recurrence after surgery. However, GGT, ALP, and PT also face the challenge of lacking universally accepted optimal cutoff values, leading to controversy in their contributions to adverse outcomes in HCC patients. Xu et al’s studyCitation33 suggested that GGT > 115 U/L and ALP > 120 U/L were independent factors influencing patient prognosis, while Ma et alCitation7 found no association between GGT, ALP, and PT levels with long-term survival when the optimal cutoff values were 50 U/L, 100 U/L, and 13.5s. Xu et al’s researchCitation6 identified GGT > 60 U/L as an independent factor affecting the 5-year overall survival of HCC patients, but GGT and ALP measurements, as well as a PT cutoff value of 17s, were not related to patient outcomes. Despite the different results from these studies, it is undeniable that the fact that HCC patients are more prone to recurrence or death after surgery in the context of abnormal liver metabolism or liver and gallbladder dysfunctionCitation34 is continuously being proven. It is worth noting that standardization of optimal cutoff values for different populations still requires further research.
Furthermore, our study found that patients who experienced super early recurrence had significantly shorter survival times compared to those who did not, indicating that the super early recurrence status of HCC patients after radical liver resection severely constrains their long-term survival. At present, clinical research widely believes that early recurrence originates from occult metastases of tumors within the liver through portal vein circulation.Citation9 This recurrence pattern suggests that perioperative treatment may offer significant benefits for improving the prognosis of HCC patients, as it allows for early multidisciplinary and systematic treatment to control the occult metastases of HCC.Citation13,Citation14 As of October 2020, there are 24 promising projects related to adjuvant therapies for HCC globally,Citation13 and studies have already shown that preoperative administration of neoadjuvant radiotherapy leads to longer disease-free survival and overall survival in HCC patients.Citation35 In addition, postoperative adjuvant therapies also have potential advantages in prolonging disease-free survival and reducing extrahepatic recurrence. Zhang et al’s reviewCitation15 summarized at least nine types of postoperative adjuvant therapies effective in preventing HCC recurrence after radical liver resection, and they suggested that antiviral therapy and postoperative adjuvant transarterial chemoembolization (TACE) significantly reduce intrahepatic recurrence rates after liver resection in HCC patients. Therefore, with the widespread application of drug development and new treatment methods, the treatment landscape for HCC patients will be different from the past. Our research may provide a reference for selecting the treatment strategy for patients with early-stage solitary HCC who plan to undergo perioperative treatment.
Our study has certain limitations. Firstly, due to the limited sample size and positive rate, we only used Bootstrap resampling for internal validation and did not conduct independent internal and external validations of the predictive model. In future research, we will increase the sample size and further adjust the predictive model. Secondly, the wide period of the study samples resulted in some patients with postoperative early-stage recurrence not receiving standardized treatments. Subsequent research will supplement the treatment plans for patients with recurrence, including repeat resection, local ablation, or other effective therapies. Thirdly, our study predicted the postoperative early-stage recurrence of solitary HCC based on LI-RADS-defined imaging features, without performing quantitative analysis of the imaging data. In future studies, we will increase the sample size and incorporate quantitative indicators obtained from imaging data. Lastly, due to the limitations of data collection in our retrospective study, the data on the novel biomarkers were incomplete. In future studies, we will consider including the novel biomarkers to further explore their relationship with HCC recurrence and prognosis.
In conclusion, our study combines clinical laboratory indicators and qualitative imaging features to construct a non-invasive diagnostic method for predicting postoperative early-stage recurrence in patients with early-stage solitary HCC. This method contributes to guiding clinical decision-makers in selecting suitable candidates for radical liver resection and making rational decisions regarding postoperative monitoring and adjuvant therapies for patients who have undergone radical liver resection. The ultimate goal is to prolong the overall survival of these patients.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Review Board of Harbin Medical University (protocol code: KY2023-59). The requirement of informed consent from the patients was waived because of the retrospective design of this study, and patients’ information was protected. And the study was performed in accordance with the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Xinxin Wang and Yanyan Yu are co-first authors for this study. The authors declare that they have no competing interests.
