Abstract
Hepatocellular carcinoma (HCC) is a global public health problem, based on it being the fifth most common cancer and third leading cause of cancer-related mortality worldwide. The approved conventional treatment methods for HCC have shown life-threatening side effects with limited or negligible success, especially in multifocal HCC. As a consequence, new therapeutic approaches are being explored, including immunoregulatory molecules that may have the potential to treat or delay the progression of HCC. A novel pharmaceutical botanical drug – Ambovex®, an immune-modulator molecule – was tested to treat or delay the progress of HCC. We conducted a 6-month randomized clinical trial with an additional 3-month washing period (no treatment) to evaluate the safety and efficacy of low-dose Ambovex oral spray in treating patients with HCC. The clinical study involved a total of 40 patients, with 33 in the treatment group and seven in the control group. The α-fetoprotein (AFP) levels were measured every month and ultrasound scans were performed at time zero and every 2 months thereafter. Computed tomography (CT) scans were performed for patients in the treatment group. Ambovex proved to be safe, as there were no significant side effects although some patients found that the drug has unpleasant taste. AFP analysis showed a significant decrease in its level (α=0.05; 95% confidence interval) in the treatment group when compared to the control group at 3 months (P=0.0031) and at 6 months (P=0.007). The ultrasound results showed improvement in the treated group, as evidenced by a significant decrease in the lesion numbers and sizes. The lesions in 38% of treated patients decreased from multiple to single with major improvements; 35% of patients exhibited a decrease from multiple lesions to multiple lesions with minor improvements, whereas 27% had stabilized lesions. CT scans in the treated group showed significant improvement, as there was complete disappearance of the lesions after 6 months of treatment with Ambovex in two patients. This clinical study showed the effective and promising results of Ambovex as an immunological modulator in treating HCC. Further exploration of Ambovex is recommended.
Background
Primary liver cancer is a global public health problem, based on it being the fifth most common cancer and third leading cause of cancer-related mortality worldwide.Citation1 Hepatocellular carcinoma (HCC) accounts for the majority of primary liver cancer, constituting 85%–90% of cases, and results in more than 650,000 deaths per year globally.Citation2,Citation3 Although the incidence of HCC is higher in regions such as sub-Saharan Africa and Asia, its incidence has also been rising in the last few decades in developed countries such as the United States, Western Europe, and Japan.Citation2,Citation4,Citation5,Citation13–Citation15
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are two established risk factors for the development of HCC.Citation3,Citation6,Citation8,Citation22 A cross-sectional study of several liver transplantation centers in the United States that examined the etiologies of HCC reported that 47% of their HCC cohort had HCV, 15% had HBV, 5% had both HBV and HCV, and 33% had neither virus.Citation8 Among Asians, HBV accounts for at least 60% of HCC.Citation8 Behavioral factors such as heavy alcohol intake and cigarette smoking are other possible risk factors for HCC.Citation3,Citation7,Citation9–Citation11,Citation16–Citation20 Mechanisms of tolerance and their implications in cancer are of central interest in immunology. The liver has an immunologically privileged status, which is a consequence of several unique immunological properties, causing antigen tolerance rather than immunityCitation29,Citation30 and relative resistance against liver allograft rejection,Citation31 allowing 20% of allotransplanted patients to be withdrawn from long-term immunosuppression.Citation32
As was reviewed by Abe and Thomson,Citation30 liver immune-privilege properties are likely due to the liver’s unique repertoire of antigen-presenting cell (APC) populations, consisting of von Kupffer cells (KCs), liver sinusoidal endothelial cells (LSECs), and dendritic cells. KCs represent 80%–90% of liver resident macrophages and are very efficient in clearing lipopolysaccharides from gut-derived blood circulation, but they are less efficient in activating CD4+ cells. LSECs were shown to efficiently separate leukocytes from hepatocytes, express factors involved in T-cell death, induce differentiation of CD4+ toward the Th2 anti-inflammatory phenotype, costimulate Tregs, and inhibit allogeneic T-cells.Citation33–Citation35
Fas ligand (FasL), a type II transmembrane protein reported to induce the apoptosis of Fas-bearing cells, was shown to confer immunological privilege to certain tissues and organs such as the eye, liver, placenta, and central nervous system. More recently, the interaction of FasL or its secreted isoform (sFASL) produced by tumor cells, with their specific Fas receptors expressed on T-lymphocytes, was implicated in tumor cell evasion from immune surveillance. Moreover, α-fetoprotein (AFP), an oncofetal protein overexpressed in some HCC, was shown to induce FasL and tumor necrosis factor (TNF)-related apoptosis expression in HCC Bel7402 cells and in the TRAIL receptor, as well as the expression of Fas in lymphocytes.Citation37
The available conventional methods of HCC treatment include surgical treatments such as resection and liver transplantation,Citation21–Citation24 and nonsurgical treatments such as ablation, chemoembolization,Citation23–Citation28 and chemotherapy using sorafenib (Nexavar®), and so on.Citation36 These approved nonsurgical treatment methods for HCC may show limited success, especially in multifocal HCC with many life-threatening side effects.Citation28
As a consequence, new therapeutic approaches are being explored, including immunoregulatory molecules that may have the potential to delay the onset or progression of HCC. Ambovex® is a plant derivative, which is an immunologic modulator.
