Abstract
Purpose
Exercise (Ex) increases reactive oxygen species and impairs antioxidant defense systems. Recent data suggest that curcumin (CW) possesses peroxisome proliferator-activated receptor gamma activity and anti-inflammatory properties. Therefore, this study was designed to investigate the effects of CW supplementation on Ex performance, endurance, and changes in serum and muscle proteins in rats after exhaustive Ex.
Materials and methods
Twenty-eight (28) male Wistar rats (age: 8 weeks and body weight: 180±20 g) were divided into four treatment groups: 1) control (C; no Ex), 2) C + CW (no Ex + CW), 3) C + Ex, and 4) C + Ex + CW (Ex + CW). CW was administered as 100 mg/kg CurcuWin®, providing 20 mg of curcuminoids daily for 6 weeks. A motor-driven rodent treadmill was used to carry out the Ex protocols. During a 5-day period, animals in chronic Ex groups were put through different regimens: day 1, 10 m/min for 10 minutes; day 2, 20 m/min for 10 minutes; day 3, 25 m/min for 10 minutes; day 4, 25 m/min for 20 minutes; and day 5, 25 m/min for 30 minutes. Animals were exercised at 25 m/min for 45 min/d for 5 d/wk for 6 weeks. Blood and muscle samples were analyzed for muscle markers, oxidative stress, and antioxidant markers.
Results
Lactate and muscle malondialdehyde levels decreased in the CW-treated groups (P<0.0001). However, activities of antioxidant enzyme levels increased in the CW-treated groups. Run to exhaustion (minutes) improved in the CW-treated groups. Muscle nuclear factor-κB (P<0.05) and heat shock protein 70 (P<0.05) levels were much lowered in the CW treated group followed by Ex group. In addition, muscle inhibitors of kappa B, peroxisome proliferator-activated receptor gamma coactivator 1-alpha, thioredoxin-1, sirtuin 1, nuclear factor (erythroid-derived 2)-like 2, and glucose transporter 4 protein levels in the Ex + CW group were higher than those in the control and Ex groups (P<0.05).
Conclusion
This study suggests that novel CW has the potential to help prevent muscle damage by regulating the nuclear factor-κB and nuclear factor (erythroid-derived 2)-like 2 pathways and improve the performance and nutritional values of CW.
Introduction
Exercise (Ex) induces inflammation, increases reactive oxygen species (ROS), and impairs antioxidant defense systems in the skeletal muscle and blood.Citation1 Antioxidant enzymes and vitamins enhance antioxidant defense systems to protect cells from ROS.Citation1,Citation2 Muscle fatigue during downhill running may lead to impaired strength and muscle damage.Citation1 The severity of muscle damage is influenced by muscle strain and muscle contractions.Citation2 Downhill running elicits a number of cellular adaptive changes in the skeletal muscle. Muscle mitochondrial biogenesis, fusion, and metabolism during Ex induce stress and changes in transcriptional genes.Citation3 Skeletal mitochondrial biogenesis and function are stimulated by stress signals.
Curcumin (CW; 1,7-bis(4-hydroxy 3-methoxy phenyl)-1,6-heptadiene-3,5-dione), a natural polyphenolic compound isolated from the plant turmeric (Curcuma longa L.), has been studied for over 3 decades, and its potential benefits have been reported for oxidative stress, cancer, diabetes, inflammatory diseases, neurodegenerative diseases, and cardiometabolic health.Citation4–Citation8
CW’s low absorption from the gut, rapid metabolism, and rapid systemic elimination have been reported, which are due to its poor water solubility.Citation9 In a recent study,Citation9 a novel formulation of CW that was made water soluble by dispersing it and antioxidants in a water-soluble carrier such as polyvinylpyrrolidone (PVP) resulted in an increased relative absorption by 46 times (CurcuWIN®) of the total curcuminoids over the unformulated standard CW form. Several studies have also indicated that antioxidants prevent oxidative stress during strenuous Ex in humans and rats.Citation10,Citation11,Citation16
Recent pilot studies have reported that CW can attenuate oxidative stress due to Ex by increasing blood’s antioxidant capacity.Citation12,Citation13 In this current study, a water-soluble CW formulation (20% curcuminoids) consisting of turmeric extract, a hydrophilic carrier, cellulosic derivatives, and natural antioxidantsCitation9 was administered to test the efficacy in a chronic Ex animal model. Therefore, the present study was undertaken in an animal model to investigate the effects of the water-soluble CW formulation on oxidative stress markers, Ex time of exhaustion, and the antioxidant status in muscles. Furthermore, we investigated the effects of CW on oxidative stress and antioxidant gene proteins such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), nuclear factor (erythroid-derived 2)-like 2 (Nrf2)/hemeoxygenase-1 (HO-1), and sirtuin 1 (SIRT1) pathways in the skeletal muscle of chronically exercised and sedentary rats (control diet group and no Ex).
