Abstract
Purpose
This study examines the safety and efficacy of transarterial chemoembolization using doxorubicin-loaded 30–60 µm QuadraSphere microspheres (DEM-TACE) for the treatment of hepatocellular carcinoma.
Materials and methods
Over 10 weeks, patients with hepatocellular carcinoma. (Child–Pugh A/B: 65%/35%) were embolized with 30–60 µm QuadraSphere microspheres. Excluded patients had previous locoregional therapy, macrovascular invasion, extrahepatic disease, Child–Pugh score >B7, ECOG performance status >0, and total bilirubin >3 mg/dL. Technical success, minor and major complications, 30-day hospital readmission rate, and 30-day mortality were assessed. α-Fetoprotein levels before and after treatment were compared. Local response was evaluated by radiologic tumor response per modified Response Evaluation Criteria in Solid Tumors 1 month after treatment.
Results
Thirty tumors (mean size, 2.3 cm; range, 1.0–4.9 cm) were treated in 20 patients (16 male and 4 female; mean age, 64.7 years). There were no major complications. Thirty-day mortality was 0%. Minor complications included postembolization syndrome in 16.7% of cases and transient rise in liver enzymes requiring no therapy. Mean α-fetoprotein levels trended down following treatment (71.8±201.9 ng/mL vs 53.4±116.7 ng/mL), but were not statistically significant. Complete response was achieved in 30% of patients, partial response in 35%, stable disease in 30%, and progression of disease in 5%. Overall objective response was 65%. Mean follow-up was 10.4 months (range, 2–16.4 months).
Conclusion
DEM-TACE with doxorubicin-loaded 30–60 µm QuadraSpheres is feasible, well tolerated, and associated with promising tumor response in early and intermediate stage disease.
Keywords:
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancyCitation1 diagnosed in over half a million people worldwide, including roughly 20,000 new cases in the United States annually.Citation2 Over the past 20 years, the incidence of HCC in the United States has nearly doubled.Citation3 Owing to late diagnosis, an overwhelming majority of patients (85%–90%) are ineligible for curative treatments at the time of diagnosis.Citation4 Transcatheter arterial chemoembolization (TACE) is the standard of care, according to the Barcelona Clinic Liver Cancer (BCLC) staging system for patients with multinodular HCC, preserved liver function, good performance status, and absence of vascular invasion or extrahepatic spread.Citation5
Conventional TACE (cTACE) using lipiodol as a delivery vehicle for chemotherapeutic agents has been shown to improve survival in patients with unresectable HCC in randomized controlled trials.Citation6–Citation8 TACE utilizing drug-eluting beads (DEB-TACE) was developed to allow a more predictable release of chemotherapeutic agents as well as to provide more sustained drug release.Citation9 In a prospective, randomized Phase II study, DEB-TACE was shown to have no significant difference in treatment related to serious adverse events at 30 days compared with cTACE, but decreased liver toxicity than the doxorubicin group.Citation10 Similarly, Sacco et alCitation11 found a significant increase in alanine aminotransferase level 24 hours after treatment, which was statistically greater after cTACE than with DEB-TACE.
The majority of studies on the safety and efficacy of DEB-TACE have utilized >300 µm LC/DC beads loaded with doxorubicin (Biocompatibles, BTG).Citation12–Citation16 A different drug-eluting platform utilizing drug-eluting microspheres for TACE (DEM-TACE), known as QuadraSpheres (Merit Medical, South Jordan, UT, USA), is available in 30–60 µm sizes when dry, with the ability to proportionally expand in size to 120–240 µm when exposed to aqueous solution. Theoretical advantages of a smaller platform include increased distal penetration and embolization of target tissue.Citation2,Citation13 Padia et alCitation2 demonstrated that the clinical advantages of smaller-diameter drug-eluting platforms over larger ones are decreased fatigue and postembolization syndromes. Yet, beyond animal studies, case studies, and a single prospective study looking at 45 patients treated with 30–60 µm HepaSpheres, there is little data on the use of DEM-TACE for the treatment of HCC.Citation17–Citation19
We report safety and efficacy results of DEM-TACE using doxorubicin-loaded 30–60 µm QuadraSpheres in the treatment of HCC at our institution.
