Abstract
Chronic liver diseases (CLDs) encompass a wide range of illnesses, including nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and viral hepatitis. Deterioration of liver capacity, with subsequent progression into cirrhosis and hepatocellular carcinoma (HCC), ultimately leads to a further decrease in the hepatic reserve. The Child–Turcotte–Pugh scoring system is the standard tool for assessing underlying liver reserve capacity in routine practice and in clinical trials of CLD and HCC. In this review, we highlight the clinical significance of insulin-like growth factor-I (IGF-I) and the growth hormone (GH) signaling pathway in HCC. IGF-I could be a marker for liver reserve capacity in CLDs and HCC in clinical practice. This approach could improve the risk assessment and stratifications of patients on the basis of their underlying liver reserve, either before active treatment in routine practice or before they are enrolled in clinical trials.
Keywords:
Insulin-like growth factors and binding proteins
Insulin-like growth factors (IGFs) were first described by Salmon and Daughaday in 1957.Citation1 The IGF axis includes several molecules: two ligands (IGF-I [somatomedin C] and IGF-II [somatomedin A]), two transmembrane receptors (IGF-I receptor [IGF-IR] and IGF-II receptor [IGF-IIR]), and eight high-affinity IGF binding proteins (IGFBPs) (IGFBP-1 to -6, along with the lesser characterized IGFBP-7 and -8) ().Citation2–Citation9 These factors stimulate musculoskeletal growth and differentiation, particularly during prenatal growth.Citation1,Citation10–Citation12 Under normal physiological conditions, all IGF axis molecules work together in a harmonized manner to maintain cellular homeostasis.
Figure 1 Insulin-like growth factor-I (IGF-I) axis and variable sources of IGF-I production.
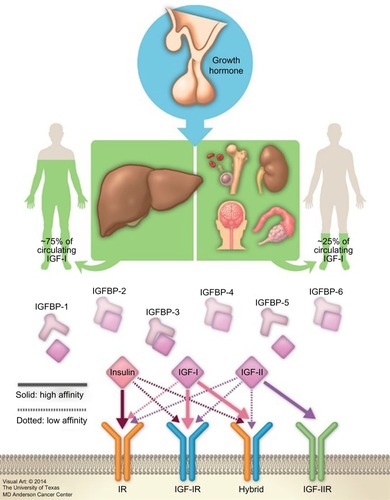
IGF-I is a hormone with a small molecular weight; it contains 70 amino acids, and unlike other peptides, 99% of it is protein bound. As the biochemical structure of IGF-IR is similar to that of the insulin receptor, the free IGF-I possesses a high affinity to bind with IGF-IR compared to that of insulin receptor, inducing cell proliferation and inhibiting apoptosis. It also binds with a high affinity to hybrid receptors, which contain an alpha–beta IGF half-receptor paired with an alpha–beta insulin half-receptor. The physiologic significance of hybrid receptors is not well defined, but they may mediate the insulin-like actions of IGF-I (). These effects can be primarily inhibited by IGFBP-3, which binds to and prevents IGF-I from binding to IGF-IR.Citation4,Citation7,Citation13
Mechanism and sources of IGF-I synthesis
Growth hormone (GH) is secreted, with diurnal variation, from the anterior pituitary gland in a pulsatile manner. This occurs under hypothalamic control through the influence of hypothalamic neuropeptides, GH-releasing hormone, and GH-inhibitory hormone (somatostatin); in addition to the influence of both IGF-I and ghrelin (a gastric hormone).Citation14,Citation15
Released GH produced from the pituitary gland is transported by GH binding protein to bind to its receptors in different tissues, including the liver, which is considered the main target of GH. This binding upregulates IGF-I synthesis through stimulation of IGF-I gene transcription.Citation8,Citation16 Approximately 75% of circulating IGF synthesized by the liver is believed to perform an “endocrine” function as it is typically used remotely.Citation17–Citation19 In contrast, approximately 25% of IGF-I that is synthesized in the bones, cartilage, central nervous system, kidneys, ovaries, and erythroid cell precursors executes autocrine and paracrine functions ( and ).Citation20–Citation25
Factors affecting plasma levels of GH/IGF-I
GH/IGF secretion can be stimulated directly by the “push effect” or indirectly by the “pull effect” by reducing the negative feedback inhibitory effect.Citation26 Normally, the circulating IGF level changes with age. During childhood, increase in the production of sex steroid hormones results in increased production of GHCitation27–Citation29 and subsequently IGF-I in both sexes.Citation27,Citation30–Citation34 GH/IGF-I levels rapidly decline during the second decade of life, followed by a slow decline until the age of 60 years.Citation35 However, the relationship between steroid hormones and GH/IGF is affected by sex. Several studies have shown that in men, regardless of age, testosterone centrally increases the GH level, followed by IGF-I production through the push effect.Citation36–Citation40 Studies were performed to determine whether testosterone enhances IGF-I directly or as a costimulatory factor to GH; testosterone alone had a very limited or no effect on circulating IGF-I levels except in the presence of GH.Citation41–Citation43 On the contrary, there is a debate concerning the relationship between estrogen and GH/IGF-I. Researchers have found that during menstruation, GH levels increase in response to an estrogen peak, with higher GH levels in premenopausal women than in postmenopausal women.Citation44,Citation45 However, most studies did not evaluate IGF-I levels and thus could not determine whether GH levels increased as the result of a pushing or pulling effect.Citation39,Citation46,Citation47 Notably, recent studies showed that estrogen indirectly stimulates GH production by inhibiting the IGF-I “pulling effect”.Citation48–Citation51
Notably, elevated glucose, insulin, cortisol, and non-stratified free fatty acid could also inhibit GH production. Amino acids, sleep, and exercise increase GH secretion levels. In all of these conditions, IGF-I is influenced by changes in the GH level. The presence of all these factors complicates the GH/IGF-I secretion control process.Citation52–Citation56 Furthermore, IGF-I that is synthesized in peripheral tissues is influenced by several factors on the basis of the site of production:Citation57,Citation58 1) bone and cartilage (parathyroid hormone [PTH] regulates IGF-I gene transcription in the bone, while GH increases IGF-I synthesis from osteoblasts and chondrocytes); 2) erythroid cell precursors (which synthesize IGF-I under the influence of erythropoietin); 3) skeletal muscles (both muscle injury and hypertrophy stimulate IGF-I synthesis); and 4) kidneys (which are an important local source of IGF-I). Notably, unilateral nephrectomy induces compensatory growth of the contralateral kidney, with a subsequent increase in IGF-I expression.