Materials and methods
Animals and feeding protocols
Male Wistar rats (N=28; n=7 per arm; age: 8 weeks and body weight: 180±20 g) were housed in a controlled standard laboratory environment (12/12-hour light/dark cycle at 22 °C) and fed with rat chow and water ad libitum. All experiments were conducted according to the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals and approved by the Ethics Committee of the Firat University. provides the composition of the basal (control) diet. Animals were divided randomly into the following four groups: 1) control (C; no Ex), 2) Control + CurcuWIN, 3) Ex + C and 4) Ex + C + Ex + CW (Ex + CW). CW was administered as 100 mg/kg CurcuWIN, providing 20 mg of CW daily for 6 weeks. A novel water-soluble CW formulation (lot number CU20DNS1-008/009) was provided by OmniActive Health Technologies, Ltd. (Pune, India). CW dose at 100 mg/kg was chosen based on previously reported value for effective antioxidant activity in rodents.Citation14,Citation15
Table 1 Composition of basal diet
Exercise protocol
The Ex protocols were performed on a motor-driven rodent treadmill (MAY-TME; Commat Ltd., Ankara, Turkey). Ex based on the treadmill protocol and tests was performed over a 5-day period. All rats were pre-trained for a week and subjected to the treadmill Ex. The Ex protocol was as follows: 1) day 1, 10 m/min for 10 minutes; 2) day 2, 20 m/min for 10 minutes; 3) day 3, 25 m/min for 10 minutes; 4) day 4, 25 m/min for 20 minutes; and 5) day 5, 25 m/min for 30 minutes. All animals were subjected to the treadmill Ex for 25 m/min for 45 min/d for 5 d/wk for 6 weeks.
Sample collection
Animals were sacrificed after the last Ex by cardiac puncture. To minimize diurnal effects, all animals were killed at the same hour. Blood muscle, and tissue samples were stored at −80° C till further analyses.
Laboratory analyses
Serum glucose, lipid profile, aspartate transaminase, alanine transaminase, urea, and creatinine levels were measured. The malondialdehyde (MDA) level in muscle tissue was measured by high-performance liquid chromatography (Shimadzu, Tokyo, Japan) using a Shimadzu UV-vis SPD-10 AVP detector and C18 ODS-3, 5 μm, 4.6 mm ×250 mm column. Superoxide dismutase (SOD), glutathione (GSH), and GSH peroxidase (GPx) were measured using commercial kits (Cayman Chemical, Ann Arbor, MI, USA).
NF-kB, inhibitors of kappa B (I-κB), heat shock protein 70 (HSP70), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), thioredoxin-1 (TRX-1), SIRT1, Nrf2, HO-1, and glucose transporter 4 (GLUT4) levels were analyzed by Western blot.Citation17 Samples were analyzed in quadruplicates for each experimental condition, and protein levels were determined densitometrically using an image analysis system (ImageJ; National Institute of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as mean ± SD The sample size was based on a power of 85% to obtain a P-value of 0.05. Seven animals per treatment were examined to see the significance of the treatments. ANOVA and Tukey tests for post hoc analyses were conducted between treatments and within treatments; P<0.05 was considered statistically significant.
Results
Body weight, endurance time, and biochemical parameters
No significant difference in body weight (P>0.05; ) was observed. As seen from , a significant difference in the time of exhaustion between the control (C) and Ex rats was observed (P<0.01). CW supplementation affected the time of exhaustion in the exercised rats (P<0.05). No significant differences in the safety end markers were found in liver and kidney function tests in all treatments (P>0.05; ).
Table 2 CW+ Ex increased the run to exhaustion time.
Table 3 Changes in liver and kidney function
shows metabolic health parameters including glucose and lipid profile in all treatments. No significant difference was found in blood glucose concentrations, but chronically exercised rats had less serum total cholesterol (P<0.001), high-density lipoprotein (HDL) (P<0.002), and triglyceride (P<0.01) concentrations than controls (P<0.0001). Additionally, the serum total cholesterol, HDL, and triglyceride levels decreased in the E+CW group significantly compared with other groups. Serum low-density lipoprotein cholesterol (LDL-C) levels were reduced in the Ex + CW treatment groups compared to those in the untreated rats (P<0.0001). The serum lactate levels in the Ex + CW group was decreased compared to those in the control and Ex groups (P<0.0001); additionally, serum lactate levels in the Ex group were much higher than those in the control group (P<0.01).
