Abstract
c-MET is the membrane receptor for hepatocyte growth factor (HGF), also known as scatter factor or tumor cytotoxic factor, a mitogenic growth factor for hepatocytes. HGF is mainly produced by cells of mesenchymal origin and it mainly acts on neighboring epidermal and endothelial cells, regulating epithelial growth and morphogenesis. HGF/MET signaling has been identified among the drivers of tumorigenesis in human cancers. As such, c-MET is a recognized druggable target, and against it, targeted agents are currently under clinical investigation. c-MET overexpression is a common event in a wide range of human malignancies, including gastric, lung, breast, ovary, colon, kidney, thyroid, and liver carcinomas. Despite c-MET overexpression being reported by a large majority of studies, no evidence for a c-MET oncogenic addiction exists in hepatocellular carcinoma (HCC). In particular, c-MET amplification is a rare event, accounting for 4%–5% of cases while no mutation has been identified in c-MET oncogene in HCC. Thus, the selection of patient subgroups more likely to benefit from c-MET inhibition is challenging. Notwithstanding, c-MET overexpression was reported to be associated with increased metastatic potential and poor prognosis in patients with HCC, providing a rationale for its therapeutic inhibition. Here we summarize the role of activated HGF/MET signaling in HCC, its prognostic relevance, and the implications for therapeutic approaches in HCC.
Introduction to c-MET and brief overview of physiological functions
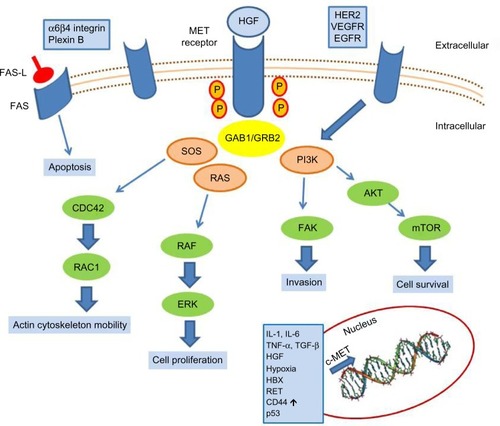
c-MET is the tyrosine kinase receptor for hepatocyte growth factor (HGF),Citation1,Citation2 also known as Scatter FactorCitation3 or Tumor Cytotoxic Factor.Citation4 It is a single-pass heterodimer made of an extracellular alpha subunit containing three functional domains (the semaphorin, plexin–semaphorin–integrin, and immunoglobulin–plexin–transcription domains) linked to a transmembrane beta subunit by a disulphide bond. The transmembrane and the intracellular subunits are made of three portions controlling the kinase activity. c-MET pathway activation may occur either upon HGF binding (canonical pathway) or following interaction with other signaling cascades, such as that triggered by epidermal growth factor receptor (EGFR) or by binding with other circulating factors such as des-gamma carboxyprothrombin.Citation5 In the canonical pathway, HGF binding leads to c-MET receptor homodimerization and autophosphorylation of tyrosine residues of the carboxy terminal domain of c-MET. These events lead to the activation of mitogen-activated protein kinase, phosphoinositide 3-kinase (PI3K)-dependent, rat sarcoma (RAS)-dependent, extracellular-signal-regulated kinase (ERK)-dependent, and RAC1-cell division control protein 42 homolog cascades,Citation6 as summarized in , which promote cell proliferation, survival, and cell motility. Another important function of c-MET is the prevention of apoptosis, which occurs through sequestering of the death receptor FAS, thus preventing its binding to FAS ligand.Citation7 The physiological activation of the c-MET–driven program occurs in embryogenesis, wound healing, and tissue repair, and it is self limiting. c-MET’s activation promotes the “invasive growth” program, which is characterized by enhanced cell motility, invasion, and reduced apoptosis.
Figure 1 c-MET activation signaling pathways.
During embryogenesis, the HGF/MET axis sustains hepatocyte proliferation and liver and placenta development. While homozygous null mice for either HGF or c-MET die in utero at day 13 and 16, respectively, due to impaired organogenesis,Citation8,Citation9 when HGF or MET are knocked down at later phases during the development, the livers of these mice are reduced in size as a result of decreased hepatocyte proliferation and increased susceptibility to apoptosis. In the adult animals, under physiological conditions, loss of c-MET is not critical for hepatocyte function.Citation10 Conversely, the role of c-MET appears to be critical when a response to injuries is required. In this regard, several experimental models have confirmed the pivotal role of MET in liver regeneration and restoration of the liver mass after partial hepatectomy.Citation10 In the setting of fulminant hepatitis in mice treated by the agonistic antibody of FAS receptor, HGF was able to prevent the onset of fulminant hepatitis by suppressing hepatocytes apoptosis.Citation11 When liver injury is induced as in FAS-induced apoptosis, the adaptive response of the liver is strongly reduced in the absence of c-MET. Mice lacking c-MET gene in hepatocytes are hypersensitive to FAS-induced apoptosis, dying as a result of a massive liver apoptosis.Citation12
Another pathological condition in which c-MET exerts its cytoprotective role is cholestasisCitation13 induced by bile duct ligation in mice. Indeed, during cholestasis the HGF/c-MET signaling provides cytoprotective effects in hepatocytes.Citation13 In line with these findings, the role of c-MET in the maintenance of the structural integrity and adaptive plasticity of the liver under adverse conditions was reported by Marquardt et al,Citation14 who explored the effects of c-MET inhibition (in c-MET conditional knockout mice) in the presence of carbon tetrachloride-induced liver damage. Loss of hepatocyte c-MET signaling altered the hepatic microenvironment and was associated with more pronounced fibrogenesis and liver damage, decreased hepatocyte proliferation, stellate cell activation, and rapid dystrophic calcification of necrotic areas. In the same setting, a transcriptomic analysis revealed an impact of c-MET on signaling pathways leading to fibrosis, chemotactic and inflammatory signaling, reorganization of the cytoskeletal network, intercellular communications and adhesion, proliferation, damage, and stress response.