Mechanism of action of IGF-I
Synthesized IGF-I is cleaved by protease enzymes before being released into the circulation. IGFBPs, which are present in all extracellular fluids, transport IGF-I by binding to approximately 99% of it with a higher affinity than IGF-IR.Citation14,Citation59 The bound form of IGF-I is mainly synthesized in the liver, while the free form, which is produced by other tissues, has a low affinity to IGFBPs and is responsible for its autocrine and paracrine effects.Citation16,Citation17
Notably, elevated serum levels of IGF-I induce a negative feedback effect on GH secretion, either directly through a local inhibitory effect on the pituitary gland or indirectly by stimulating somatostatin release. Thus, IGF-I and GH work cooperatively as IGF-I regulates GH effects, which in turn control the release of IGF-I.Citation60–Citation67
The role of GH receptor (GHR) in harmonizing the association between GH elevationCitation68 and IGF-I suppressionCitation69 has been reported in previous studies. Chang et alCitation70 studied the correlation between these changes and GHRs, which are present on hepatocyte cell membranes. They determined the presence of GHR in human HCC, cirrhosis, and normal tissue samples using radio-receptor assays and discovered that GHR was absent in both cirrhotic and HCC samples, which explains the persistent decrease in the serum level of IGF-I with elevated levels of GH.
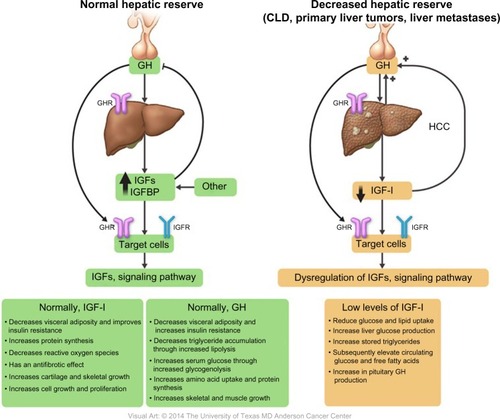
Normally, both GH and IGF-I have an anabolic effect, promoting lipolysis and protein synthesis by stimulating amino acid uptake, stimulating cell growth and differentiation, increasing muscle mass through sarcomere hyperplasia, and stimulating the immune system by restoring a normal nitrogen balance and causing a 25% increase in GFR. IGF-I also decreases blood glucose levels, improves insulin resistance, decreases reactive oxygen species, and has an antifibrotic effect.Citation71–Citation73
Molecular role of IGF-I in cancer development
In 1990, the role of IGF-I in the process of tumorigenesis was revealed. Since then, a major research focus has been to better understand the nature and role of the IGF axis in the pathogenesis of various neoplasms.Citation11
Recently, there has been renewed interest in the roles of GH/IGF-I in cancer development because of an increase in cancer incidence, including breast, thyroid, colon, and prostate cancers, in acromegalic cancer patients with elevated serum IGF-I secondary to GH-producing pituitary tumors.Citation74 Elevated serum IGF-I and GH levels were reported in non-acromegalic cancer patients. GH enhances cancer development through several pathways:Citation75–Citation81 1) it binds to GHR and activates several intracellular signal pathways; 2) it stimulates IGF-I production from the liver; and 3) it induces peripheral tissue insulin resistance, with subsequent elevation of serum insulin levels.
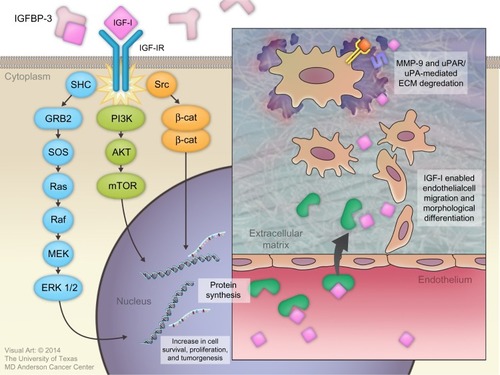
Binding of IGF-I to the alpha subunit of IGF-IR leads to auto-activation of tyrosine kinase and the auto-phosphorylation of tyrosines, with subsequent phosphorylation of insulin receptor substrate-1 (IRS-I) and insulin receptor substrate 2 (IRS-II).Citation82,Citation83 IRS-I stimulates several kinase pathways, such as phosphatidylinositol 3 kinase (PI3K), SHC, and Src. Through the SHC pathway, Grb-2 forms a complex that activates son of sevenless (SOS) protein. This complex activates p21 Ras, which is a mitogen-activated protein kinase pathway. Activation of this pathway is important for stimulating cell growth.Citation83 IRS-I also activates the PI3K/mitogen-activated protein kinase (mTOR) pathway, which is important for stimulating protein synthesis, glucose transportation, cell motility, and apoptosis inhibition.Citation84
IGF-I plays an important role in cancer development by regulating angiogenesis, lymphangiogenesis, degradation of the extracellular matrix (ECM), tumor invasion into both the ECM and blood vessels, and maintenance of tumor cell survival and proliferation.Citation7,Citation85
Several basic science studies showed that IGF-I regulates angiogenesis and lymphangiogenesis by activating vascular endothelial growth factor and stimulating the expression of hypoxia-inducible factor 1 via the PI3K/Akt and Ras/mTOR pathways.Citation86–Citation89 IGF-I is transported across the vascular endothelial cell lining through a paracellular route where it binds to the subendothelial ECM to stimulate the migration and morphological differentiation of endothelial cells.Citation90–Citation92 Subsequently, IGF-I activates matrix metalloproteinase-9, which is a type IV collagenase.Citation93 IGF-I also increases the binding of single-chain urokinase-type plasminogen activator (uPA) to the cell-surface uPA receptor (uPAR). This combination converts serum plasminogen to plasmin, which is a broad-spectrum serum protease enzyme. Both metalloproteinase-9 and uPAR/uPA are major molecular mediators that play a significant role in ECM proteolysis and degradation, followed by tumor invasion ().Citation94
Figure 3 Roles of insulin-like growth factor-I (IGF-I) in cancer development.