Materials and methods
This was a retrospective study approved by a local Institutional Review Board. We conducted a search through our clinical database to identify patients having undergone chemoembolization for HCC with DEM-TACE over a 10-week period beginning in October 2012.
Included patients had a diagnosis of HCC based on imaging criteria recommended by American Association for the Study of Liver Diseases (AASLD) guidelines – contrast-enhanced multidetector CT (MDCT) or magnetic resonance imaging (MRI) demonstrating “intense arterial uptake followed by ‘washout’ of contrast on venous-delayed phases”.Citation5 Stratification followed the BCLC staging system.Citation20 All the cases were discussed in a multidisciplinary meeting involving surgical, medical, and interventional specialties. Included patients were those with stage B disease who had not yet undergone locoregional therapy. Patients with BCLC stage A disease not amenable to curative treatment with resection or ablation due to lesion location, such as high dome lesions and those adjacent to the heart or bowel, and those awaiting transplant requiring locoregional control in the interim, were also included in this study. Our liver transplantation surgeons’ preference is not to pursue ablation for potential transplant candidates as the waitlist in New York State for orthotopic liver transplant is in excess of 18 months.
Exclusion criteria included previous locoregional therapy, macrovascular invasion, extrahepatic disease, Child–Pugh score >B7, ECOG >0, and total bilirubin >3 mg/dL. Demographic data was collected, including sex, age, etiology of cirrhosis, and Child–Pugh score for cirrhosis mortality. Pre-DEM-TACE α-fetoprotein (AFP) and liver enzyme levels and synthetic function tests were obtained. Immediate post-treatment liver enzyme and synthetic function tests (within 3 days) as well as the same tests performed closest to 1 month from intervention were collected. Two consecutive follow-up AFP levels were averaged and defined as the posttreatment level. Additional data including technical success, minor and major complications based on SIR Classification,Citation21 length of in-patient stay, 30-day hospital readmission rate, and 30-day mortality were reviewed.
Tumor characteristics at baseline and local response following treatment were assessed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines.Citation22 Selected target HCC lesions were those described as RECIST-measurable – well-delineated lesions that can be accurately measured in at least one dimension as 1 cm or more, suitable for remeasurement, and demonstrating definitive arterial enhancement.Citation22 All imaging evaluation was performed by a single, board-certified diagnostic radiologist from the abdominal imaging section with 8 years of experience.
Baseline imaging and follow-up imaging assessment at approximately 5 weeks post-DEM-TACE was performed with contrast-enhanced MDCT or MRI. Pre-imaging and initial follow-up imaging was performed with the same modality. Imaging acquisition was based on AASLD guidelines for imaging-based diagnosis of HCC. MDCT of the abdomen was performed with 5 mm thick contiguous slices. Noncontrast scan and postcontrast scans timed for arterial and portal venous enhancement in the liver were performed. In a subset, equilibrium phase scan was also obtained. MRI of the abdomen was performed with a 1.5 or 3 T magnet per departmental liver mass protocol, including HASTE, fat-saturated T2, T2*, T1 in- and opposed-phase, diffusion-weighted and dynamic fat-suppressed VIBE-LAVA sequences before and after the administration of Eovist (postcontrast scans at 20, 60, and 180 seconds and delayed scans at 10 and 20 minutes).
DEM-TACE was performed using a fixed protocol. Arterial access was achieved via the transfemoral or transradial approach. Celiac and superior mesenteric arteriograms were performed to assess arterial anatomy, as well as to evaluate tumor vascularity and portal vein patency prior to treatment. DEM-TACE was performed via segmental or subsegmental catheterization of the hepatic artery branch(es) feeding the tumor with a microcatheter (Renegade Hi-Flo, Boston Scientific or Progreat, Terumo) followed by delivery of the chemoembolic agent. Each 30–60 µm vial of Quadra-Spheres was preloaded with 50 mg doxorubicin. After reconstituting the doxorubicin powder into 20 mL 0.9% normal saline, half the doxorubicin liquid, 10 mL (25 mg), was injected into the 30–60 µm QuadraSphere vial and allowed to stand for 10 minutes. The entire contents of the microsphere/doxorubicin admixture were then drawn into a syringe that contained the remaining half of the reconstituted doxorubicin liquid. Per manufacturer suggestion, 1 hour was allotted to optimize the uptake of the doxorubicin into the microspheres prior to delivery.