Table 4 Effect of E+CW on cardio-metabolic health markers and lactate
Muscle MDA and antioxidant enzymes
Muscle MDA concentration was decreased by 15.5% (P<0.0001; ) in the Ex group. CW treatment reduced the serum MDA concentration by 43.5% (P<0.001). Exercised rats had higher muscle SOD (0.35 vs 0.26; P<0.0001), GPx (161 vs 148; P< 0.003) activities (U/mg protein), and muscle GSH (11.8 μg/mg vs 8.8 μg/mg protein; P<0.0001) levels than controls. A decrease in MDA and an increase in muscle superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GHS-Px) concentrations in response to CW treatment were more notable than in the other groups (P<0.001 for all; ).
Table 5 Effect of CW supplementation in the Ex group on muscle oxidative stress metabolites and antioxidant enzymes
Muscle protein levels
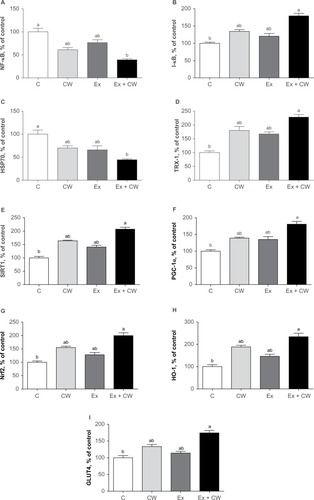
Clear bands for different muscle proteins were observed in the CW-treated groups with or without Ex compared to the control group (). Oxidative stress protein levels of muscle NF-κB () and HSP70 () were decreased in the CW-treated groups. Antioxidant muscle protein levels of I-κB (), TRX-1 (), SIRT1 (), PGC-1α (), Nrf2 (), HO-1 (), and GLUT4 () were increased in the CW group compared to those in the other groups (P<0.05; ).
Figure 1 Effect of different treatments on protein expression levels (Western blot strips) of muscle tissues.
Notes: The intensity of the bands was quantified by densitometric analysis. Data are expressed as the ratio of control (sedentary untreated rats) value (set to 100%). The bar represents standard deviation of mean. Blots were repeated at least three times (n=3) and a representative blot is shown. Protein loading was controlled using β-actin.
Abbreviations: GLUT4; glucose transporter 4; HO-1, hemeoxygenase-1; HSP70, heat shock protein 70; I-κB, inhibitors of kappa B; NF-kB; nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor (erythroid-derived 2)-like 2; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; SIRT1, sirtuin 1; TRX-1; thioredoxin-1; C, control; CW, curcumin, Ex, exercise.
Figure 2 Effect of different treatments on NF-kB (A), I-kB (B), HSP70 (C), TRX-1 (D), SIRT1 (E), PGC-1α (F), Nrf2 (G), HO-1 (H), and GLUT4 (I) protein expression levels of muscle.
Note: Superscripts differ by alphabets represent significance at P<0.05.
Abbreviations: GLUT4; glucose transporter 4; HO-1, hemeoxygenase-1; HSP70, heat shock protein 70; I-κB, inhibitors of kappa B; NF-kB; nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2, nuclear factor (erythroid-derived 2)-like 2; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; SIRT1, sirtuin 1; TRX-1; thioredoxin-1; C, control; CW, curcumin, Ex, exercise.