GH/IGF-I as an indicator of hepatic reserve
A previous article reported an IGF-I deficiency in CLDs such as nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, viral hepatitis, cirrhosis, and HCC; it occurs through several mechanisms, including insulin resistance, oxidative stress, mitochondrial dysfunction, and the inflammatory cascade.Citation95
The correlation between GH and IGF-I levels in liver cirrhosis has been previously evaluated; elevated plasma levels of GH were found in cirrhosis patients, with a pulse frequency and plasma half-life that were more than twice those in the control group; this was partially explained by the associated hyperglycemia.Citation96
In 1993, Buzzelli et alCitation63 studied changes in the GH/IGF-I circadian rhythm in cirrhosis patients, regardless of the presence or absence of associated HCC. They concluded that, compared to the control group, patients with cirrhosis had lower serum IGF-I levels and higher GH levels. These changes remained stable for 24 hours, resulting in loss of GH/IGF-I circadian rhythm.Citation63 This phenomenon was explained by the decrease in GHRs and their binding capacity in damaged liver tissue compared with in normal liver tissue.
A low serum IGF-I level leads to several metabolic changes induced by reduced peripheral glucose and lipid uptake, increased liver glucose production, increased stored triglyceride hydrolysis, and subsequently elevated circulating glucose and free fatty acid levels ().Citation62 Furthermore, a few studies showed that, under normal conditions, IGF-I stimulates hepatocyte growth factor (HGF) production from hepatic stellate cells, and that administration of human recombinant HGF suppressed the onset of liver fibrosis/cirrhosis in animal models.Citation97–Citation99 Thus, low levels of free IGF-I lead to a loss of antifibrotic effects. Reactive oxygen species, different cytokines, and inflammatory mediators can easily activate hepatic stellate cells and induce fibrosis.Citation100
Further studies showed a lower rate of IGF-I expression in patients diagnosed with either nonalcoholic fatty liver disease or nonalcoholic steatohepatitis ().Citation101,Citation102
Table 1 Clinical studies of circulating IGF-I in CLDs
Notably, liver cirrhosis, which is a chronic disease in which the liver tissue is irreversibly replaced by fibrous tissue, necrosis, and regenerating nodules, leads to the deterioration of normal hepatic function.Citation103 Systematically, cirrhosis patients experience several clinical manifestations of their decreased metabolic liver capacity and subsequent IGF-I deficiency and GH elevation.
Several studies reported decreased serum IGF-I levels in patients with diseased liver compared to normal population. This suggests that circulating levels of IGF-I are a surrogated marker for assessment of liver dysfunction ().Citation101,Citation102,Citation104–Citation119
Collectively, these findings support the hypothesis that plasma IGF-I levels reflect hepatic synthetic function and hence should be considered a surrogate marker for determining the hepatic reserve.
IGF-I as an assessment tool for liver reserve capacity in HCC
Currently, surgical resection and liver transplantation are the only curative treatments for HCC.Citation120,Citation121 Unfortunately, most patients are not surgical candidates because of an advanced tumor stage at presentation or advanced under lying CLDs.Citation122,Citation123 These factors have a significant effect on treatment decisions and outcomes (including overall survival [OS]) and prognostic stratification for clinical trial enrollment.
Several HCC prognostic systems are used to assess underlying CLD status, predict treatment outcome and OS, and stratify patients in clinical trials. However, the standard system for assessing hepatic reserve in HCC staging systems is the Child-Turcotte-Pugh (CTP) score, which depends on two subjective parameters (encephalopathy and ascites) and three objective parameters (serum albumin, serum bilirubin, and prothrombin time or the international normalized ratio).Citation107,Citation111,Citation113,Citation116,Citation117,Citation119 Despite its limitations, the CTP score has remained the standard tool for predicting the degree of underlying CLD in HCC patients before active therapy or trial entry, using CTP class A (CTP-A) as the standard treatable patient population.Citation124–Citation131 Several studies have concluded that patients classified as CTP-B or CTP-C have significantly shorter survival duration than do those classified as CTP-A because of deterioration in their hepatic function. Therefore, CTP-A patients are the only patients who are eligible for active treatment and clinical trialsCitation122,Citation123,Citation132 and are the only population approved by the US Food and Drug Administration (FDA) for sorafenib therapy on the basis of the results of the first international, randomized, double-blind, placebo-controlled, multicenter Phase III study.Citation121
The CTP-A group is heterogeneous (especially nonsurgical patients, who constitute the main pool in clinical practice and clinical trials, as described at the most recent international expert consensus conference),Citation123 and post-therapeutic decline in liver functions is still a major challenge to outcome prediction. Furthermore, the survival benefit of sorafenib was associated with only a 2.3% objective response rate, as defined by Response Evaluation Criteria in Solid Tumors. Sorafenib is an expensive treatment; it is not affordable to many patients and may increase health care costs, especially in low- and middle-income countries. National and international guidelines recommend sorafenib only in patients with CTP-A to avoid potential severe adverse effects and death due to hepatic failure.Citation133–Citation135 However, hepatic failure and sorafenib intolerance still occur in patients with CTP-A. Thus, there is a critical and immediate need for more sensitive tools than the CTP score to predict survival duration in HCC patients undergoing treatment and in selected patients who are not eligible for enrollment in clinical trials.