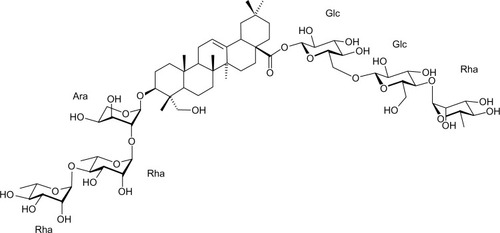
The structural formula of Ambovex
Ambovex is a botanical derivative that is an immunologic modulator. Ambovex has been demonstrated to enhance the function of T-cells, increase CD4 counts, and increase natural killer (NK) cells. In addition, Ambovex prevents the differentiation of progenitor (stellate) cells to myofibroblasts while encouraging the differentiation of stem cells to leukocytes.
The drug product is an oral solution containing 8% of a mixture of chemical species with allowance for ±1.0% conjugated polysaccharide drug substance. Excipients are water, ethyl alcohol, sodium benzoate, potassium phosphate dibasic, methyl paraben, propyl paraben, butyl paraben, ethylenediaminetetraacetic acid (EDTA), potassium carbonate, potassium bicarbonate, benzyl alcohol, glycerin, and flavor.
As a botanical drug product, the drug substance is a mixture of chemical species (polysaccharides, glycosides, minerals, and other related components), and it features activities that are considered to arise from the whole mixture and not from an individual component. It is not known if the total activity from the individual ingredients is additive or synergistic. Therefore, it is important to assure that these components are present at least at the levels that were seen in clinical batches to address efficacy, but not above those levels to address safety. So far, stability data at long-term storage does not show a substantial negative trend for conjugated polysaccharides ().
Figure 1 Molecular ion at m/z 1,393.
Ambovex has been demonstrated to enhance the function of T-cells, increase CD4/CD8 counts, and increase NK cells. In addition, Ambovex prevents the differentiation of progenitor (stellate) cells to myofibroblasts while encouraging the differentiation of stem cells to leukocytes. Ambovex has also shown the ability to decrease AFP and result in the disappearance of some focal lesions of cancer, while increasing the T4/T8 cell ratio.Citation38–Citation40 Therefore, it is important to assure that these components are present at least at the levels that were seen in clinical batches to address efficacy, but not above those levels to address safety. So far, stability data at long-term storage does not show a substantially negative trend for conjugated polysaccharides.
The objectives of this study were: 1) to conduct a 6-month pilot clinical trial with an additional 3-month washing period (no treatment) to evaluate the safety and ease of administration of Ambovex; 2) to determine the efficacy of low-dose Ambovex oral spray when treating patients with HCC; and 3) evaluate the efficacy of Ambovex as a low-cost, nontoxic oral spray to treat or delay HCC progression in patients with chronic liver diseases.
The study protocol
Included in this study were 47 patients with post-HCV liver cirrhosis and HCC documented by two imaging techniques, as well as elevated AFP levels (>200 ng/mL). Inclusion criteria were patients with post-HCV liver cirrhosis with HCC, both sexes, ages ranging from 35–65 years, with no other options for curative therapy for their HCC. Exclusion criteria were patients with other organ affection, other neoplasms, pregnant and lactating females, those with pancytopenia or concomitant infections, or those with an inability to provide written consent.