Each vial of doxorubicin-loaded QuadraSpheres was diluted with 20 mL of nonionic iodinated contrast medium, with 10 minutes allotted to allow for microsphere expansion. The solution was gently agitated utilizing a three-way stopcock prior to delivery. The suspension was delivered in 1 mL aliquots at an approximate rate of 1–3 mL/min. The endpoint for delivery of QuadraSpheres to the target lesion was obliteration of the neovascularity and near stasis of the branch vessel. A completion angiogram was performed several minutes post-initial DEM-TACE, and DEM-TACE was reinitiated until previous endpoint if neovascularity was noted. For patients with bilobar disease, a staged embolization was performed to treat all targeted lesions (two treatments approximately 3 weeks apart). Patients were admitted for overnight observation.
Results
A total of 30 tumors (mean size, 2.3 cm; range, 1.0–4.9 cm) were treated in 20 patients (16 male and 4 female; mean age, 64.7 years). Etiology of cirrhosis included hepatitis C virus (HCV; n=10), hepatitis B virus (HBV; n=5), alcohol (n=2), combined HCV/HBV (n=1), and cryptogenic (n=3). Five patients (25%) underwent hepatic resection prior to DEM-TACE, and one patient underwent previous orthotopic liver transplantation. Child–Pugh class was A for 65% of patients (n=13) and B for 35% (n=7). Sixteen patients (80%) were BCLC stage A, and four were stage B (20%). Patient demographics and clinical characteristics are listed in . In two cases, patients were treated with a staged approach, with two sessions less than 1 month apart (17 and 28 days). Technical success was achieved in all cases.
Table 1 Patient demographics and tumor characteristics
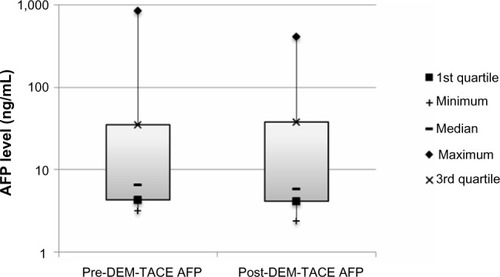
There were no major complications during follow-up. Minor complications in the cohort included postembolization syndrome in four of 24 cases (16.7%), and transient rise in liver enzymes requiring no therapy. Liver enzyme and synthetic function tests and AFP levels are shown in –. There was no statistically significant difference between pre- and post-DEM-TACE liver synthetic function tests (prothrombin time and albumin) or liver enzyme levels. The mean pre-DEM-TACE AFP levels were 71.8±201.9 ng/mL. Mean values after embolization were 53.4±116.7 ng/mL, which was not a statistically significant decrease (unpaired t-test, P=0.7). All patients were discharged on postoperative day 1 as initially planned. There were no hospital readmissions within 30 days, and 30-day mortality was 0%.
Figure 1 Box plot of total bilirubin (mg/dL) levels at baseline, immediately following DEM-TACE, and 1 month post-DEM-TACE.
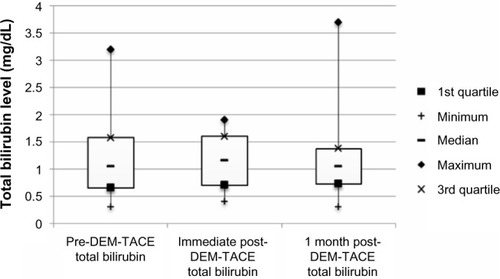
Figure 2 Box plot of ALT (IU/L) levels at baseline, immediately following DEM-TACE, and 1 month post-DEM-TACE.