Integrating IGF-I levels into HCC management
Since the liver produces >75% of circulating IGF-I, and IGF-I’s role is documented in other CLDs, several studies have investigated whether IGF-I levels can be used to assess hepatic capacity in HCC patients and detect its correlation with HCC prognosis and survival outcome ().Citation136–Citation139 The decline in serum levels of IGF-I in HCC is likely mediated through the decreased synthetic capacity of normal liver cells, which have been replaced by tumor cells.Citation109,Citation140–Citation142
We recently reported the utility of plasma IGF-I as a molecular biomarker for assessing liver reserve in HCC patients.Citation136,Citation137,Citation143 In addition, two recent studies reported that low pretreatment IGF-I levels independently correlated with poor outcome in the form of a shorter TTP and OS in patients with HCC who underwent TACE.Citation144,Citation145
On the basis of the widely adopted American Association for the Study of Liver Diseases guidelines, HCC can be diagnosed using a noninvasive imaging approach.Citation123,Citation146 There is a critical need to develop a blood-based biomarker strategy to assess hepatic reserve and predict patients’ survival and treatment outcomes. This approach will improve the personalization of HCC treatment by allowing us to select the best candidates for specific therapeutic modalities and avoid unnecessary harm and health care expenses.
Developing the IGF-I score
The CTP score is the standard tool currently used for assessing hepatic reserve in HCC staging systems. Recently, our research group studied the value of incorporating IGF-I into the CTP system to replace the two subjective parameters, ascites and encephalopathy ().Citation143 Our results indicated that the IGF-CTP score significantly improved OS prediction and patient risk stratification compared to the CTP score in both the training and validation cohorts (P=0.003 and P=0.005, respectively, when measured by the C-index) (). Differences between the C-indices were not large but were statistically significant as the C-index computes the ability to predict OS for all patients in the cohort, including those whose CTP and IGF-CTP scores are different and those whose scores are the same. Interestingly, patients with CTP-A that was reclassified as IGF-CTP-B had significantly shorter OS than did patients whose IGF-CTP-A classification remained unchanged in both the training and validation cohorts (P=0.03 and P<0.001, respectively) ( and ).
Figure 4 Kaplan–Meier curves for IGF classification of Child–Turcotte–Pugh (CTP) class A hepatocellular carcinoma patients.
Abbreviation: IGF, insulin-like growth factor.
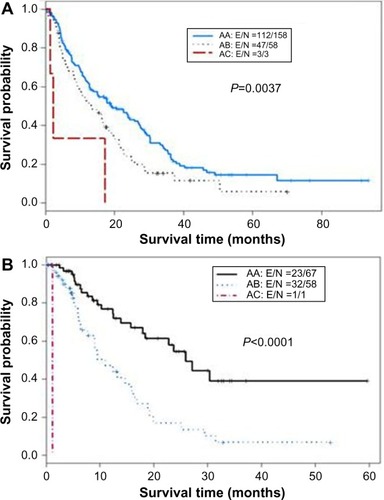
Table 2 Original CTP scoring system replaced by the new IGF-I CTP scoring system
Table 3 Ranking of scoring systems by C-index
Table 4 Rearrangement of originally CTP class A according to the IGF-CTP score
Conclusion
Classification of the degree of liver reserve is critical to HCC management and for selecting patients for clinical trials. CTP is the most commonly used clinical tool to assess hepatic reserve, but it has multiple limitations, including the use of two subjective variables (ascites and encephalopathy) that are difficult to assess and may change daily under the influence of medications, nutritional status, and comorbidities. In addition, these subjective variables and their arbitrary cutoff points have been randomly selected. Emerging data about the GH/IGF-I axis in HCC by our research group and others indicate that plasma IGF-I should be incorporated in assessment of the liver reserve capacity. In our recent studies, we incorporated plasma IGF-I into the objective parameters of CTP to create an exclusively objective blood-based score and reported the results from two independent cohorts at our institution. The score is currently undergoing independent multicenter and international validation. We anticipate that IGF-I use will enhance the accuracy of selecting appropriate patients for active therapy in routine practice and for enrollment in clinical trials. Importantly, a rigorous analysis of the interactions and correlations between the GH/IGF-I axis in HCC will help advance our current understanding of the complex pathogenesis of HCC development and progression. The emerging data on upregulating GH in patients with cirrhosis and HCC is intriguing, given the potential to target this pathway for HCC prevention and treatment. Future validation studies of this approach are warranted.
Acknowledgments
The current study is supported, in part, by National Institutes of Health through grants CA170035-01 (to AOK), CA106458-01 (to MMH), and CA151533 (to HMA). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. RA is supported by a scholarship from the Egyptian Ministry of Higher Education.
Disclosure
The authors report no conflicts of interest in this work.