Very recently, Kroy et alCitation15 showed that c-MET deletion in the methionine–choline-deficient mouse model of non-alcoholic steato-hepatitis (NASH) triggers NASH progression, due to fatty acid accumulation, early progression of fibrosis, and increased apoptosis. Hepatocyte-specific deletion of c-MET (occurring in the postnatal period in a conditional knockout mice) leads to the development of severe NASH in mice.Citation15 One of the molecular mechanisms linking c-MET to NASH is the ability of c-MET to sequester the death receptor FAS, preventing its binding to FAS ligand. In NASH, FAS ligand is produced in excess and the protective effect of MET is not effective, resulting in increased apoptotic death of hepatocytes.Citation16 In liver cirrhosis, Kim et alCitation17 showed how HGF/MET activation is able to suppress hepatocyte apoptosis and, at the same time, to trigger apoptosis of alpha-smooth muscle positive and portal myofibroblasts, outlining the contribution of this signaling to the resolution of cirrhotic changes in animal models of cirrhosis. This study showed that, while c-MET is undetectable in quiescent hepatic stellate cells, its expression becomes relevant in activated hepatic stellate cells and in liver myofibroblasts expressing alpha-smooth muscle actin. In vitro, HGF inhibited the activation of ERK1/2 pathway, induced c-Jun N-terminal kinase (JNK)1 phosphorylation, and promoted apoptosis in cultures of rat portal myofibroblasts. Similarly, in vivo, during diethylnitrosamine-induced rat liver injury, HGF inhibited proliferation and induced apoptosis of alpha-smooth muscle actin-positive portal myofibroblasts, reducing liver fibrosis.
All of these findings are in line with a critical role of the HGF/c-MET axis in the regulation of liver regeneration and survival, in the adaptive response of the liver, and in tissue remodeling.
Brief overview of physiological functions and patient outcomes
The role of the HGF/MET axis in liver development has been underscored by c-MET and HGF knockout mice, which display an impaired liver development with reduced size. Similarly, experimental models of liver regeneration in response to acute and chronic damage outline the role of c-MET as a strong mitogenic and antiapoptotic stimulus.Citation18,Citation19 In line with these findings, deregulated c-MET signaling was observed in hepatocelluar carcinoma (HCC) tissues and its deregulated expression was associated with clinical and pathological characteristics in HCC patients.Citation20 c-MET and HGF expression in tumor tissue has been evaluated in many studies, with contrasting findings probably due to the heterogeneous and small populations assayed, the different etiologies, the intrinsic heterogeneous nature of HCC (whose molecular classification is still poorly defined) and the use of different techniques, such as Northern blot, Western blot, reverse-transcription polymerase chain reaction (RT-PCR), and immunohistochemistry (IHC) – this last being the most commonly used method for examination of c-MET expression in human tissues.
c-MET overexpression was observed in human HCC samples by means of Northern blot analysis and an IHC study. Northern blot analysis revealed c-MET mRNA expression in the tumors of 6/19 patients (31.6%); in the IHC study, high c-MET expression was detected in 16/23 patients (69.9%). Both methods revealed c-MET overexpression in HCC compared with the surrounding normal liver.Citation20 Tavian et alCitation21 performed an IHC study and RT-PCR in 24 patients with HCC and showed c-MET overexpression in most of the cases, but low levels of HGF. c-MET overexpression was observed by Western blot analysis in 62 patients with HCC and was associated with an increased incidence of intrahepatic metastases and worse survival. Patients with high expression of c-MET in HCC tissue had a significantly shorter survival than patients with low c-MET expression (33% versus 80.3%, respectively).Citation22 These findings were further confirmed by Kaposi-Novak et al,Citation23 who identified a c-MET-driven gene-expression signature in all HCC metastases but in only a subgroup of primary HCCs.
Most reports suggest that c-MET overexpression is significantly associated with clinicopathological features of HCC, such as tumor grade,Citation20,Citation24 vascular invasion or thrombosis, tumor recurrence,Citation25 metastases,Citation25,Citation26 and worse prognosis with impaired 5-year survival.Citation22,Citation27 However, other studies reported contradictory findings, in particular with respect to tumor stage.Citation28,Citation29 A recent large retrospective study of 194 patients with HCC treated by hepatectomy or microwave ablation revealed that c-MET overexpression was associated with unfavorable clinical outcomes.Citation27 Recently, Lee et alCitation30 assayed c-MET expression and amplification in 287 patients with HCC that was associated with hepatitis B virus infection in 75% of cases. They reported a c-MET overexpression in about 30% of patients with HCC that was not associated with any clinicopathological variable (histopathological grade, size, microvascular and macrovascular invasion, stage, recurrence-free survival, overall survival).
A higher consensus can be found among studies testing genetic alterations of c-MET genomic region, including c-MET amplification and activating point mutations. c-MET amplification and mutation seem to be rare events in most of the studies performed in HCC. A low frequency of c-MET amplification was reported in the HCC series examined by Takeo et al,Citation31 who identified only one case out of 20 HCCs with c-MET amplification, as well as in the 59 patients with HCC examined by Kondo et alCitation26 and in the large series of 286 patients with HCC assayed by Wang et al, where only 4%–5% of cases displayed c-MET amplification.Citation32 Conversely, in this same study, no patient with HCC displayed an amplification of HGF. Concerning activating point mutations, Guichard et alCitation33 did not identify any c-MET point mutations in 24 patients with HCC analyzed by whole exome sequencing.