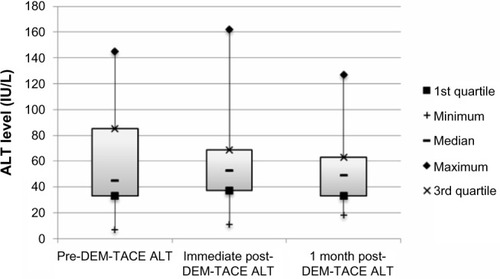
Figure 3 Box plot of AST (IU/L) levels at baseline, immediately following DEM-TACE, and 1 month post-DEM-TACE.
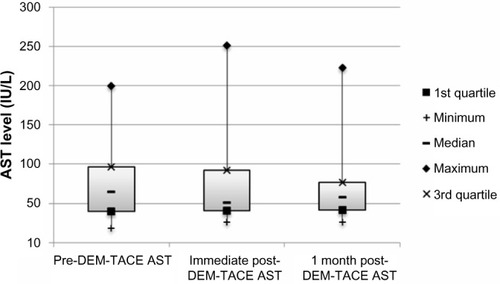
Figure 4 Box plot of prothrombin time (seconds) at baseline and following DEM-TACE.
Abbreviation: DEM-TACE, drug-eluting microspheres transcatheter arterial chemoembolization.
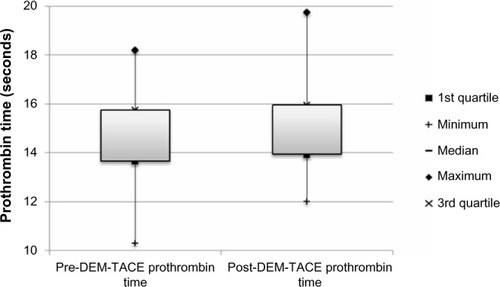
Figure 5 Box plot of albumin (g/dL) at baseline and following DEM-TACE.
Abbreviation: DEM-TACE, drug-eluting microspheres transcatheter arterial chemoembolization.
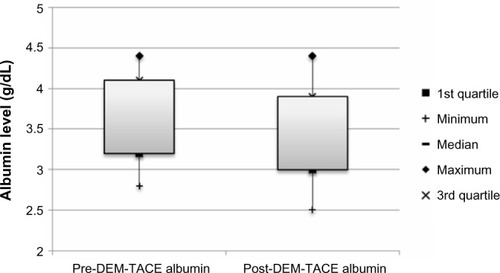
Figure 6 AFP (ng/mL) levels at baseline and following DEM-TACE.
Abbreviations: AFP, α-fetoprotein; DEM-TACE, drug-eluting microspheres transcatheter arterial chemoembolization.
Posttreatment imaging was performed at a mean of 37 days (range, 20–60 days). Local response rates are presented in . Complete response (CR) was achieved in 30% of patients, partial response (PR) was seen in 35%, stable disease (SD) in 30%, and progression of disease (PD) in 5%. Overall objective response (CR + PR) was seen in 65% of cases. Overall mean follow-up time was 10.4 months (range, 2–16.4 months), with one patient lost to follow-up after initial posttreatment imaging.
Table 2 Rates of local overall response according to the mRECIST criteria at follow-up
Discussion
Several studies have shown that a smaller drug-eluting platform may have advantages compared with a larger-caliber drug-eluting platform or conventional TACE with lipiodol.Citation2,Citation8,Citation13,Citation18,Citation23 Varela et alCitation8 found that peak serum doxorubicin levels were significantly lower following DEB-TACE than after cTACE, suggesting that there is a higher concentration of the chemotherapeutic agent sustained within the target lesion. Similarly, van Malenstein et alCitation23 found this to be true for DEM-TACE as compared with cTACE. A more recent study by Malagari et alCitation18 compared peak serum doxorubicin levels in 24 patients after DEM-TACE with 30–60 µm Hepa-Spheres to three patients treated by cTACE and supported the same finding. While cTACE works by rapid release of a chemotherapeutic agent from a lipiodol solution (t½ =1 hour), DEB-TACE exploits ion exchange, which allows for slow, sustained release of the chemotherapeutic agent over a longer period (t½ =150 hours for 100–300 µm microspheres). This is thought to increase the time of interaction between the chemotherapeutic drug and tumor cells, while simultaneously decreasing systemic concentrations of the drugs, leading to greater tolerability.Citation24
Regarding comparisons of QuadraSpheres with DEBs, Sottani et al compared several pharmacokinetic parameters of patients treated with epirubicin loaded HepaSpheres with patients treated with loaded DC beads over a 24-hour period. The study found a statistically significant higher peak of serum epirubicin concentration at 5 minutes in patients treated with DC beads relative to those treated with HepaSpheres. Both had detectable serum concentrations at 24 hours, suggesting that both types of beads are capable of sustained release kinetics.Citation25