Discussion
This study was carried out to explain the efficacy and potential mechanism of action of a water-soluble CW formulation (20% curcuminoids) on muscle proteins, oxidative stress, andconcentration associated with modulation of the antioxidant Nrf2 and decreased oxidative stress expression of NF-κB. CW supplementation enhances the antioxidant activity by increasing serum SOD, GPx, and GSH () compared to the other groups. The molecular basis of the antioxidant and anti-inflammatory properties of CW is linked to transcription factors, growth regulators, and cellular signaling molecules.Citation4,Citation16,Citation18,Citation19 Several studies have reported that CW inhibits the scavenging of superoxide radicals, hydrogen peroxide, and nitric oxide from activated macrophages, reducing the iron complex and inhibiting the lipid peroxidation.Citation20–Citation22 Results of our study suggest that Ex may enhance lipid peroxidation and reduce the oxidative damage of proteins and DNA.Citation23 Reduced oxidative stress results from an enhanced antioxidant defense system.Citation24,Citation25 Chronic Ex reduces oxidative stress by upregulating the activity of antioxidant enzymes.Citation26 Belviranli et alCitation25 have reported that plasma MDA levels were lowered in chronically exercised groups compared to controls, as well as by an antioxidant (grape seed extract) supplementation. They also reported the plasma activities of SOD, an antioxidant defense for superoxide radicals that catalyzes the dismutation of superoxide and the formation of H2O2 and GPx. The levels of these enzymes improved after chronic Ex and antioxidant supplementation (grape seed extract). Takahashi et alCitation27 reported that the serum biological antioxidant potential concentrations after Ex were much higher in single and double CW supplementation trials compared to those before Ex. CW supplementation can attenuate Ex-induced oxidative stress and increase blood’s antioxidant capacity. CW directly influences the activity of inflammatory regulators.Citation18,Citation19,Citation28,Citation29 Total cholesterol and LDL-C were reduced in the Ex + CW groups. Ramírez-Tortosa et alCitation30 reported that the administration of turmeric extract inhibits the oxidation of LDL and potential hypocholesterolemic effect. A study by ArafaCitation31 in experimental animals fed with high-cholesterol diet indicated that CW had a hypocholesterolemic effect by reducing the serum total cholesterol and LDL-C and increasing the HDL cholesterol. In order to understand the mechanism of lowering cholesterol in a CW diet, the activity of hepatic cholesterol-7a-hydroxylase and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG Co A) reductase was measured. Hepatic cholesterol-7a-hydroxylase level and cholesterol catabolism rate were significantly higher in diabetic rats fed with CWCitation32 Yiu et alCitation33 demonstrated an increase in the expression of cholesterol 7 α-hydroxylase, HO-1, and LDL receptors but a decrease in 3-hydroxy-3-methyl-glutaryl-CoA reductase level in high-cholesterol diet. These results suggest that turmeric prevents hypercholesterolemia.
Our study demonstrates different regulatory pathways to prevent muscle damage and soreness and inflammation, as shown in . CW bioavailability is very important to realize the efficacy of molecular physiological effects. CW uptake in cells is significant.Citation34 Ex induces transient changes in metabolic genes in human skeletal muscle and enhances redox regulation of NF-κB and expression of antioxidant enzymes.Citation35 NF-κB is a transcription factor that controls gene expression and many inflammatory proteins, cellular growth, and apoptosis.Citation36 Increased NF-κB signaling decreases insulin action and promotes muscle wasting. NF-κB is activated in a redox-sensitive manner during muscular contraction due to an increased oxidant production. These data suggest that the novel CW form downregulates NF-κB and upregulates Nrf2.
In the present study, HSP70 expression decreased after CW treatment compared to the other groups (). The upregulation of HSP70 potentially contributes to muscle fiber integrity, muscle regeneration, and recovery. HSP70 is an indicator of cellular stress and a molecular chaperone, maintains cellular homeostasis and apoptosis, influences energy metabolism, facilitates cellular processes of muscular adaptation, and interacts with signaling pathways.Citation37 Evidence supports that the loss of HSP70 drives muscle atrophy, contractile dysfunction, and reduced regenerative capacityCitation38 CW-treated groups showed upregulation of the stress protein HSP70. TRX system, an antioxidant system, controls the cellular redox status.Citation39 TRX-1 has antioxidative and anti-apoptosis properties.Citation40 CW-treated groups showed upregulation of TRX-1, which when combined with Ex was highly significant over control (). Due to the limitation of the literature on the effect of CW on TRX-1, TRX-1 data are not comparable. However, TRX-1 ameliorates the depletion of GSH and restores the GSH/GSSG ratio.Citation41
SIRT1 promotes mitochondrial biogenesis via deacetylation of PGC-1α and mitochondrial biogenesis.Citation42 SIRT1 levels increase in skeletal muscles in response to chronic Ex, in parallel to the upregulation of mitochondrial content.Citation42 CW activates SIRT1 and potentially enhances mitochondrial biogenesis and fatty acid oxidation in adipocytes and myotubes.Citation43,Citation44 CW regulates mitochondrial biogenesis (SIRT1), including PGC-1α. Consistent with our results, Ray Hamidie et alCitation44 have shown that CW and Ex increased cytosol and the NAD+/NADH ratio and SIRT1 protein in muscle. In addition, some polyphenols, including CW, activate SIRT1 directly or indirectly, as shown in a variety of research models.Citation45 Our study demonstrates an increase in SIRT1 () and PGC-1α () levels in skeletal muscle in response to CW treatment compared to the other groups.