On the other hand, HGF expression was not increased in tumor tissues in most of the studies performed on human HCCs.Citation21,Citation22 This last evidence suggests an HGF-independent c-MET activation. Remarkably, abnormal c-MET pathway activation can occur from the interaction with adhesive receptors, tyrosine kinases receptors, such as EGFR and vascular endothelial growth factor receptor (VEGFR), proapoptotic FAS, and, finally, by binding with des-gamma-carboxy prothrombin secreted from HCC cells.Citation5
Because no standardized quantification approach can be recommended to assay c-MET expression in HCC, and no scoring system can be suggested, c-MET quantification in HCC largely depends on the analytical approach adopted by each individual study, assessing either mRNA or protein expression. In addition, because HCC is an extremely heterogeneous cancer, the analysis of small groups with different etiologies and different clinical and pathological characteristics may contribute at least in part to the contradictory results reported in the literature. Meanwhile, the discovery of microRNAs as regulators of c-MET expression makes the quantification of mRNA questionable as a biomarker to assess c-MET expression in HCC. In this perspective, the use of c-MET–dependent downstream factors or pathways as biomarkers reflecting c-MET pathway activation might help in the choice of patients more likely to benefit from c-MET-inhibitors. Moreover, most of the data reported in the literature are derived primarily from surgical series of patients who underwent hepatectomy for early HCCCitation32 andwho were usually previously untreated.
Remarkably, few data on c-MET expression are available in advanced HCC and, especially, in previously treated patients, as several therapeutic approaches (such as transarterial chemoembolization or antiangiogenic drugs) induce hypoxia and might be responsible for increased c-MET expression in tumor tissue. Hypoxia, occurring during tumor progression or as a result of specific treatments, is a well-known factor able to activate the transcription of the c-MET proto-oncogene, as proven both in vitro and in vivo.Citation34 Although low partial oxygen tension represents a limiting factor for tumor growth, it nonetheless acts as a positive stimulus by inducing neoangiogenesis,Citation35 by selecting cells that are more resistant to apoptosis,Citation36 and by triggering invasive growth through the increased transcription of the c-MET proto-oncogene and the HGF signaling stimulation.
Role of c-MET in cancer
c-MET overexpression is a common event in a wide range of human malignancies, including gastric, lung, breast, ovary, colon, kidney, thyroid and liver carcinomas.Citation37 The first evidence underscoring the driver effect of c-MET in tumorigenesis was the discovery of the germline activating mutation in patients with hereditary papillary renal cancer.Citation38 Similarly, activating germline point mutations have been identified in patients affected by childhood HCC,Citation39 gastric carcinoma,Citation40 and squamous cell cancers.Citation41 Spontaneous somatic mutations remain a rare event, accounting for no more than 3%–4% of cases.Citation42 Another relevant finding confirming the role of c-MET in cancer was represented by the identification of c-MET as the protein product of a chromosomal rearrangement in an osteosarcoma cell line treated with a chemical carcinogen.Citation43 This rearrangement results in a constitutively active fused oncogene, the translocated promoter region (TPR)-MET. In a transgenic mouse model, the enforced expression of TPR-MET leads to the development of mammary tumors.Citation44 In humans, the TPR-MET rearrangement has been detected in some cases of gastric cancer.Citation45
Different molecular alterations were found to determine c-MET activation in human tumors: point mutations, gene amplifications, enhanced transcription, autocrine activation. In HCC, the aberrant activation of c-MET signaling results mostly from ligands binding or from its overexpression due to enhanced transcription, rather than from gene mutations.Citation46,Citation47 In turn, c-MET enhanced transcription can be triggered by various factors, including: 1) cytokines such as interleukin (IL)-1, IL-6, tumor necrosis factor-α, and transforming growth factor-β;Citation48 2) stimulation by HGF;Citation49 3) hypoxia and in particular by HIF1-α;Citation34 4) cross-talking with oncogenic pathways such as those of RAS,Citation50 ETS,Citation51 and RET;Citation52 5) adhesive receptors such as CD44Citation53 and α6β4 integrins;Citation54 6) cross-talk with the downstream pathways regulated by receptor tyrosine kinases such as EGFR, human epidermal growth factor receptor 2, and VEGFR;Citation55,Citation56 7) cross-talk with plasma membrane proteins such as Caveolin1, involved in the modulation of signal transduction;Citation57 or, 8) as a result of tumor-suppressor gene inactivation, such as TP53.Citation58
Besides these events responsible for c-MET overexpression, the contribution of microRNAs as modulators of c-MET expression was recently outlined. In particular, miR-1, miR-34b and c,Citation59 and miR-199aCitation60 were demonstrated to directly bind c-MET transcript and to modulate its expression as well as its biological effects. miR-199a downregulation is a common and quantitatively relevant event in HCCCitation61 resulting from abnormal histone methylation. In HCC, c-MET overexpression resulting from miR-199a downregulation is responsible for increased proliferation and increased invasion capability.Citation60 Korhan et alCitation62 recently demonstrated that c-MET is a direct target of miR-181a-5p, whose downregulation in HCC leads to enhanced motility, invasion, and branching morphogenesis. Another microRNA-dependent modulation of the HGF/c-MET pathway is operated by miR-26a in HCC. Indeed miR-26a directly targets HGF, thus suppressing angiogenesis.Citation63 Recently, Takeda et alCitation64 demonstrated that mixed-lineage leukemia (MLL), the human homologue of the trithorax in Drosophila, activates matrix metalloproteinase (MMP)-1 and MMP-3 transcription via H3K4 methylation. MLL-ETS2 complex, which is responsible for histone H3K4 methylation, is stabilized by HGF/MET signaling, conferring invasive and metastatic potential to HCC cells. Indeed, deregulated extracellular proteolysis is an essential element contributing to cancer-cell metastatic spread and it mainly relies upon MMP activity. Ozaki et alCitation65 described an upregulation of MMP-1, MMP-3, MMP-7 as well as c-MET both in primary HCCs and in HCC-derived cell lines. They also determined the role of HGF as an inducer of MMP-1, MMP-3, MMP-7, and c-MET via ETS-1 binding to MMP promoters in HCC.Citation65 Conversely, no increase of MMP-2, MMP-9 or HGF mRNA expression could be found.