In addition to achieving lower peak serum concentrations, and thereby, presumably, higher intratumoral concentrations, Padia et alCitation2 found that patients treated with smaller caliber LC beads (100–300 vs 300–500 µm) had a significantly lower incidence of postembolization syndromes and a trend toward a higher instance of EASL CR relative to those treated with larger beads. Gupta et al compared the pharmacokinetic profile of TACE with doxorubicin-loaded QuadraSpheres (DEM-TACE) with that of TACE with doxorubicin, lipiodol, and Embospheres. A third group underwent direct hepatic arterial infusion (HAI) of doxorubicin. The QuadraSpheres group demonstrated the lowest peak plasma concentration of doxorubicin of the three – an indication of higher tumor retention. Furthermore, the intratumoral level of doxorubicin was greater in the QuadraSpheres group at each time point measured with extended intratumoral detection of doxorubicin beyond the time frame of the other two groups (14 days vs 1 and 3 days for the HAI and TACE groups, respectively).Citation26–Citation28
Although promising as a drug-eluting delivery agent, little data exists on the feasibility of employing 30–60 µm Quadra-Spheres for the treatment of HCC in clinical practice. Only a single prospective study of 45 patients with HCC treated with HepaSpheres (a study by Malagari et alCitation18) is present in the literature. Furthermore, the majority of patients in that study had HBV as the underlying etiology for their HCC. For their patient population, Malagari et alCitation18 found that the treatment was well tolerated with an acceptable safety profile and a good rate of objective response (68.9%). In contrast, the majority of patients in our cohort had HCV. Our study helps confirm the feasibility and safety of a novel drug-eluting platform in a broader patient population.
In our single-center study, 30 tumors were treated with 30–60 µm QuadraSpheres in 21 patients. The patient population consisted of a male majority, with HCV being the most common etiology for HCC. Overall, although only short-term follow-up was available for our cohort, DEM-TACE did prove to be well tolerated. All patients were discharged on postoperative day 1, with the only complications being postembolization syndrome in four patients and minor (type A) complications related to a transient rise in liver enzymes. Overall, there were no statistically significant differences between baseline laboratory values and postembolization laboratory values. Although not a statistically significant decline, there was a downward trend in AFP levels following treatment, suggesting response to embolization. CR was achieved in 30% of patients, and the overall objective response rate was 65%, comparable to the response found by Malagari et al.
There are several limitations to this study. This is a single-institution, retrospective study with a small number of patients and does not offer a comparison with cTACE or DEB-TACE. The majority of patients were BCLC A, which may result in a higher objective response rate according to mRECIST. Most significantly, this is only a short-term follow-up study.
Despite these limitations, our initial single-center experience demonstrates that DEM-TACE utilizing doxorubicin-loaded 30–60 µm QuadraSpheres is feasible and well tolerated, and is associated with promising tumor response in early and intermediate stage disease.
Disclosure
AM Fischman is a consultant/speaker in Terumo Interventional Systems, Surefire Medical Inc., and Philips Healthcare. RA Lookstein is a consultant in Cordis Corp and Bayer Healthcare. He is also the Speaker of Boston Scientific Corp. Advisory Board and a DSM at WL Gore and Associates, Inc. E Kim is on the Advisory Board of Onyx Pharmaceuticals and Nordion. He is also a consultant in Philips Healthcare. The authors report no other conflicts of interest in this work.