Nrf2 is a transcription factor that binds to antioxidant response element, thereby increasing a variety of cytoprotective genes.Citation46,Citation47 The levels of Nrf2 and HO-1 are increased in CW and Ex groups over the control group (). Consistent with our results, Ex stimulates transcription factors, decreases oxidative stress, and increases antioxidantdefenses.Citation35
GLUT4 isoform of insulin-regulated glucose transporter increases during Ex. GLUT4 may enhance mitochondrial biogenesis.Citation43 GLUT4 is found in heart tissue, skeletal muscles, and adipose tissues.Citation48 The data suggest the immunomodulatory properties of CW and its potential for altering the expression of inflammatory genes. In summary, the combination of Ex and CW with a unique water-soluble formulationCitation9 may accelerate mitochondrial biogenesis in the skeletal muscle and regulate the NF-kB, Nrf2, SIRT1, and PGC-1α pathways. CW’s protective effects are significant for cholesterol metabolism, improved antioxidant status, and reduction of oxidative stress metabolites.
Acknowledgments
The authors thank OmniActive Health Technologies Inc. (Morristown, NJ, USA) for financial support. This work was also supported in part by the Turkish Academy of Sciences (KS). This article was presented at the Experimental Biology Meeting, Boston, 2015.
Disclosure
VJ is an employee of OmniActive Health Technologies Inc. The authors report no other conflicts of interest in this work.
References
- BanerjeeAKMandalAChandaDChakrabortiSOxidant, antioxidant and physical exerciseMol Cell Biochem20132531–230731214619981
- NewhamDJJonesDAClarksonPMRepeated high-force eccentric exercise: effects on muscle pain and damageJ Appl Physiol (1985)1987634138113863693172
- MichailidisYKaragounisLGTerzisGThiol-based antioxidant supplementation alters human skeletal muscle signaling and attenuates its inflammatory response and recovery after intense eccentric exerciseAm J Clin Nutr201398123324523719546
- GuptaSCPatchvaSAggarwalBBTherapeutic roles of curcumin: lessons learned from clinical trialsAAPS J201315119521823143785
- GuptaSCPatchvaSKohWAggarwalBBDiscovery of curcumin, a component of golden spice, and its miraculous biological activitiesClin Exp Pharmacol Physiol201239328329922118895
- AggarwalBBTargeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticalsAnnu Rev Nutr20103017319920420526
- AggarwalBBHarikumarKBPotential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseasesInt J Biochem Cell Biol2009411405918662800
- GoelAAggarwalBBCurcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organsNutr Cancer201062791993020924967
- JägerRLoweryRPCalvaneseAVJoyJMPurpuraMWilsonJMComparative absorption of curcumin formulationsNutr J2014131124461029
- HeunksLMViñaJvan HerwaardenCLFolgeringHTGimenoADekhuijzenPNXanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary diseaseAm J Physiol19992776 Pt 2R1697R170410600916
- MastaloudisAMorrowJDHopkinsDWDevarajSTraberMGAntioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runnersFree Radic Biol Med200436101329134115110397
- SciberrasJNGallowaySDFenechAThe effect of turmeric (curcumin) supplementation on cytokine and inflammatory marker responses following 2 hours of endurance cyclingJ Int Soc Sports Nutr2015121525628521
- DrobnicFRieraJAppendinoGReduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva®): a randomised, placebo-controlled trialJ Int Soc Sports Nutr2014113124982601
- MaJLiuJYuHWangQChenYXiangLCurcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injuryNeurosci Lett2013547263123669643
- AnandPSundaramCJhuraniSKunnumakkaraABAggarwalBBCurcumin and cancer: an “old-age” disease with an “age-old” solutionCancer Lett2008267113316418462866
- LiuJYeoHCOvervik-DoukiEChronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidantsJ Appl Physiol (1985)2000891212810904031
- SahinKTuzcuMOrhanCAnti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocinBr J Nutr2013110219720523211098
- AggarwalBBKumarABhartiACAnticancer potential of curcumin: preclinical and clinical studiesAnticancer Res2003231A36339812680238
- GuptaSCTyagiAKDeshmukh-TaskarPHinojosaMPrasadSAggarwalBBDownregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenolsArch Biochem Biophys2014559919924946050