To date it is commonly accepted that the HGF/c-MET signaling pathway regulates multiple cellular processes, notably leading to increased cell growth, protection from apoptosis, scattering and migration, invasion, and angiogenesis, through interaction with a plethora of downstream stress and survival association molecules.Citation66 These complex events, defined as “invasive growth” program,Citation67 are involved in a wide variety of physiological and pathological contexts, such as embryonic development during gastrulation and nervous system expansion, adult tissue regeneration after injuries and organ failure,Citation68,Citation69 and, finally, in pathological conditions, in the mechanisms of growth and invasion that occur during tumor development and progression.Citation70 While in physiological events c-MET activation is a transient occurrence, during tumor onset and progression c-MET signaling can be constitutively active. Experimental evidence linking the constitutive activation of c-MET to the malignant phenotype and in particular to liver cancer have been reported both in vitro and in vivo. Indeed, c-MET overexpression increases the tumorigenic potential of HCC cell lines injected into nude mice. Xenografts obtained with HCC-derived cell lines manipulated for enhanced or inhibited expression of c-MET clearly outline the relevance of MET overexpression as an event promoting the tumorigenic properties of these cells.Citation29 Experimental conditions that mimic the spontaneous amplification of the c-MET proto-oncogene observed in human tumors, such as transgenic mice overexpressing c-MET, confirmed the development of hepatocellular carcinomas.Citation71,Citation72 In addition, c-MET cooperates with other known oncogenes involved in HCC development, such as c-Myc and beta-catenin, to generate more aggressive tumors in mice.Citation73
The advantage conferred by the activation of c-MET pathway to neoplastic cells during tumor progression has been linked mainly to their increased capability to disaggregate from surrounding tumor cells, destroy the basement membranes, and enhance cell motility and metastatic potential. The involvement of the HGF/MET axis in facilitating tumor metastasis is sustained by experimental evidence obtained in vitro, in animal models, and indirectly confirmed by studies on human HCC specimens. The induction of proteases such as urokinase-type plasminogen activator and MMPs is responsible for the breakdown of the extracellular matrix which, in turn, facilitates the invasion capability of cancer cells. Several studies performed on human HCC samples seem to confirm the possible role of c-MET in tumor progression and metastasis, since MET overexpression is associated with poor-to-moderate differentiation,Citation20,Citation25 presence of intrahepatic metastasis,Citation22,Citation25 and shorter survival.Citation21 In line with these findings, c-MET inhibition in experimental settings was shown to reduce HCC growth and invasion capability.Citation74,Citation75
However, the studies analyzing the role of HGF/MET signaling in hepatocarcinogenesis have also provided contrasting evidence. Even though it is widely accepted that c-MET is required for normal liver regeneration,Citation10,Citation12 Takami et alCitation76 reported that liver-specific c-MET-/- mice displayed a greater number of HCCs that were also larger in size and earlier in the development following a treatment with N-nitrosodiethylamine.Citation76 Interestingly, the growth advantage conferred by the abrogation of c-MET signaling was observed in the early stages of hepatocarcinogenesis. In this model of HCC induced by N-nitrosodiethylamine, c-MET knockout was associated with increased lipid peroxidation, decreased ratio of reduced glutathione to oxidized glutathione, and upregulation of superoxide dismutase 1 and heat shock protein 70, all consistent with a compensatory response to increased oxidative stress and with a role of MET in the maintenance of a normal redox homeostasis. In addition, the transcriptomic analysis performed by microarray confirmed an upregulation of genes associated with cell proliferation and stress responses in c-MET mutant livers.
Thus, HGF/MET signaling may elicit opposing proliferative responses in normal and transformed hepatocytes.Citation77 The loss of functional c-MET in hepatocytes was thus responsible for a state of chronic oxidative stress with increased reactive oxygen species production triggering hepatocarcinogenesis. Indeed, in this experimental setting, dietary supplementation with the thiol-containing antioxidant N-acetyl-L-cysteine rescued the pro-oxidative effect of c-MET deficiency, reducing hepatocarcinogenesis. Similar findings were reported by Marx-Stoelting et alCitation78 in a N-nitrosodiethylamine and phenobarbital-induced model of HCC obtained in a different strain of mice that lacked a functional c-MET. As reported in the previous studies, conditional c-MET knockout mice developed an increased number of preneoplastic and neoplastic liver lesions when compared to controls, again outlining how impaired c-MET signaling participates in HCC induction. These findings supporting tumor-promoting effects of c-MET deficiency should be taken into consideration for their possible implications in the planning of c-MET targeted treatment in specific subgroups of patients.