After the institutional review board approval of the ethical committee of Cairo University, and after obtaining informed patient consent, the patients were divided into one of two groups: group 1 included 40 patients with HCC who received Ambovex oral spray (20 sublingual push/puffs, three times a day [tid]) for 4 months. The patients in this group were divided into one of two subgroups: group 1A included 33 patients who continued on Ambovex at the same dose for a further 2 months; and group 1B included seven patients who were followed for a further 2 months after stopping treatment to assess relapse.
Group 2 included seven patients with HCC who were well matched for age and sex; they served as a control group and received the standard line of care, as they were unfit for any active intervention for 6 months. The criteria for HCC progression were followed up by AFP levels measured on a monthly basis and the use of imaging techniques (abdominal ultrasonographic [US] examination/computed tomography [CT] scan before the start of the study, and after 3 months and 6 months, respectively).
Descriptive methods were used. Time-to-event data (time to progression, progression-free survival, and overall survival) were calculated using the Kaplan–Meier method; the comparisons between the two treatment groups were done using the log-rank test and the hazard ratio was derived from the Cox model. Further tests were used according to the needs of the study. In addition, any covariates may have been added into the model as an exploratory analysis. Regarding the sample size, considering the 95% confidence interval (1-alpha) with an α-error of 5%, the 80% power of the study, that the ratio of group 1A to group 1B is 1:1, and that a target improvement in group 1A would have to be 80% according to Kelsey et al, the total number of patients included in the study was 47.
The patient randomizations were performed in the following way: matched-pairs randomized block designs were applied based on the age and sex of the participants; then, within each pair, participants were randomly assigned to either the Ambovex (group 1) or control (group 2) group. Within Ambovex group 1, all of the patients received the drug for 4 months, and then a matched-pairs randomized block design was again applied based on the age and sex of the participants in the group; within each pair, participants were randomly assigned to either control group for a further 2 months. The participants in group 2 received the standard line of treatment.
In this study, we used the following criteria for the evaluation and for the definition of endpoints. The endpoints after 6 months of Ambovex oral spray therapy were measured as follows: 1) evaluate the patient’s general condition and performance status; 2) evaluate the AFP level; and 3) evaluate the HCC lesions by US and CT. The data were collected, tabulated, and statistically evaluated. The following off-agent criteria were used and the participants may stop taking the study agent for the following reasons – 1) completed the protocol-prescribed intervention; 2) adverse event or serious adverse event, unacceptable toxicity; 3) tumor disease progression (either an increase in tumor size or further elevation of AFP); 4) increasing viral load (more than the initial load level before enrolment in the study), or worsening of the liver’s condition (downgrading of the Child–Pugh score); patients who developed severe toxicity may be enrolled again with a dose reduction (ten puffs tid) and gradually increase the dose with close follow-up; 5) inadequate agent supply, noncompliance, or concomitant medications; 6) medical contraindication or female participants becoming pregnant; and 7) participants who are not willing to continue follow-up for safety reasons, according to the schedule of events. These laboratory parameters were also used for off-agent criteria to determine deterioration: for the CBC, a hemoglobin level <10 g/dL; a total leukocyte count <3,000 mm; and a platelet count <50,000. The following renal function tests were also conducted: serum creatinine; blood urea nitrogen; serum Na; and serum K. After stopping the drug administration, the patients were followed up both clinically to detect signs of liver or kidney failure, and by laboratory investigations. If normalization of the laboratory investigations in these critical patients was achieved within 1–2 weeks, redosing with Ambovex was given in the following way: 20 puffs/push once per day for 1 week, followed by 20 puffs/push twice per day for another week. If deterioration of the studied laboratory parameters occurred, then the dose was decreased to ten puffs twice per day and the patient was maintained on this dose.
Health hazards and side effect information
Adverse effects
Some adverse reactions that were noted included headache, a slight increase in temperature, and feelings of nausea.
Overdose effects
Symptoms of mild overdose include nausea, influenza-like symptoms, and musculoskeletal pain. In a preclinical study, rats were injected with an overdose on a daily basis to establish the median lethal dose (LD50), and no known side effects were observed.
Drug interaction
The interaction between Ambovex and other drugs has not been fully evaluated, although it has been evaluated through previous limited clinical trials conducted with hepatitis C patients at Saint Joseph’s Hospital (Paterson, NJ, USA) who were on other drugs (blood pressure, diabetes, and other medications). None of the patients had discontinued the regimen or the drugs for any of the diseases that they were being treated for.