References
- SalmonWDJrDaughadayWHA hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitroJ Lab Clin Med195749682583613429201
- BaxterRCInsulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivitiesAm J Physiol Endocrinol Metab20002786E967E97610826997
- ClemmonsDRUse of mutagenesis to probe IGF-binding protein structure/function relationshipsEndocr Rev200122680081711739334
- KosteckaYBlahovecJInsulin-like growth factor binding proteins and their functions (minireview)Endocr Regul1999332909410467430
- LeRoithDWernerHBeitner-JohnsonDRobertsCTJrMolecular and cellular aspects of the insulin-like growth factor I receptorEndocr Rev19951621431637540132
- MurphyLJInsulin-like growth factor-binding proteins: functional diversity or redundancy?J Mol Endocrinol1998212971079801453
- SamaniAAYakarSLeRoithDBrodtPThe role of the IGF system in cancer growth and metastasis: overview and recent insightsEndocr Rev2007281204716931767
- SridharSSGoodwinPJInsulin-insulin-like growth factor axis and colon cancerJ Clin Oncol200927216516719064959
- ZarrilliRBruniCBRiccioAMultiple levels of control of insulin-like growth factor gene expressionMol Cell Endocrinol19941011–2R1R149397969
- DaughadayWHThe possible autocrine/paracrine and endocrine roles of insulin-like growth factors of human tumorsEndocrinology19901271142163304
- MakiRGSmall is beautiful: insulin-like growth factors and their role in growth, development, and cancerJ Clin Oncol201028334985499520975071
- MasoodiMAghazadehRSomiMHShavakhiHShabestariAAZaliMRSerum insulin-like growth factor-I and tumor size in patients with metastatic liver cancerHepat Mon200883179183
- LeRoithDRobertsCTThe insulin-like growth factor system and cancerCancer Lett2003195212713712767520
- RosenbloomALGuevara-AguirreJRosenfeldRGPollockBHGrowth in growth hormone insensitivityTrends Endocrinol Metab19945729630318407222
- SmithRGJiangHSunYDevelopments in ghrelin biology and potential clinical relevanceTrends Endocrinol Metab200516943644216213742
- VajdosFFUltschMSchafferMLCrystal structure of human insulin-like growth factor-1: detergent binding inhibits binding protein interactionsBiochemistry20014037110221102911551198
- OhlssonCMohanSSjögrenKThe role of liver-derived insulin-like growth factor-IEndocr Rev200930549453519589948
- FroeschERSchmidCSchwanderJZapfJActions of insulin-like growth factorsAnnu Rev Phsiol198547443467
- SjögrenKLiuJLBladKLiver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in miceProc Natl Acad Sci U S A199996127088709210359843
- BasergaRPeruzziFReissKThe IGF-1 receptor in cancer biologyInt J Cancer2003107687387714601044
- BasergaRPorcuPRubiniMSellCCell cycle control by the IGF-1 receptor and its ligandsAdv Exp Med Biol19933431051128184731
- CosmanDLymanSDIdzerdaRLA new cytokine receptor superfamilyTrends Biochem Sci19901572652702166365
- CruickshankJGrossmanDIPengRKFamulaTROberbauerAMSpatial distribution of growth hormone receptor, insulin-like growth factor-I receptor and apoptotic chondrocytes during growth plate developmentJ Endocrinol2005184354355315749813
- SchwanderJCHauriCZapfJFroeschERSynthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone statusEndocrinology198311312973056190641
- WernerHLeRoithDThe role of the insulin-like growth factor system in human cancerAdv Cancer Res1996681832238712068
- HoKYVeldhuisJDJohnsonMLFasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in manJ Clin Invest19888149689753127426
- GiustinaAScalviniTTassiCMaturation of the regulation of growth hormone secretion in young males with hypogonadotropic hypogonadism pharmacologically exposed to progressive increments in serum testosteroneJ Clin Endocrinol Metab1997824121012199100598
- KeenanBSRichardsGEPonderSWDallasJSNagamaniMSmithERAndrogen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed pubertyJ Clin Endocrinol Metab199376499610018473416
- LocheSColaoACappaMThe growth hormone response to hexarelin in children: reproducibility and effect of sex steroidsJ Clin Endocrinol Metab19978238618649062497
- KerriganJRRogolADThe impact of gonadal steroid hormone action on growth hormone secretion during childhood and adolescenceEndocr Rev19921322812981618164
- JospeNOrlowskiCCFurlanettoRWComparison of transdermal and oral estrogen therapy in girls with Turner’s syndromeJ Pediatr Endocrinol Metab1995821111167584704
- HarrisDAVan VlietGEgliCASomatomedin-C in normal puberty and in true precocious puberty before and after treatment with a potent luteinizing hormone-releasing hormone agonistJ Clin Endocrinol Metab19856111521593889035
- MansfieldMJRudlinCRCriglerJFJrChanges in growth and serum growth hormone and plasma somatomedin-C levels during suppression of gonadal sex steroid secretion in girls with central precocious pubertyJ Clin Endocrinol Metab1988661392961786
- BlumWFAlbertsson-WiklandKRosbergSRankeMBSerum levels of insulin-like growth factor I (IGF-I) and IGF binding protein 3 reflect spontaneous growth hormone secretionJ Clin Endocrinol Metab1993766161016167684744
- BrabantGvon zur MühlenAWüsterCSerum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter studyHorm Res2003602536012876414
- GiustiMTorreRCavagnaroPAttanasioRTraversoLGiordanoGThe effect of long-term pulsatile GnRH administration on the 24-hour integrated concentration of GH in hypogonadotropic hypogonadic patientsActa Endocrinol (Copenh)198912067247282499152
- WeissbergerAJHoKKActivation of the somatotropic axis by testosterone in adult males: evidence for the role of aromatizationJ Clin Endocrinol Metab1993766140714128501143
- BondanelliMAmbrosioMRMarguttiAFranceschettiPZatelliMCdegli UbertiECActivation of the somatotropic axis by testosterone in adult men: evidence for a role of hypothalamic growth hormone-releasing hormoneNeuroendocrinology200377638038712845224
- LiuLMerriamGRSherinsRJChronic sex steroid exposure increases mean plasma growth hormone concentration and pulse amplitude in men with isolated hypogonadotropic hypogonadismJ Clin Endocrinol Metab19876446516563546349