- CaiWZhangBDuanDWuJFangJCurcumin targeting the thioredoxin system elevates oxidative stress in HeLa cellsToxicol Appl Pharmacol2012262334134822634334
- BayomiSMEl-KashefHAEl-AshmawyMBSynthesis and biological evaluation of new curcumin derivatives as antioxidant and antitumor agentsMed Chem Res201322311471162
- SankarPTelangAGManimaranAProtective effect of curcumin on cypermethrin-induced oxidative stress in Wistar ratsExp Toxicol Pathol201264548749321130633
- LeeuwenburghCJiLLGlutathione and glutathione ethyl ester supplementation of mice alter glutathione homeostasis during exerciseJ Nutr199812812242024269868190
- BloomerRJGoldfarbAHAnaerobic exercise and oxidative stress: a reviewCan J Appl Physiol200429324526315199226
- BelviranlıMGökbelHOkudanNBaşaralıKEffects of grape seed extract supplementation on exercise-induced oxidative stress in ratsBr J Nutr2012108224925622011589
- GreathouseKLSamuelsMDiMarcoNMCriswellDSEffects of increased dietary fat and exercise on skeletal muscle lipid peroxidation and antioxidant capacity in male ratsEur J Nutr200544742943515633018
- TakahashiMSuzukiKKimHKEffects of curcumin supplementation on exercise-induced oxidative stress in humansInt J Sports Med201435646947524165958
- AggarwalBBGuptaSCSungBCurcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkersBr J Pharmacol201316981672169223425071
- MishraLSMertiaPNNandedkarTCurcuWIN™ cellular uptake in human glioblastoma cells further proves bioavailability4th World Ayurveda Congress & AROGYA EXPOBangalore2010
- Ramírez-TortosaMCMesaMDAguileraMCOral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosisAtherosclerosis1999147237137810559523
- ArafaHMCurcumin attenuate diet-induced hypercholesterolemia in ratsMed Sci Monit2005117BR228BR23415990684
- BabuPSSrinivasanKHypolipidemic action of curcumin, the active principle of turmeric (Curcuma longa) in streptozotocin induced diabetic ratsMol Cell Biochem19971661–21691759046034
- YiuWFKwanPLWongCYAttenuation of fatty liver and prevention of hypercholesterolemia by extract of Curcuma longa through regulating the expression of CYP7A1, LDL-receptor, HO-1, and HMG-CoA reductaseJ Food Sci2011763H80H8921535835
- AngeloLSWuJYMengFCombining curcumin (diferuloylmethane) and heat shock protein inhibition for neurofibromatosis 2 treatment: analysis of response and resistance pathwaysMol Cancer Ther201110112094210321903608
- GeorgeLAsgharMLokhandwalaMFExercise stimulates transcription factors (Nrf2 & NFκB), increases antioxidant defenses, decreases oxidative stress, and restores renal dopamine D1 receptor function in agingFASEB J2008221159.6
- AliSMannDASignal transduction via the NFκB pathway: a targeted treatment modality for infection, inflammation and repairCell Biochem Funct2004222677915027095
- SenfSMSkeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disordersFront Physiol2013433024273516
- MandalMNPatlollaJMZhengLCurcumin protects retinal cells from light- and oxidant stress-induced cell deathFree Radic Biol Med200946567267919121385
- LuJHolmgrenAThioredoxin system in cell death progressionAntioxid Redox Signal201217121738174722530689
- KuoJJChangHHTsaiTHLeeTYCurcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosisInt J Mol Med201230364364922692588
- IwataSHoriTSatoNAdult T cell leukemia (ATL)-derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by L-cystine and glutathione depletion: possible involvement of thiolmediated redox regulation in apoptosis caused by pro-oxidant stateJ Immunol19971587310831179120263
- SuwaMNakanoHRadakZKumagaiSEndurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscleMetabolism200857798699818555842
- HolloszyJORegulation of mitochondrial biogenesis and GLUT4 expression by exerciseCompr Physiol20111292194023737207
- Ray HamidieRDYamadaTIshizawaRSaitoYMasudaKCurcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levelsMetabolism201564101334134726278015
- QueenBLTollefsbolTOPolyphenols and agingCurr Aging Sci201031344220298168
- NairSLiWKongANNatural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cellsActa Pharmacol Sin200728445947217376285
- NaHKSurhYJModulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCGFood Chem Toxicol20084641271127818082923
- StenbitAETsaoTSLiJGLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetesNat Med1997310109611019334720