Use in pregnancy and nursing mothers
Safe use of Ambovex in humans has not been established; therefore, Ambovex should not be used during pregnancy. It is also not known whether this drug is secreted in human milk. Therefore, Ambovex should not be used in nursing mothers.
Pediatric use
Safety and effectiveness have not been established in patients below the age of 18 years. Therefore, Ambovex should not be used in pediatrics (patients younger than 18 years).
Results
Ambovex sublingual oral spray was given to the patients three times per day before meals for 6 months. Patients treated with Ambovex showed minimal side effects – mainly disgust for the drops in ten patients – but no one stopped the drops. The treatment group with Ambovex showed a unique therapeutic effect in comparison to the control group. The immunomodulation produced by Ambovex was quite evident, as there was a distinguished and significant decrease (P-value <0.05 at a 95% confidence interval) in the AFP values among the patients treated with Ambovex (). In brief, one of the male patients (aged 62 years) had multifocal HCC and an AFP level of 38,909 ng/mL at time zero before the Ambovex treatment. After the patient was put on Ambovex treatment, the AFP level of the patient reduced significantly to 16,300 ng/mL after 3 months, and finally to 546 ng/mL after 6 months of treatment. Another male patient (aged 58 years) with liver cirrhosis, multifocal areas, portal vein thrombosis, and an AFP level of 66,339 ng/mL at time zero was put on Ambovex treatment. After 1 month of treatment, the patient’s AFP level reduced significantly to 17,500 ng/mL and eventually to 1,498 ng/mL by the end of 6 months, showing the utmost level of efficiency of Ambovex in reducing AFP levels.
Table 2 AFP levels for patients at time zero, and after 2 months, 3 months, 4 months, 5 months, and 6 months, respectively, on Ambovex® treatment therapy
A third patient with liver cirrhosis and multifocal HCC occupying most of the right lobe with thrombosis of the right branch of the portal vein also had two basal lung metastatic nodules and an AFP level of 334,000 ng/mL. After the Ambovex drops, the AFP level dropped to 25,367 ng/mL after 1 month and to 5.870 ng/mL after 3 months with a recanalized portal vein and disappearance of metastatic nodules in the lung, but at 4 months, the AFP increased again to 13,452 ng/mL and at 6 months to 83,969 ng/mL with another increase in tumor size.
In parallel to the decrease in AFP level, the US scan showed a significant reduction in the size and number of HCC lesions (P-value <0.05 at a 95% confidence interval) in the treated patient via a significant decrease in the lesion number and size. The lesions in 38% of treated patients decreased from multiple to single lesions showing major improvement, 35% of patients went from multiple lesions to decreased multiple lesions with minor improvement, whereas 27% of patients had stabilized lesions (). At time zero, before the treatment, four patients were identified with single lesions (numbered and denoted as 1) and 25 patients for multiple lesions (numbered and denoted as 5). After 6 months of the study, the US scan revealed that the Ambovex was extremely effective at either resulting in major or minor improvements or stabilization of the lesions ( and ). In brief, at time zero, four patients had single lesions and 24 patients had multiple lesions. After the 6 months of Ambovex treatment, the number of single lesion patients significantly increased from four to 14, whereas the number of patients with multiple lesions significantly decreased from 25 to nine, and there were nine patients with decreased multiple lesions (). The CT scan of the patients also showed a positive and significant effect on the patients treated with Ambovex, consistent with the results found from the AFP levels and US scan for the focal lesions. Before the treatment at time zero, the patient had the following findings: the liver was enlarged showing cirrhotic changes; and there was a large heterogeneous focal lesion in the right lobe, meeting the criteria for a primary hepatic neoplasm with malignant infiltration of the right portal branch; there were also bilateral basal lung deposits (). After 6 months of Ambovex treatment therapy, CT scan showed a marked regression of the right lobe masses with a patent main portal vein and its left branch, as well as an indistinct right portal branch for the Doppler correlation, and no pulmonary metastases were detected. This indicated the great effectiveness of Ambovex oral spray for treating this life-threatening disease.
Figure 2 Postcontrast CT examination of the abdomen with a triphasic liver study of a patient before and after Ambovex® therapy showing marked tumor regression.