References
- BoschFXRibesJDiazMCleriesRPrimary liver cancer: worldwide incidence and trendsGastroenterology20041275 Suppl 1S5S1615508102
- PadiaSAShivaramGBastawrousSSafety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particlesJ Vasc Intervent Radiol2013243301306
- El-SeragHBHepatocellular carcinoma: recent trends in the United StatesGastroenterology20041275 Suppl 1S27S3415508094
- HungHTreatment modalities for hepatocellular carcinomaCurr Cancer Drug Targets20055213113815810877
- BruixJShermanMManagement of hepatocellular carcinoma: an updateHepatology20115331020102221374666
- LlovetJMRealMIMontanaXBarcelona Liver Cancer GroupArterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trialLancet200235993191734173912049862
- LlovetJMBruixJSystematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survivalHepatology200337242944212540794
- VarelaMSalaMLlovetJMBruixJTreatment of hepatocellular carcinoma: is there an optimal strategy?Cancer Treat Rev20032929910412670452
- KettenbachJStadlerAKatzlerIVDrug-loaded microspheres for the treatment of liver cancer: review of current resultsCardiovasc Intervent Radiol200831346847618228095
- LammerJMalagariKVoglTProspective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V studyCardiovasc Intervent Radiol2010331415219908093
- SaccoRBargelliniIBertiniMConventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinomaJ Vasc Intervent Radiol2011221115451552
- ReyesDKVossenJAKamelIRSingle-center phase II trial of transarterial chemoembolization with drug-eluting beads for patients with unresectable hepatocellular carcinoma: initial experience in the United StatesCancer J200915652653220010173
- MalagariKChatzimichaelKAlexopoulouETransarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patientsCardiovasc Intervent Radiol200831226928017999110
- DhanasekaranRKhannaVKoobyDAThe effectiveness of locoregional therapies versus supportive care in maintaining survival within the Milan criteria in patients with hepatocellular carcinomaJ Vasc Intervent Radiol201021811971204 quiz 204
- PrajapatiHJDhanasekaranREl-RayesBFSafety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinomaJ Vasc Intervent Radiol2013243307315
- PrajapatiHJSpiveyJRHanishSImRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE)Ann Oncol201324496597323223331
- LeeKHLiapiEACornellCDoxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancerCardiovasc Intervent Radiol201033357658220087738
- MalagariKPomoniMMoschourisHChemoembolization of hepatocellular carcinoma with hepasphere 30–60 µm. Safety and efficacy studyCardiovasc Intervent Radiol201437116517524263774
- JarzabekMJargielloTPyraKBudzynskaAPrzyszlakMSzczerbo-TrojanowskaMChemoembolization (DEM-TACE) in hepatocellularcarcinoma. Report of a case and review of treatment standards in advanced stage diseasePrzegl Lek201269738638923276044
- LlovetJMDi BisceglieAMBruixJPanel of Experts in HCC-Design Clinical TrialsDesign and endpoints of clinical trials in hepatocellular carcinomaJ Natl Cancer Inst20081001069871118477802
- SacksDMcClennyTECardellaJFLewisCASociety of Interventional Radiology clinical practice guidelinesJ Vasc Intervent Radiol2003149 Pt 2S199S202
- LencioniRLlovetJMModified RECIST (mRECIST) assessment for hepatocellular carcinomaSemin Liver Dis2010301526020175033
- van MalensteinHMaleuxGVandecaveyeVA randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinomaOnkologie201134736837621734423
- EmerichDFSnodgrassPLafreniereDSustained release chemotherapeutic microspheres provide superior efficacy over systemic therapy and local bolus infusionsPharm Res20021971052106012180539
- SottaniCPoggiGQuarettiPSerum pharmacokinetics in patients treated with transarterial chemoembolization (TACE) using two types of epirubicin-loaded microspheresAnticancer Res20123251769177422593459
- GuptaSWrightKCEnsorJVan PeltCSDixonKAKundraVHepatic arterial embolization with doxorubicin-loaded superabsorbent polymer microspheres in a rabbit liver tumor modelCardiovasc Intervent Radiol20113451021103021479746
- BilbaoJIde LuisEGarcia de JalonJAComparative study of four different spherical embolic particles in an animal model: a morphologic and histologic evaluationJ Vasc Intervent Radiol2008191116251638
- NamurJCitronSJSellersMTEmbolization of hepatocellular carcinoma with drug-eluting beads: doxorubicin tissue concentration and distribution in patient liver explantsJ Hepatol20115561332133821703190