Even more controversial data have been reported in studies exploring the functions of HGF in in vitro and in vivo models of HCC. Indeed, HGF, originally identified as a factor sustaining mitogenic, morphogenic, antiapoptotic, and motogenic properties of hepatocytes, is also able to inhibit proliferation and cell growth in hepatic stellate cells. It is likely that the final effects of HGF/MET axis activation mainly rely upon the cell type, the simultaneous functional status of other signaling pathways cross-talking with it, such as ERK phosphorylation,Citation79 or activation of JNK1.Citation80
Critical analysis of the potential for targeting c-MET in hepatocellular carcinoma
Based on the therapeutic rationale to target c-MET, various c-MET inhibitors are currently being developed as potential treatments for a variety of cancers.Citation81 Clinical trials targeting c-MET in hepatocellular carcinoma are summarized in .
Table 1 Clinical trials targeting c-MET in hepatocellular carcinoma
Tivantinib, an oral selective c-MET receptor tyrosine kinase inhibitor, has shown promising results in Phase I and II studies as monotherapy for the treatment of advanced HCC.Citation82,Citation83
In a multicenter, single-arm, Phase IB study, 21 cirrhotic patients (Child–Pugh A or B) with advanced HCC for whom prior systemic therapy had failed were treated with tivantinib at a dose of 360 mg twice daily.Citation82 Treatment was associated with disease stabilization in 56% of 16 evaluable patients. Twenty patients (95%) had at least one drug-related adverse event (AE); the most common of any grade were neutropenia (52%), anemia (48%), asthenia (48%), leukopenia (38%), anorexia (38%), and diarrhea (29%). The most-frequent grade 3/4 AEs were neutropenia (38%), anemia (24%), and leukopenia (19%). Serious AEs occurred in four (19%) patients and included anemia, neutropenia, and one fatal septic shock secondary to neutropenia.
More recently, tivantinib was studied in a randomized Phase II trial in patients with advanced HCC and Child–Pugh A cirrhosis who had radiological progression or intolerance to first line systemic therapy.Citation83 Patients were randomized (2:1) to receive tivantinib (360 mg twice daily) or placebo until disease progression. The primary endpoint was time to progression (TTP) in the intention-to-treat population. c-MET expression was assessed in archival or recent tumor samples by IHC, and samples that scored ≥2 in at least 50% of tumor cells were considered as having high c-MET expression. Importantly, the tivantinib dose was amended to 240 mg twice daily because of high incidence of treatment-related grade ≥3 neutropenia (21%) observed with the starting dose of 360 mg twice daily. Four deaths occurred in patients receiving tivantinib, three in the 360 mg twice daily group and one in the 240 mg twice daily group, all of which were related to severe neutropenia.
From an antitumor standpoint, in molecularly unselected patients (71 tivantinib versus 36 placebo), median TTP was longer for those treated with tivantinib (1.6 months [95% confidence interval {CI} =1.4–2.8]) than placebo (1.4 months [95% CI =1.4–1.5]; hazard ratio [HR] =0.64; P=0.04) with no significantly different survival (median overall survival was 6.6 months for patients in the tivantinib group and 6.2 months for patients in the placebo group; HR =0.90; P=0.63).
Interestingly, in the post hoc analysis of a c-MET-high subgroup, the tivantinib group (n=22) had a median TTP of 2.7 months (95% CI =1.4–8.5 months) compared to 1.4 months (95% CI =1.4–1.6 months; HR =0.43; P=0.03) in the placebo group (n=15), with a significantly longer median overall survival (7.2 months [95% CI =3.9–14.6 months] versus 3.8 months [95% CI =2.1–6.8 months], respectively; HR =0.38; P=0.01). Furthermore, the comparison of outcomes by c-MET status in the placebo group showed that the c-MET-high subgroup had a significantly shorter median overall survival than did c-MET-low patients (3.8 months [95% CI =2.1–6.8 months] versus 9.0 months [95% CI =3.7–24.0 months], respectively; HR =2.94; P=0.02), thus suggesting that c-MET overexpression is an independent negative prognostic factor for overall survival in a population of previously treated HCC patients.
Given these promising results, a Phase III, randomized, placebo-controlled trial to evaluate the efficacy of tivantinib in patients with c-MET-high HCC for whom sorafenib therapy had failed was initiated. The confirmation that HCC patients with high c-MET expression treated with tivantinib have a better outcome than those with low MET expression might offer the background for the first attempt at personalized therapy in HCC patients selected on a molecular basis.
Other c-MET inhibitors are currently under investigation in HCC.Citation84 Cabozantinib is an oral small-molecule tyrosine kinase inhibitor that blocks phosphorylation of c-MET and VEGFR2.Citation85 In a randomized discontinuation Phase II study, 41 HCC patients (Child–Pugh A) who experienced tumor progression after one prior systemic therapy received cabozantinib at a dose of 100 mg daily over a 12 week lead-in stage.Citation86 Treatment continuation past week 12 was based on response: patients with partial response (PR) continued open-label cabozantinib, patients with stable disease (SD) were randomized to cabozantinib versus placebo, and those with progressive disease discontinued the treatment. The primary endpoint in the randomized phase was progression-free survival.
Twenty-nine (71%) patients completed the lead-in stage. The overall disease control rate (DCR = PR + SD) at week 12 was 68%. Median progression-free survival was 4.2 months (95% CI =3.0–5.6 months). Most common grade 3/4 AEs were diarrhea (17%), palmar-plantar erythrodysesthesia (15%), and thrombocytopenia (10%). Based on the encouraging antitumor activity of cabozantinib, a Phase III evaluation in HCC patients where sorafenib failed or could not be tolerated is currently underway.