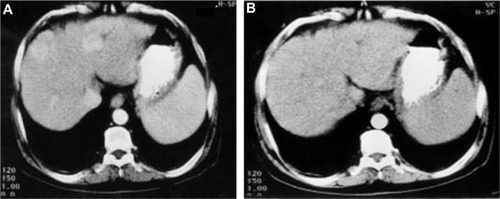
Figure 3 The proposed immunomodulation effects of Ambovex® and the released cytokines and free radicals.
Abbreviations: APC, antigen-presenting cells; HSC, hematopoietic stem cells; IL, interleukin; IFN, interferon; NK, natural killer; MCSF, macrophage colony-stimulating factor; GMCSF, granulocyte macrophage colony-stimulating factor; ROS, reactive oxygen species; TNF, tumor necrosis factor.
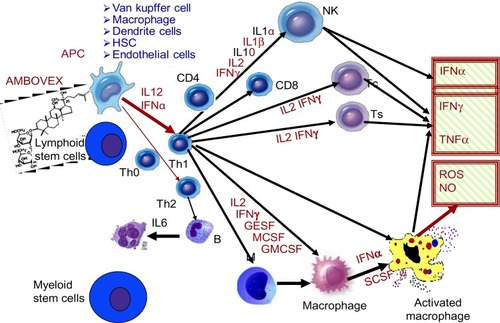
Figure 4 Pre- and postultrasound examination, time zero and after 3 months of the patient on Ambovex® treatment therapy.
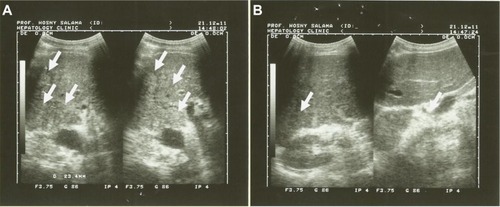
Figure 5 Pre- and postbiopsy examination of tissues at time zero and after 6 months of a patient on Ambovex® treatment therapy.
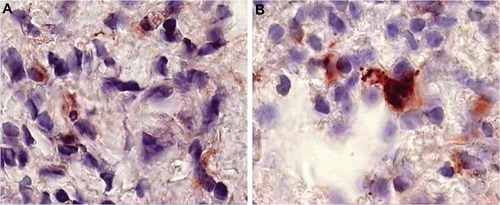
Figure 6 Pre- and postbiopsy examination of tissues at time zero and after 6 months of a patient on Ambovex® treatment therapy.
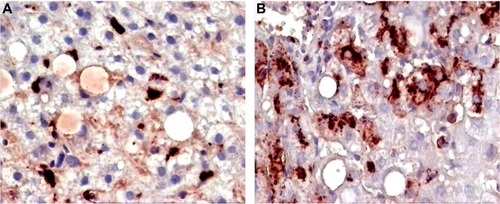
Table 1 US scan showing the number of focal lesions and their changes at time zero, as well as after 3 months and 6 months of Ambovex® treatment
Discussion
Primary liver cancer is a global public health problem, based on it being the fifth most common cancer and third leading cause of cancer-related mortality worldwide.Citation1 HCC accounts for the majority of primary liver cancers, constituting 85%–90% of cases, and resulting in more than 650,000 deaths per year globally.Citation2,Citation3
The available conventional methods for HCC treatment include surgical treatments such as resection and liver transplantation,Citation21,Citation23,Citation24 and nonsurgical treatments such as ablation, chemoembolization,Citation23–Citation28 polykinase inhibitors such as sorafenib (Nexavar®), and so on.Citation36 The approved conventional treatment methods for HCC have shown many life-threatening side effects with sometimes minimal or negligible success, especially in multifocal HCC.Citation27,Citation28
As a consequence, new therapeutic approaches are being explored, including immunoregulatory molecules that may have the potential to delay the onset or progression of HCC. Ambovex is a plant derivative that is an immunologic modulator. Ambovex has been demonstrated to enhance the function of T-cells, increase CD4/CD8 counts, and increase the number of NK cells.Citation40 In addition, Ambovex prevents the differentiation of progenitor (stellate) cells to myofibroblasts while encouraging the differentiation of stem cells to leukocytes. Ambovex has also shown the ability to decrease AFP, as well as result in the disappearance of local focal lesions of cancer and increase the T4/T8 cell ratio.Citation38 The drug product is an oral solution containing 8.0% of a mixture of chemical species with an allowance of ±1.0% of the conjugated polysaccharide drug substance. Therefore, it is important to assure that these components are present at least at the levels that were seen in clinical batches to address their efficacy, but not above those levels to address safety.Citation38,Citation39
From this pilot study, Ambovex showed good tolerability with minimal side effects throughout the whole study period. Concerning the efficacy of Ambovex in HCC cases, there was a dramatically good response according to the US examination where the lesions in 38% of treated patients decreased from multiple to single, showing major improvement; in addition, 35% of patients went from multiple lesions to decreased multiple lesions with minor improvement, while 27% of patients had stabilized lesions. CT scan showed a dramatic decrease in the tumor sizes in two cases, where the tumors showed a marked reduction in size with disappearance of basal lung metastasis in one case and a recanalized portal vein branch in the other case.