Additional information
Funding
References
- Villanueva A, Longo DL. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late Phase Intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi:10.1016/s0168-8278(02)00360-4
- Du ZG, Wei YG, Chen KF, Li B. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution’s experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int. 2014;13(2):153–161. doi:10.1016/s1499-3872(14)60025-4
- Zhang Z, Jiang H, Chen J, et al. Hepatocellular carcinoma: radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging. 2019;19(1):22. doi:10.1186/s40644-019-0209-5
- Xing H, Zhang WG, Cescon M, et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB. 2020;22(5):677–689. doi:10.1016/j.hpb.2019.09.006
- Xu W, Liu F, Shen X, Li R. Prognostic nomograms for patients with Hepatocellular carcinoma after curative hepatectomy, with a focus on recurrence timing and post-recurrence management. J Hepatocell Carcinoma. 2020;7:233–256. doi:10.2147/JHC.S271498
- Ma L, Deng K, Zhang C, et al. Nomograms for predicting Hepatocellular carcinoma recurrence and overall postoperative patient survival. Front Oncol. 2022;12:843589. doi:10.3389/fonc.2022.843589
- Li Q, Wei Y, Zhang T, et al. Predictive models and early postoperative recurrence evaluation for hepatocellular carcinoma based on gadoxetic acid-enhanced MR imaging. Insights Imaging. 2023;14(1):4. doi:10.1186/s13244-022-01359-5
- Nevola R, Ruocco R, Criscuolo L, et al. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol. 2023;29(8):1243–1260. doi:10.3748/wjg.v29.i8.1243
- Inoue Y, Suzuki Y, Ota M, et al. The management of recurrence of Hepatocellular carcinoma occurring within 6 months after hepatic resection: a Comparative Study using a propensity score matching analysis. J Gastrointest Cancer. 2022;53(2):272–281. doi:10.1007/s12029-021-00585-2
- Zheng J, Cai J, Tao L, et al. Comparison on the efficacy and prognosis of different strategies for intrahepatic recurrent hepatocellular carcinoma: a systematic review and Bayesian network meta-analysis. Int J Surg. 2020;83:196–204. doi:10.1016/j.ijsu.2020.09.031
- Serenari M, Han KH, Ravaioli F, et al. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. 2020;73(4):855–862. doi:10.1016/j.jhep.2020.04.032
- Liu D, Song T. Changes in and challenges regarding the surgical treatment of hepatocellular carcinoma in China. Biosci Trends. 2021;15(3):142–147. doi:10.5582/bst.2021.01083
- Yin Z, Chen D, Liang S, Li X. Neoadjuvant therapy for Hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:929–946. doi:10.2147/JHC.S357313
- Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med. 2021;15(2):155–169. doi:10.1007/s11684-021-0848-3
- Feng LH, Sun HC, Zhu XD, et al. Prognostic nomograms and risk classifications of outcomes in very early-stage hepatocellular carcinoma patients after hepatectomy. Eur J Surg Oncol. 2021;47(3 Pt B):681–689. doi:10.1016/j.ejso.2020.10.039
- Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–1293. doi:10.1016/j.jhep.2018.08.027
- Zhang C, Yang R, Wang X, et al. LI-RADS morphological type predicts prognosis of patients with Hepatocellular carcinoma after radical resection. Ann Surg Oncol. 2023. doi:10.1245/s10434-023-13494-4
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with Hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
- Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of Hepatocellular carcinoma in at-risk patients. Radiology. 2018;289(3):816–830. doi:10.1148/radiol.2018181494
- Chernyak V, Santillan CS, Papadatos D, Sirlin CB. LI-RADS® algorithm: CT and MRI. Abdom Radiol. 2018;43(1):111–126. doi:10.1007/s00261-017-1228-y
- Mulé S, Galletto Pregliasco A, Tenenhaus A, et al. Multiphase liver MRI for identifying the macrotrabecular-massive subtype of Hepatocellular carcinoma. Radiology. 2020;295(3):562–571. doi:10.1148/radiol.2020192230
- Chen J, Xia C, Duan T, et al. Macrotrabecular-massive hepatocellular carcinoma: imaging identification and prediction based on gadoxetic acid-enhanced magnetic resonance imaging. Eur Radiol. 2021;31(10):7696–7704. doi:10.1007/s00330-021-07898-7
- Kang HJ, Kim H, Lee DH, et al. Gadoxetate-enhanced MRI features of proliferative Hepatocellular carcinoma are prognostic after surgery. Radiology. 2021;300(3):572–582. doi:10.1148/radiol.2021204352
- Xu XF, Wu H, Li JD, et al. Association of tumor morphology with long-term prognosis after liver resection for patients with a solitary huge hepatocellular carcinoma-a multicenter propensity score matching analysis. Hepatobiliary Surg Nutr. 2023;12(3):314–327. doi:10.21037/hbsn-21-423
- Hui AM, Takayama T, Sano K, et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J Hepatol. 2000;33(6):975–979. doi:10.1016/s0168-8278(00)80131-2
- Murakata A, Tanaka S, Mogushi K, et al. Gene expression signature of the gross morphology in Hepatocellular carcinoma. Ann Surg. 2011;253(1):94–100. doi:10.1097/SLA.0b013e3181f9bc00
- Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
- Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in Hepatocellular carcinoma. Liver Int. 2019;39(12):2214–2229. doi:10.1111/liv.14223
- Lu Z, Zuo B, Jing R, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous Hepatocellular carcinoma mouse models. J Hepatol. 2017;67(4):739–748. doi:10.1016/j.jhep.2017.05.019
- Lee JH, Lee Y, Lee M, et al. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with Hepatocellular carcinoma. Br J Cancer. 2015;113(12):1666–1676. doi:10.1038/bjc.2015.430
- Nakagawa H, Mizukoshi E, Kobayashi E, et al. Association between high-avidity T-cell receptors, induced by α-fetoprotein-derived peptides, and anti-tumor effects in patients with Hepatocellular carcinoma. Gastroenterology. 2017;152(6):1395–1406.e10. doi:10.1053/j.gastro.2017.02.001
- Xu XS, Wan Y, Song SD, et al. Model based on γ-glutamyl transferase and alkaline phosphatase for Hepatocellular carcinoma prognosis. World J Gastroenterol. 2014;20(31):10944–10952. doi:10.3748/wjg.v20.i31.10944
- Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223–234. doi:10.1136/postgradmedj-2015-133715
- Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable Hepatocellular carcinoma with portal vein tumor thrombus: a Randomized, Open-Label, Multicenter Controlled Study. J Clin Oncol. 2019;37(24):2141–2151. doi:10.1200/JCO.18.02184