Conclusion
Understanding the signaling pathways driving the malignant phenotype opens the possibility of their therapeutic inhibition. The molecular events contributing to the c-MET–induced “invasive growth” program are being identified in different experimental settings. Conversely, the full understanding of their role and their therapeutic targeting in HCC patients is still in progress. This has to be ascribed to several factors, among which is the high molecular heterogeneity of HCC, which is even more complex when advanced and previously treated tumors are considered. Anticancer treatments are in fact well-known triggers of genetic, epigenetic, and transcriptomic changes. In addition, a consensus on tissue biomarkers identifying tumors in which the c-MET–dependent “invasive growth” program is a driver event are still poorly defined. Indeed, activating point mutations, gene amplification, c-MET–enhanced mRNA and protein expression, as well as c-MET–dependent signatures, have been assayed in different studies, but a correlation between a specific biomarker(s) and the likelihood of response to c-MET inhibition is still under evaluation. The identification of shared assays reflecting the activation of c-MET signaling will give useful information, especially if the final effectors of the signaling pathways will be tested.
Indeed, the complexity of cross-talk with other intracellular cascades makes the molecular scenario quite complex. This is more and more evident if we consider that HCC candidates for c-MET inhibition are those previously treated by both locoregional and systemic approaches. In these HCCs, a molecular classification is not available. Preclinical findings, however, clearly outline the role of hypoxia-inducing treatments in the selection of more aggressive clones with high migration and invasion capability. In these settings it will be crucial to investigate whether the combinations between c-MET inhibitors or HGF antagonists might prevent the escape mechanisms described under hypoxic conditions.Citation87 In addition, it should be kept in mind that in several cases a lack of correlation was reported between HGF levels and c-MET expression and activation. This finding outlines the role of alternative ways of triggering of c-MET signaling, other than HGF, and might play a role in the resistance to c-MET receptor targeted treatments.
Disclosure
The authors have no competing interest to declare. The authors report no other conflicts of interest in this work.
References
- NakamuraTNawaKIchiharaAPartial purification and characterization of hepatocyte growth factor from serum of hepatectomized ratsBiochem Biophys Res Commun19841223145014596477569
- RussellWEMcGowanJABucherNLPartial characterization of a hepatocyte growth factor from rat plateletsJ Cell Physiol198411921831926715416
- StokerMGherardiEPerrymanMGrayJScatter factor is a fibroblast-derived modulator of epithelial cell mobilityNature198732761192392422952888
- ShimaNNagaoMOgakiFTsudaEMurakamiAHigashioKTumor cytotoxic factor/hepatocyte growth factor from human fibroblasts: cloning of its cDNA, purification and characterization of recombinant proteinBiochem Biophys Res Commun103119911802115111581835383
- SuzukiMShirahaHFujikawaTDes-gamma-carboxy prothrombin is a potential autologous growth factor for hepatocellular carcinomaJ Biol Chem225200528086409641515582995
- GherardiEBirchmeierWBirchmeierCVande WoudeGTargeting MET in cancer: rationale and progressNat Rev Cancer12420121228910322270953
- WangXDeFrancesMCDaiYA mechanism of cell survival: sequestration of Fas by the HGF receptor MetMol Cell220029241142111864613
- SchmidtCBladtFGoedeckeSScatter factor/hepatocyte growth factor is essential for liver developmentNature223199537365166997027854452
- UeharaYMinowaOMoriCPlacental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factorNature223199537365167027057854453
- BorowiakMGarrattANWüstefeldTStrehleMTrautweinCBirchmeierCMet provides essential signals for liver regenerationProc Natl Acad Sci U S A720200410129106081061315249655
- KosaiKMatsumotoKNagataSTsujimotoYNakamuraTAbrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factorBiochem Biophys Res Commun327199824436836909535725
- HuhCGFactorVMSánchezAUchidaKConnerEAThorgeirssonSSHepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repairProc Natl Acad Sci U S A3302004101134477448215070743
- GiebelerABoekschotenMVKleinCc-Met confers protection against chronic liver tissue damage and fibrosis progression after bile duct ligation in miceGastroenterology720091371297308308.e1419208365
- MarquardtJUSeoDGómez-QuirozLELoss of c-Met accelerates development of liver fibrosis in response to CCl(4) exposure through deregulation of multiple molecular pathwaysBiochim Biophys Acta620121822694295122386877
- KroyDCSchumacherFRamadoriPHepatocyte specific deletion of c-Met leads to the development of severe non-alcoholic steatohepatitis in miceJ Hepatol10201461488389024845607
- ZouCMaJWangXLack of Fas antagonism by Met in human fatty liver diseaseNat Med920071391078108517704785
- KimWHMatsumotoKBesshoKNakamuraTGrowth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosisAm J Pathol4200516641017102815793283
- ZarnegarRMichalopoulosGPurification and biological characterization of human hepatopoietin A, a polypeptide growth factor for hepatocytesCancer Res61519894912331433202524251