Possible mechanisms of action of Ambovex
Ambovex sublingual oral spray was given to the patients to locally activate the rich throat lymphoid tissue and avoid gastrointestinal digestion or liver metabolism. The previous immunological and clinical studies carried out by the authors for patients with fibrosis and cirrhosis indicated a significant increase in and activation of macrophages, as well as an increase in NK cells, which increased more than threefold, accompanied by an increase in and activation of the macrophages and KCs ( and ).Citation39,Citation40 This has to be considered, as the immune system is governed by a very careful and meticulous cascade; autoregulation provides the balance to maintain a healthy state in individuals. The released TNFα from the NK cells and the activated macrophages attack the infected cancer cells by releasing TOS and NO as active radicals destroying the cells, particularly their cell membrane. Another mechanism that takes place during such an attack is the process of apoptosis, where the cell dies silently and the debris is engulfed by the macrophages and KCs. The results of this clinical trial gave further evidence regarding the immunomodulatory properties of the drug, as there was a marked increase in and activation of the immunocellular reaction presented by the increase of NK cells, as well as by the increase in and activation of the macrophages systemically and locally around the pathological lesions during the liver biopsies. The interaction between Ambovex and the NK cells stimulated the NK cells. The stimulated NK cells kill the cancerous cells; however, the advantage of NK cells is that they recognize the normal cells in comparison to the cancerous cells via a binding mechanism at the site where they are activated. Therefore, the activation of NK cells will not harm the normal cells.
Antigen processing cell (APC) receptors are present in the liver in many forms (KCs, hepatic stellate cells, dendritic cells, endothelial cells, and macrophages) (). Once the APC receptors are activated, a cascade of cellular reactions take place where activation of Th1 cells occurs, followed by activation of the NK cells, monocytes, macrophages, and activated macrophages. The activation of macrophages and the increase in NK cells are considered to be most important, as this is when the triggering of almost all of the entire cellular immune system takes place. This provides evidence that the peripheral and local immune response around the hepatic sinusoids is activated, as seen in the liver needle biopsies taken before and after Ambovex therapy.
The release of these cytokines occurs very closely to the target infected liver cells around the hepatic sinusoids, which are close to and within the space of Disse. Such immunocellular reactions attack the infected live cells, and they also attack some of the neighboring healthy cells ().Citation39
After 6 months of the study, the US scan revealed that Ambovex was extremely effective in leading to either major or minor improvements or stabilization of the lesions (). As seen in the US scan (), before the treatment (), the patients’ scans showed multiple lesions. However, after Ambovex therapy for 3 months, multiple lesions reduced to single lesions (). Other US scans showed multiple lesions for a patient at time zero before the treatment; after Ambovex therapy, the US scan showed a decrease in the number of multiple lesions. Similar results were seen for most of the patients (38%). The authors have shown only a couple of US scans to provide a view and better idea of the results for patients after Ambovex treatment. However, we performed a US scan on all the patients, and the tabulated data are shown in .