- VeldhuisJDKeenanDMMielkeKMilesJMBowersCYTestosterone supplementation in healthy older men drives GH and IGF-I secretion without potentiating peptidyl secretagogue efficacyEur J Endocrinol2005153457758616189179
- MaurasNRiniAWelchSSagerBMurphySPSynergistic effects of testosterone and growth hormone on protein metabolism and body composition in prepubertal boysMetabolism200352896496912898459
- SaggeseGCesarettiGFranchiGStartariLTestosterone-induced increase of insulin-like growth factor I levels depends upon normal levels of growth hormoneEur J Endocrinol199613522112158810735
- GibneyJWolthersTJohannssonGUmplebyAMHoKKGrowth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary menAm J Physiol Endocrinol Metab20052892E266E27115727949
- OvesenPVahlNFiskerSVeldhuisJDChristiansenJSJørgensenJOIncreased pulsatile, but not basal, growth hormone secretion rates and plasma insulin-like growth factor I levels during the periovulatory interval in normal womenJ Clin Endocrinol Metab1998835166216679589674
- WeissbergerAJHoKKLazarusLContrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal womenJ Clin Endocrinol Metab19917223743811991807
- MollGWJrRosenfieldRLFangVSAdministration of low-dose estrogen rapidly and directly stimulates growth hormone productionAm J Dis Child198614021241273946321
- SchoberEFrischHWaldhauserFBieglmayrCInfluence of estrogen administration on growth hormone response to GHRH and L-Dopa in patients with Turner’s syndromeActa Endocrinol (Copenh)198912044424462497615
- LeungKCJohannssonGLeongGMHoKKEstrogen regulation of growth hormone actionEndocr Rev200425569372115466938
- FriendKEHartmanMLPezzoliSSClaseyJLThornerMOBoth oral and transdermal estrogen increase growth hormone release in postmenopausal women – a clinical research center studyJ Clin Endocrinol Metab1996816225022568964860
- GibneyJJohannssonGLeungKCHoKKComparison of the metabolic effects of raloxifene and oral estrogen in postmenopausal and growth hormone-deficient womenJ Clin Endocrinol Metab20059073897390315855258
- LiuPYHoeyKAMielkeKLVeldhuisJDKhoslaSA randomized placebo-controlled trial of short-term graded transdermal estradiol in healthy gonadotropin-releasing hormone agonist-suppressed pre- and postmenopausal women: effects on serum markers of bone turnover, insulin-like growth factor-I, and osteoclastogenic mediatorsJ Clin Endocrinol Metab20059041953196015623811
- RothJGlickSMYalowRSBersonSASecretion of human growth hormone: physiologic and experimental modificationMetabolism19631257757913975314
- ImakiTShibasakiTShizumeKThe effect of free fatty acids on growth hormone (GH)-releasing hormone-mediated GH secretion in manJ Clin Endocrinol Metab19856022902933917457
- MerimeeTJRabinowtitzDFinebergSEArginine-initiated release of human growth hormone. Factors modifying the response in normal manN Engl J Med196928026143414385786514
- MelmedSInsulin suppresses growth hormone secretion by rat pituitary cellsJ Clin Invest1984735142514336371058
- VahlNJørgensenJOSkjaerbaekCVeldhuisJDOrskovHChristiansenJSAbdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adultsAm J Physiol19972726 Pt 1E1108E11169227458
- ButlerAALe RoithDControl of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent rolesAnnu Rev Physiol20016314116411181952
- PhilippouAHalapasAMaridakiMKoutsilierisMType I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophyJ Musculoskelet Neuronal Interact20077320821817947802
- NyombaBLBerardLMurphyLJFree insulin-like growth factor I (IGF-I) in healthy subjects: relationship with IGF-binding proteins and insulin sensitivityJ Clin Endocrinol Metab1997827217721819215291
- BerelowitzMSzaboMFrohmanLAFirestoneSChuLHintzRLSomatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitaryScience19812124500127912816262917
- BertheratJBluet-PajotMTEpelbaumJNeuroendocrine regulation of growth hormoneEur J Endocrinol1995132112247850005
- BonefeldKMøllerSInsulin-like growth factor-I and the liverLiver Int201131791191921733081
- BuzzelliGDattoloPPinzaniMBrocchiARomanoSGentiliniPCirculating growth hormone and insulin-like growth factor-I in nonalcoholic liver cirrhosis with or without superimposed hepatocarcinoma: evidence of an altered circadian rhythmAm J Gastroenterol19938810174417488213718
- DaughadayWHRotweinPInsulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrationsEndocr Rev198910168912666112
- HollyJMWassJAInsulin-like growth factors; autocrine, paracrine or endocrine? New perspectives of the somatomedin hypothesis in the light of recent developmentsJ Endocrinol198912236116182478648
- TannenbaumGSGuydaHJPosnerBIInsulin-like growth factors: a role in growth hormone negative feedback and body weight regulation via brainScience1983220459277796338593
- UnderwoodLED’ErcoleAJClemmonsDRVan WykJJParacrine functions of somatomedinsClin Endocrinol Metab198615159773514004
- MuggeoMTiengoAFedeleDCrepaldiGAltered control of growth hormone secretion in patients with cirrhosis of the liverArch Intern Med19791391011571160485748
- SchimpffRMLebrecDDonnadieuMSomatomedin production in normal adults and cirrhotic patientsActa Endocrinol (Copenh)1977862355362578625
- ChangTCLinJJYuSCChangTJAbsence of growth-hormone receptor in hepatocellular carcinoma and cirrhotic liverHepatology19901111231262153093
- StratikopoulosESzabolcsMDragatsisIKlinakisAEfstratiadisAThe hormonal action of IGF1 in postnatal mouse growthProc Natl Acad Sci U S A200810549193781938319033454
- KupferSRUnderwoodLEBaxterRCClemmonsDREnhancement of the anabolic effects of growth hormone and insulin-like growth factor I by use of both agents simultaneouslyJ Clin Invest19939123913967679407
- ClemmonsDRMetabolic actions of insulin-like growth factor-I in normal physiology and diabetesEndocrinol Metab Clin North Am2012412425443viiviii22682639
- RenehanAGBrennanBMAcromegaly, growth hormone and cancer riskBest Pract Res Clin Endocrinol Metab200822463965718971124