- NakamuraTNishizawaTHagiyaMMolecular cloning and expression of human hepatocyte growth factorNature1123198934262484404432531289
- SuzukiKHayashiNYamadaYExpression of the c-met protooncogene in human hepatocellular carcinomaHepatology111994205123112367927256
- TavianDDe PetroGBenettiAPortolaniNGiuliniSMBarlatiSu-PA and c-MET mRNA expression is co-ordinately enhanced while hepatocyte growth factor mRNA is down-regulated in human hepatocellular carcinomaInt J Cancer91200087564464910925356
- UekiTFujimotoJSuzukiTYamamotoHOkamotoEExpression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinomaHepatology419972548628669096589
- Kaposi-NovakPLeeJSGòmez-QuirozlCoulouarnCFactorVMThorgeirssonSSMet – regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotypeJ Clin Invest6200611661582159516710476
- D’ErricoAFiorentinoMPonzettoALiver hepatocyte growth factor does not always correlate with hepatocellular proliferation in human liver lesions: its specific receptor c-met doesHepatology7199624160648707284
- DaveauMScotteMFrançoisAHepatocyte growth factor, transforming growth factor alpha, and their receptors as combined markers of prognosis in hepatocellular carcinomaMol Carcinog3200336313014112619035
- KondoSOjimaHTsudaHClinical impact of c-Met expression and its gene amplification in hepatocellular carcinomaInt J Clin Oncol4201318220721322218908
- WangZLLiangPDongBWYuXLYu deJPrognostic factors and recurrence of small hepatocellular carcinoma after hepatic resection or microwave ablation: a retrospective studyJ Gastrointest Surg2200812232733717943391
- ShimomuraTKondoJOchiaiMActivation of the zymogen of hepatocyte growth factor activator by thrombinJournal of Biological Chemistry19932683022927229328226803
- BirchmeierCBirchmeierWGherardiEVande WoudeGFMet, metastasis, motility and moreNature Reviews Molecular Cell Biology200341291592514685170
- LeeSJLeeJSohnIMaoMKaiWParkCKLimHYA survey of c-MET expression and amplification in 287 patients with hepatocellular carcinomaAnticancer Res11201333115179518624222167
- TakeoSAraiHKusanoNExamination of oncogene amplification by genomic DNA microarray in hepatocellular carcinomas: comparison with comparative genomic hybridization analysisCancer Genet Cytogenet10151996130212713211675133
- WangKLimHYShiSGenomic landscape of copy number aberrations enables the identification of oncogenic drivers in hepatocellular carcinomaHepatology8201358270671723505090
- GuichardCAmaddeoGImbeaudSIntegrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinomaNat Genet56201244669469822561517
- PennacchiettiSMichieliPGalluzzoMMazzoneMGiordanoSComoglioPMHypoxia promotes invasive growth by transcriptional activation of the met protooncogeneCancer Cell20034343476112726861
- HarrisALHypoxia-a key regulatory factor in tumor growthNature Rev Cancer2002384811902584
- YuJLRakJWCoomberBLEffect of p53 status on tumor response to antiangiogenic therapyScience29520021526152811859195
- SierraJRTsaoMSc-MET as a potential therapeutic target and biomarker in cancerTher Adv Med Oncol11201131 SupplS213522128285
- SchmidtLDuhFMChenFGermline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomasNat Genet5199716168739140397
- ParkWSDongSMKimSYSomatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomasCancer Res11519995923073109927037
- LeeJHHanSUChoHA novel germ line juxtamembrane Met mutation in human gastric cancerOncogene1012200019434947495311042681
- AebersoldDMLandtOBerthouSPrevalence and clinical impact of Met Y1253D-activating point mutation in radiotherapy-treated squamous cell cancer of the oropharynxOncogene1120200322528519852314627992
- Wellcome Trust Sanger InstituteCOSMIC-Catalogue of Somatic Mutations in Cancer [webpage on the Internet]Hinxton, Cambridge, UKWellcome Trust Sanger Institute, Genome Research limited2010 Available from: http//www.sanger.ac.uk/genetics/CGP/cosmic/Accessed January 5, 2015
- CooperCSParkMBlairDGMolecular cloning of a new transforming gene from a chemically transformed human cell lineNature96–111984311598129336590967
- LiangTJReidAEXavierRCardiffRDWangTCTransgenic expression of tpr-met oncogene leads to development of mammary hyperplasia and tumorsJ Clin Invest61519969712287228778675700
- SomanNRCorreaPRuizBAWoganGNThe TPR-MET oncogenic rearrangement is present and expressed in human gastric carcinoma and precursor lesionsProc Natl Acad Sci U S A6119918811489248962052572
- Danilkovitch-MiagkovaAZbarBDysregulation of Met receptor tyrosine kinase activity in invasive tumors.[Review]Clin Invest420021097863867 Review
- CorsoSComoglioPMGiordanoSCancer therapy: can the challenge be MET? [Review]Trends Mol Med6200511628429215949770
- TrusolinoLBertottiAComoglioPMMET signalling: principles and functions in development, organ regeneration and cancer.[Review]Nat Rev Mol Cell Biol122010111283484821102609
- BoccaccioCGaudinoGGambarottaGGalimiFComoglioPMHepatocyte growthn factor (HGF) receptor expression is inducible and is part of the delayed-early response to HGFJ Biol Chem42919942691712846128518175699
- WebbCPTaylorGAJeffersMEvidence for a role of Met-HGF/SF during Ras-mediated tumorigenesis/metastasisOncogene102219981716201920259798673
- GambarottaGBoccaccioCGiordanoSAndŏMStellaMCComoglioPMEts up-regulates MET transcriptionOncogene1171996139191119178934537
- IvanMBondJAPratMComoglioPMWynford-ThomasDActivated ras and ret oncogenes induce over-expression of c-met (hepatocyte growth factor receptor) in human thyroid epithelial cellsOncogene52219971420241724239188856