In a previous study conducted by the authors, Ambovex was used to treat chronic HCV infection among Egyptian patients. The authors also performed pre- and postbiopsy for the examination of liver tissues before and after the Ambovex treatment. The tissues were evaluated for the following parameters: percentage of Ban B; percentage of Ban T; percentage of macrophages and KCs; activation of macrophages and KCs; and the percentage of NK cells (). The immunological data for the blood examination of patients with cirrhosis and fibrosis (data from the previous study), and not those with HCC, are shown in . The data are expressed as the mean ±1 standard deviation. Statistical analysis was performed to assess the significant difference at 95% and 99% confidence intervals. shows the baseline nonactivated macrophages and KCs (activation at time zero), and shows the activated macrophages and KCs after 6 months. The results from the examination clearly show a significant increase in the activation of macrophages and KCs after 6 months in comparison to time zero before treatment.Citation39 shows the base percentage of NK cells and shows the percentage of NK cells after 6 months of Ambovex treatment. The results from the examination clearly show a significant increase in the percentage of NK cells from 10% to 60%. Similarly, these results were also seen for other patients. There was a significant increase in the activated macrophages and KCs, and an increase in the percentage of NK cells after 6 months of Ambovex treatment therapy.Citation40
Table 3 Immunological data from the blood examination of patients with cirrhosis and fibrosis (data from our previous study), but not cancer
Conclusion
Results from the 6-month randomized clinical trial with an additional 3-month washing period (no treatment) showed that Ambovex was effective and nontoxic as a sublingual oral spray to treat patients with HCC. Low-dose Ambovex used in the randomized clinical trials showed no significant side effects on the patients treated, indicating the safe administration of Ambovex in humans. Some minor adverse reactions that were noted included headache, mild fever, nausea, and disgust. The results from the AFP level measurement showed a significant decrease (after 6 months of treatment) among the patients treated with Ambovex in comparison to the AFP level at time zero. A significant and positive difference was also observed between the patients treated with Ambovex when compared to control (those not treated with Ambovex). The US scan revealed major improvements in 38% of patients (the lesions decreased from multiple to single), minor improvements in 35% of patients (multiple lesions to decreased multiple lesions), and 27% of patients were stabilized (stable lesions, no increase or decrease) when treated with Ambovex for 6 months. The results from the patients’ CT scans showed significant improvements, as there was complete disappearance of the lesion after 6 months of treatment with Ambovex. The clinical study shows effective and promising results for Ambovex as an immunological modulator in treating HCC. Further exploration of Ambovex is recommended.
Acknowledgments
The authors would like to thank the Hepatology Department of Cairo University and the National Cancer Institute, Egypt for conducting the study and performing the analysis. Patent inventors (HA and IE) and the other authors would also like to thank Saint Joseph Hospital (Paterson, NJ, USA) and Montclair State University (Montclair, NJ, USA).
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkinDMGlobal cancer statistics in the year 2000Lancet Oncol20012953354311905707
- MazzantiRGramantieriLBolondiLHepatocellular carcinoma: epidemiology and clinical aspectsMol Aspects Med2008291–213014318061252
- CookGCHepatocellular carcinoma: one of the world’s most common malignanciesQ J Med1985572237057083001812
- El-SeragHBRudolphKLHepatocellular carcinoma: epidemiology and molecular carcinogenesisGastroenterology200713272557257617570226
- NguyenMHKeeffeEBGeneral managementBest Pract Res Clin Gastroenterol200519116117415757811
- KanwalFEl-SeragHBHepatocellular cancer care: cost is important but only one factor of disease burdenJ Hepatol2009501101219019482
- DonatoFTaggerAGelattiUAlcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and womenAm J Epidemiol2002155432333111836196
- Di BisceglieAMLyraACSchwartzMLiver Cancer NetworkHepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic statusAm J Gastroenterol20039892060206314499788
- ParkinDMThe global health burden of infection-associated cancers in the year 2002Int J Cancer2006118123030304416404738
- El-SeragHBHepatocellular carcinoma: recent trends in the United StatesGastroenterology20041275 Suppl 1S27S3415508094