- BougenNMSteinerMPertzigerMAutocrine human GH promotes radioresistance in mammary and endometrial carcinoma cellsEndocr Relat Cancer201219562564422807498
- BougenNMYangTChenHLobiePEPerryJKAutocrine human growth hormone reduces mammary and endometrial carcinoma cell sensitivity to mitomycin COncol Rep201126248749321567106
- MazzoccoliGSothernRBPazienzaVCircadian aspects of growth hormone-insulin-like growth factor axis function in patients with lung cancerClin Lung Cancer2012131687421729653
- MinoiaMGentilinEMolèDGrowth hormone receptor blockade inhibits growth hormone-induced chemoresistance by restoring cytotoxic-induced apoptosis in breast cancer cells independently of estrogen receptor expressionJ Clin Endocrinol Metab2012976E907E91622442272
- PerryJKEmeraldBSMertaniHCLobiePEThe oncogenic potential of growth hormoneGrowth Horm IGF Res2006165–627728917101287
- WuZSYangKWanYTumor expression of human growth hormone and human prolactin predict a worse survival outcome in patients with mammary or endometrial carcinomaJ Clin Endocrinol Metab20119610E1619E162921849525
- ZatelliMCMinoiaMMolèDGrowth hormone excess promotes breast cancer chemoresistanceJ Clin Endocrinol Metab200994103931393819622619
- WernerHWeinsteinDBentovISimilarities and differences between insulin and IGF-I: structures, receptors, and signalling pathwaysArch Physiol Bochem200811411722
- LeeYHWhiteMFInsulin receptor substrate proteins and diabetesArch Pharm Res200427436137015180298
- ClemmonsDMaileLXiGShenXRadhakrishnanYIgf-I signaling in response to hyperglycemia and the development of diabetic complicationsCurr Diabetes Rev20117423524521707534
- ChambersAFGroomACMacDonaldICDissemination and growth of cancer cells in metastatic sitesNat Rev Cancer20022856357212154349
- FeldserDAganiFIyerNVPakBFerreiraGSemenzaGLReciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2Cancer Res199959163915391810463582
- ZelzerELevyYKahanaCShiloBZRubinsteinMCohenBInsulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNTEMBO J19981717508550949724644
- FukudaRHirotaKFanFJungYDEllisLMSemenzaGLInsulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cellsJ Biol Chem2002277413820538201112149254
- ClarkeKSmithKGullickWJHarrisALMutant epidermal growth factor receptor enhances induction of vascular endothelial growth factor by hypoxia and insulin-like growth factor-1 via a PI3 kinase dependent pathwayBr J Cancer200184101322132911355942
- ShigematsuSYamauchiKNakajimaKIijimaSAizawaTHashizumeKIGF-1 regulates migration and angiogenesis of human endothelial cellsEndocr J199946SupplS59S6212054122
- LeeOHBaeSKBaeMHIdentification of angiogenic properties of insulin-like growth factor II in in vitro angiogenesis modelsBr J Cancer200082238539110646893
- Grulich-HennJRitterJMesewinkelSHeinrichUBettendorfMPreissnerKTTransport of insulin-like growth factor-I across endothelial cell monolayers and its binding to the subendothelial matrixExp Clin Endocrinol Diabetes20021102677311928068
- MiraEMañesSLacalleRAMárquezGMartínezACInsulin-like growth factor I-triggered cell migration and invasion are mediated by matrix metalloproteinase-9Endocrinology199914041657166410098500
- DunnSETorresJVNiheiNBarrettJCThe insulin-like growth factor-1 elevates urokinase-type plasminogen activator-1 in human breast cancer cells: a new avenue for breast cancer therapyMol Carcinog2000271101710642432
- SmithBWAdamsLANon-alcoholic fatty liver diseaseCrit Rev Clin Lab Sci20114839711321875310
- CuneoRCHickmanPEWallaceJDAltered endogenous growth hormone secretory kinetics and diurnal GH-binding protein profiles in adults with chronic liver diseaseClin Endocrinol (Oxf)19954332652757586594
- SkrticSWalleniusKGressnerAMJanssonJOInsulin-like growth factor signaling pathways in rat hepatic stellate cells: importance for deoxyribonucleic acid synthesis and hepatocyte growth factor productionEndocrinology1999140125729573510579338
- SkrticSWalleniusVEkbergSBrenzelAGressnerAMJanssonJOInsulin-like growth factors stimulate expression of hepatocyte growth factor but not transforming growth factor beta1 in cultured hepatic stellate cellsEndocrinology199713811468346899348194
- MatsudaYMatsumotoKYamadaAPreventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosisHepatology199726181899214455
- PucheJECastilla- CortázarIHuman conditions of insulin-like growth factor-I (IGF-I) deficiencyJ Transl Med20121022423148873
- ArturiFSuccurroEProcopioCNonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-IJ Clin Endocrinol Metab20119610E1640E164421816784
- VölzkeHNauckMRettigRAssociation between hepatic steatosis and serum IGF1 and IGFBP-3 levels in a population-based sampleEur J Endocrinol2009161570571319690083
- AnthonyPPIshakKGNayakNCPoulsenHEScheuerPJSobinLHThe morphology of cirrhosis: definition, nomenclature, and classificationBull World Health Organ1977554521540304393
- AssyNHochbergZEnatRBaruchYPrognostic value of generation of growth hormone-stimulated insulin-like growth factor-I (IGF-I) and its binding protein-3 in patients with compensated and decompensated liver cirrhosisDig Dis Sci1998436131713219635625
- AssyNPruzanskyYGaitiniDShen OrrZHochbergZBaruchYGrowth hormone-stimulated IGF-1 generation in cirrhosis reflects hepatocellular dysfunctionJ Hepatol2008491344218456366
- CaregaroLAlberinoFAmodioPNutritional and prognostic significance of insulin-like growth factor 1 in patients with liver cirrhosisNutrition19971331851909131676
- CastroGRCoelhoJCParolinMBMatiasJEde FreitasACInsulin-like growth factor I correlates with MELD and returns to normal level after liver transplantationAnn Transplant201318576223792502
- DehghaniSMKaramifarHHamzaviSSHaghighatMMalek-HosseiniSASerum insulinlike growth factor-1 and its binding protein-3 levels in children with cirrhosis waiting for a liver transplantExp Clin Transplant201210325225722631062
- DonaghyAJDelhantyPJHoKKWilliamsRBaxterRCRegulation of the growth hormone receptor/binding protein, insulin-like growth factor ternary complex system in human cirrhosisJ Hepatol200236675175812044524