- Orian-RousseauVChenLSleemanJPHerrlichPPontaHCD44 is required for two consecutive steps in HGF/c-Met signalingGenes Dev121200216233074308612464636
- TrusolinoLBertottiAComoglioPMA signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growthCell11302001107564365411733063
- BergströmJDWestermarkBHeldinNEEpidermal growth factor receptor signaling activates met in human anaplastic thyroid carcinoma cellsExp Cell Res8252000259129329910942601
- LuKVChangJPParachoniakCAVEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complexCancer Cell7102012221213522789536
- KorhanPErdalEKandemişEReciprocal activating crosstalk between c-Met and caveolin 1 promotes invasive phenotype in hepatocellular carcinomaPLoS One822201498e10527825148256
- RongSDonehowerLAHansenMFMet proto-oncogene product is overexpressed in tumors of p53-deficient mice and tumors of Li-Fraumeni patientsCancer Res511995559196319707728766
- MiglioreCPetrelliAGhisoEMicroRNAs impair MET-mediated invasive growthCancer Res121520086824101281013619074879
- FornariFMilazzoMChiecoPMiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cellsCancer Res615201070125184519320501828
- HouJLinLZhouWIdentification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinomaCancer Cell215201119223224321316602
- KorhanPErdalEAtabeyNMiR-181a-5p is downregulated in hepatocellular carcinoma and suppresses motility, invasion and branching-morphogenesis by directly targeting c-MetBiochem Biophys Res Commun88201445041304131225058462
- YangXZhangXFLuXMicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathwayHepatology520145951874188524259426
- TakedaSLiuHSasagawaSHGF-MET signals via the MLL-ETS2 complex in hepatocellular carcinomaJ Clin Invest71201312373154316523934123
- OzakiIMizutaTZhaoGInduction of multiple matrix metalloproteinase genes in human hepatocellular carcinoma by hepatocyte growth factor via a transcription factor Ets-1Hepatol Res12200327428930114662117
- ComoglioPMTrusolinoLInvasive growth: from development to metastasis. [Review]J Clin Invest42002109785786211927611
- TrusolinoLComoglioPMScatter-factor and semaphorin receptors: cell signalling for invasive growth. [Review]Nat Rev Cancer420022428930012001990
- MichalopoulosGKDeFrancesMCLiver regeneration.[Review]Science441997276530960669082986
- RabkinRFervenzaFTsaoTHepatocyte growth factor receptor in acute tubular necrosisJ Am Soc Nephrol3200112353154011181801
- BoccaccioCComoglioPMInvasive growth: a MET-driven genetic programme for cancer and stem cells. [Review]Nat Rev Cancer820066863764516862193
- WangRFerrellLDFaouziSMaherJJBishopJMActivation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic miceJ Cell Biol528200115351023103411381087
- SakataHTakayamaHSharpRRubinJSMerlinoGLaRochelleWJHepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse liversCell Growth Differ111996711151315238930401
- AmiconeLTerradillosOCalvoLSynergy between truncated c-Met (cyto-Met) and c-Myc in liver oncogenesis: importance of TGF-beta signalling in the control of liver homeostasis and transformationOncogene22120022191335134511857077
- ZhangSZPanFYXuJFKnockdown of c-Met by adenovirus-delivered small interfering RNA inhibits hepatocellular carcinoma growth in vitro and in vivoMol Cancer Ther1020054101577158416227408
- XieBXingRChenPDown-regulation of c-Met expression inhibits human HCC cells growth and invasion by RNA interferenceJ Surg Res82010162223123819765730
- TakamiTKaposi-NovakPUchidaKLoss of hepatocyte growth factor/c-Met signaling pathway accelerates early stages of N-nitrosodiethylamine induced hepatocarcinogenesisCancer Res1015200767209844985117942915
- LiuMLMarsWMMichalopoulosGKHepatocyte growth factor inhibits cell proliferation in vivo of rat hepatocellular carcinomas induced by diethylnitrosamineCarcinogenesis419951648418437728965
- Marx-StoeltingPBorowiakMKnorppTBirchmeierCBuchmannASchwarzMHepatocarcinogenesis in mice with a conditional knockout of the hepatocyte growth factor receptor c-MetInt J Cancer415200912481767767219123478
- KimWHMatsumotoKBesshoKNakamuraTGrowth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosisAm J Pathol4200516641017102815793283
- ConnerEATeramotoTWirthPJKissAGarfieldSThorgeirssonSSHGF-mediated apoptosis via p53/bax-independent pathway activating JNK1Carcinogenesis4199920458359010223185
- SmythECSclafaniFCunninghamDEmerging molecular targets in oncology: clinical potential of MET/hepatocyte growth-factor inhibitors.[Review]Onco Targets Ther612201471001101424959087
- SantoroASimonelliMRodriguez-LopeCA Phase-1b study of tivantinib (ARQ 197) in adult patients with hepatocellular carcinoma and cirrhosisBr J Cancer11520131081212423287988
- SantoroARimassaLBorbathITivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 studyLancet Oncol12013141556323182627
- GoyalLMuzumdarMDZhuAXTargeting the HGF/c-MET pathway in hepatocellular carcinoma.[Review]Clin Cancer Res5120131992310231823388504
- XiangQChenWRenMWangJZhangHDengDYZhangLShangCChenYCabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and METClin Cancer Res61201420112959295724700742
- VerslypeCCohnALKelleyRKActivity of cabozantinib (XL184) in hepatocellular carcinoma: Results from a phase II randomized discontinuation trial (RDT)American Society of clinical oncology 2012 Annual MeetingJune 1–5, 2012Chicago, ILJ Clin Oncol201230suppl Abstract #4007
- KnudsenBSVande WoudeGShowering c-MET-dependent cancers with drugsCurr Opin Genet Dev22008181879618406132