- GomaaAIKhanSAToledanoMBWakedITaylor-RobinsonSDHepatocellular carcinoma: epidemiology, risk factors and pathogenesisWorld J Gastroenterol200814274300430818666317
- NguyenMHWhittemoreASGarciaRTRole of ethnicity in risk for hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosisClin Gastroenterol Hepatol20042982082415354283
- ChinPLChuDZClarkeKGOdom-MaryonTYenYWagmanLDEthnic differences in the behavior of hepatocellular carcinomaCancer19998591931193610223232
- HassanMMHwangLYHattenCJRisk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitusHepatology20023651206121312395331
- MukaiyaMNishiMMiyakeHHirataKChronic liver diseases for the risk of hepatocellular carcinoma: a case-control study in Japan. Etiologic association of alcohol consumption, cigarette smoking and the development of chronic liver diseasesHepatogastroenterology19984524232823329951917
- ChenZMLiuBQBorehamJWuYPChenJSPetoRSmoking and liver cancer in China: case-control comparison of 36,000 liver cancer deaths vs. 17,000 cirrhosis deathsInt J Cancer2003107110611212925964
- MizoueTTokuiNNishisakaKProspective study on the relation of cigarette smoking with cancer of the liver and stomach in an endemic regionInt J Epidemiol200029223223710817118
- TanakaKHirohataTTakeshitaSHepatitis B virus, cigarette smoking and alcohol consumption in the development of hepatocellular carcinoma: a case-control study in Fukuoka, JapanInt J Cancer19925145095141318264
- La VecchiaCNegriEDecarliAD’AvanzoBFranceschiSRisk factors for hepatocellular carcinoma in northern ItalyInt J Cancer19884268728762847988
- EvansAAChenGRossEAShenFMLinWYLondonWTEight-year follow-up of the 90,000-person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differencesCancer Epidemiol Biomarkers Prev200211436937611927497
- ChenMFHwangTLJengLBJanYYWangCSChouFFHepatic resection in 120 patients with hepatocellular carcinomaArch Surg19891249102510282549912
- LiawYFTaiDIChuCMEarly detection of hepatocellular carcinoma in patients with chronic type B hepatitis. A prospective studyGastroenterology19869022632672416625
- YoshimiFNagaoTInoueSComparison of hepatectomy and transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma: necessity for prospective randomized trialHepatology19921637027061324216
- CastellsABruixJBruCTreatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injectionHepatology1993185112111268225217
- KotohKSakaiHSakamotoSThe effect of percutaneous ethanol injection therapy on small solitary hepatocellular carcinoma is comparable to that of hepatectomyAm J Gastroenterol19948921941988304302
- BuscariniLDi StasiMBuscariniEClinical presentation, diagnostic work-up and therapeutic choices in two consecutive series of patients with hepatocellular carcinomaOncology19965332042098643222
- FarmerDGRosoveMHShakedABusuttilRWCurrent treatment modalities for hepatocellular carcinomaAnn Surg199421932362478147605
- OhtoMYoshikawaMSaishoHEbaraMSugiuraNNonsurgical treatment of hepatocellular carcinoma in cirrhotic patientsWorld J Surg199519142467740809
- LüthSHuberSSchrammCEctopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific TregsJ Clin Invest2008118103403341018802476
- AbeMThomsonAWAntigen processing and presentation in the liverGerswhinMEVierlingJMMannsMPLiver Immunology: Principles and PracticeTotowa, NJHumana Press Inc2007486
- KamadaNDaviesHSRoserBReversal of transplantation immunity by liver graftingNature198129258268408427022223
- Seyfert-MargolisVTurkaLAMarking a path to transplant toleranceJ Clin Invest200811882684268618654643
- De MinicisSSekiEUchinamiHGene expression profiles during hepatic stellate cell activation in culture and in vivoGastroenterology200713251937194617484886
- FriedmanSLLiver fibrosis – from bench to bedsideJ Hepatol200338Suppl 1S38S5312591185
- LimmerASacherTAlferinkJFailure to induce organ-specific autoimmunity by breaking of tolerance: importance of the microenvironmentEur J Immunol1998288239524069710217
- LlovetJMRicciSMazzaferroVSHARP Investigators Study GroupSorafenib in advanced hepatocellular carcinomaN Engl J Med2008359437839018650514
- JodoSKobayashiSNakajimaYElevated serum levels of soluble Fas/APO-1 (CD95) in patients with hepatocellular carcinomaClin Exp Immunol199811221661719649177
- Phase I clinical trial to evaluate the safety, tolerability and clinical pharmacology profile of Ambovex ampoulesHeidar Ghaleb IND of Ambovex200927–48203294
- Phase II clinical trial to evaluate the safety and efficacy of Ambovex Ampules given for 3 years to patients with chronic HCV infectionAmr Helmy, IASL Meeting in Cairo, Sept 2006 IND of Ambovex295415
- An open label phase II study in hepatitis C infected patients, co-infected with human immune-deficiency virus (HIV) who have failed, intolerant or having contra-indications for standard therapyMichael Lange, MDMPH IND of Ambovex2010416521