- ElsammakMYAminGMKhalilGMRagabWSAbazaMMPossible contribution of serum activin A and IGF-1 in the development of hepatocellular carcinoma in Egyptian patients suffering from combined hepatitis C virus infection and hepatic schistosomiasisClin Biochem200639662362916624274
- JeyaratnaganthanNGrønbaekHHolland-FischerPAscites from patients with alcoholic liver cirrhosis contains higher IGF-I bioactivity than serumClin Endocrinol (Oxf)201072562563219769623
- Lorenzo-ZúñigaVBartolíRMasnouHMontoliuSMorillasRMPlanasRSerum concentrations of insulin-like growth factor-I (igf-I) as a marker of liver fibrosis in patients with chronic hepatitis CDig Dis Sci200752113245325017410466
- MazziottiGSorvilloFMoriscoFSerum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a prospective studyCancer200295122539254512467068
- MøllerSGrønbaekMMainKBeckerUSkakkebaekNEUrinary growth hormone (U-GH) excretion and serum insulin-like growth factor 1 (IGF-1) in patients with alcoholic cirrhosisJ Hepatol19931733153208315259
- RehemRNEl-ShikhWMSerum IGF-1, IGF-2 and IGFBP-3 as parameters in the assessment of liver dysfunction in patients with hepatic cirrhosis and in the diagnosis of hepatocellular carcinomaHepatogastroenterology201158107–10894995421830422
- RonsoniMFLazzarottoCFayadLIGF-I and IGFBP-3 serum levels in patients hospitalized for complications of liver cirrhosisAnn Hepatol201312345646323619263
- SandahlTDAagaardNKThomsenKLEffects of insulin-like growth factor-I administration on in vivo regulation of urea synthesis in normal subjects and patients with cirrhosisLiver Int201131113213721040412
- VyzantiadisTTheodoridouSGioulemeOHarsoulisPEvgenidisNVyzantiadisASerum concentrations of insulin-like growth factor-I (IGF-I) in patients with liver cirrhosisHepatogastroenterology2003505181481612828091
- WuYLYeJZhangSZhongJXiRPClinical significance of serum IGF-I, IGF-II and IGFBP-3 in liver cirrhosisWorld J Gastroenterol200410182740274315309731
- ChengALKangYKChenZEfficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trialLancet Oncol2009101253419095497
- LlovetJMRicciSMazzaferroVSHARP Investigators Study GroupSorafenib in advanced hepatocellular carcinomaN Engl J Med2008359437839018650514
- LlovetJMDi BisceglieAMBruixJPanel of Experts in HCC-Design Clinical TrialsDesign and endpoints of clinical trials in hepatocellular carcinomaJ Natl Cancer Inst20081001069871118477802
- WilsonSRGreigPKasebAOPretreatment assessment of hepatocellular cancer: expert consensus conferenceHPB (Oxford)201012530030120590902
- ChevretSTrinchetJCMathieuDRachedAABeaugrandMChastangCA new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome HepatocellulaireJ Hepatol199931113314110424293
- KudoMChungHOsakiYPrognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score)J Gastroenterol200338320721512673442
- LeungTWTangAMZeeBConstruction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patientsCancer20029461760176911920539
- LlovetJMBrúCBruixJPrognosis of hepatocellular carcinoma: the BCLC staging classificationSemin Liver Dis199919332933810518312
- OkudaKOhtsukiTObataHNatural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patientsCancer19855649189282990661
- PonsFVarelaMLlovetJMStaging systems in hepatocellular carcinomaHPB (Oxford)200571354118333159
- TateishiRYoshidaHShiinaSProposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patientsGut200554341942515710994
- No authors listedA new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigatorsHepatology19982837517559731568
- JørgensenJOBlumWFMøllerNRankeMBChristiansenJSCircadian patterns of serum insulin-like growth factor (IGF) II and IGF binding protein 3 in growth hormone-deficient patients and age- and sex-matched normal subjectsActa Endocrinol (Copenh)199012332572621700568
- National Comprehensive Cancer Network clinical practice guidelines: Hepatobiliary Cancers http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf2015 version 2Accessed July 24, 2015
- ThomasMBJaffeDChotiMMHepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning MeetingJ Clin Oncol201028253994400520679622
- VerslypeCRosmorducORougierPESMO Guidelines Working GroupHepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol2012237vii414822997453
- KasebAOAbbruzzeseJLVautheyJNI-CLIP: improved stratification of advanced hepatocellular carcinoma patients by integrating plasma IGF-1 into CLIP scoreOncology2011805–637338121822028
- KasebAOMorrisJSHassanMMClinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinomaJ Clin Oncol201129293892389921911725
- StuverSOKuperHTzonouAInsulin-like growth factor 1 in hepatocellular carcinoma and metastatic liver cancer in menInt J Cancer200087111812110861461
- SuWWLeeKTYehYTAssociation of circulating insulin-like growth factor 1 with hepatocellular carcinoma: one cross-sectional correlation studyJ Clin Lab Anal201024319520020486202
- AssyNHochbergZAmitTShen-OrrZEnatRBaruchYGrowth hormone-stimulated insulin-like growth factor (IGF) I and IGF-binding protein-3 in liver cirrhosisJ Hepatol19972757968029382965
- De BenedettiFAlonziTMorettaAInterleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammationJ Clin Invest19979946436509045866
- JonesJIClemmonsDRInsulin-like growth factors and their binding proteins: biological actionsEndocr Rev19951613347758431
- KasebAOXiaoLHassanMMDevelopment and validation of insulin-like growth factor-1 score to assess hepatic reserve in hepatocellular carcinomaJ Natl Cancer Inst20141065
- ElmashadNIbrahimWSMayahWWPredictive value of serum insulin-like growth factor-1 in hepatocellular carcinomaAsian Pac J Cancer Prev201516261361925684496
- ChoEKimHCLeeJHSerum insulin-like growth factor-1 predicts disease progression and survival in patients with hepatocellular carcinoma who undergo transarterial chemoembolizationPLoS One201493e9086224595361
- BruixJShermanMPractice Guidelines Committee, American Association for the Study of Liver DiseasesManagement of hepatocellular carcinomaHepatology20054251208123616250051