Abstract
Activation of the nuclear hormone receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) has been shown to be immunoregulatory in autoimmune diseases by inhibiting production of a number of inflammatory mediators. We investigated whether PPAR-γ gene deletion in hematopoietic cells would alter disease pathogenesis in the antiglomerular basement membrane (anti-GBM) mouse model. PPAR-γ+/+ and PPAR-γ−/− mice were immunized with rabbit antimouse GBM antibodies and lipopolysaccharide and evaluated for two weeks. Although both the PPAR-γ+/+ and PPAR-γ−/− mice had IgG deposition in the glomerulus and showed proteinuria two weeks after injection, glomerular and tubulointerstitial disease in PPAR-γ−/− mice were significantly more severe compared with the PPAR-γ+/+ animals. We observed that the PPAR-γ−/− mice had decreased CD4+CD25+ regulatory T cells and an increased CD8+:CD4+ ratio as compared with the PPAR-γ+/+ mice, suggesting that PPAR-γ has a role in the regulation of T cells. Furthermore, plasma interleukin-6 levels were significantly increased in the PPAR-γ−/− mice at two weeks as compared with the PPAR-γ+/+ animals. Taken together, these studies show that the lack of PPAR-γ expression enhances inflammatory renal disease in the anti-GBM antibody-induced glomerulonephritis mouse model and suggests targeting PPAR-γ may have therapeutic efficacy.
Introduction
Antiglomerular basement membrane (anti-GBM) disease is an autoimmune disorder that affects the glomeruli of the kidneys.Citation1 In this disease, glomerular capillaries become targets of autoantibodies, or anti-GBM antibodies, which are directed against an antigen normally present in the GBM and alveolar basement membrane.Citation2 The GBM antigen that is responsible for this disease is a component of the alpha-3 chain of Type IV collagen. The resulting clinical syndrome encompasses a spectrum ranging from mild or no renal involvement to rapidly progressive glomerulonephritis. Most patients have both pulmonary and kidney involvement.Citation3 Clinically, anti-GBM disease that induces both glomerulonephritis and pulmonary hemorrhage is termed Goodpasture’s syndrome.Citation1
Lupus nephritis is an immune-mediated disease in which T cells, B cells, and innate immune cells have been shown to have pathogenic roles. In mouse models of spontaneous lupus nephritis, disease takes 6–12 months to manifest, necessitating the development of models that will induce lupus-like disease over a quicker timeframe. One such model to study spontaneous lupus nephritis is the experimental anti-GBM mouse.Citation4,Citation5 GBM glomerulonephritis, characterized by crescent formation and necrotizing inflammation of the glomerular capillary, is the most severe form of glomerulonephritis. When given anti-GBM antibodies and inflammatory stimulation, these animals develop glomerular basement nephritis that in many ways resembles lupus nephritis and Goodpasture’s disease. The studies of spontaneous lupus nephritis in mouse models and experimental anti-GBM disease have provided valuable insights into the underlying mechanisms of human lupus nephritis.Citation4,Citation6
Peroxisome proliferator-activated receptors (PPARs) belong to the 48-member superfamily of nuclear receptor proteins that function as transcription factors, regulating gene expression. PPARs are receptors for endogenous lipid molecules and are the molecular targets for drugs against Type 2 diabetes.Citation7,Citation8 They represent promising new targets for the treatment and prevention of inflammatory and autoimmune disorders, such as inflammatory bowel disease and systemic lupus erythematosus.Citation9–Citation11 Treatment with rosiglitazone, a pharmacologic agonist for PPAR-γ of the thiazolidinedione class of insulin-sensitizing drugs, has therapeutic efficacy: it reduces autoantibody production, atherosclerosis, and renal injury in lupus nephritis mice, showing reduced glomerular scarring and reduced inflammation in the renal cortex.Citation12,Citation13 There are three known PPARs (α, β, and γ) that each differ in their tissue distribution and functional activity.Citation14 PPAR-γ is expressed in T and B cells, monocytes/macrophages, dendritic cells, and epithelial cells.Citation15,Citation16 There are three isoforms of PPAR-γ;Citation17 the first and third are identical when fully translated and only differ in their splice variants, whereas the second differs from the other isoforms at the N-terminus.Citation18 All three isoforms have been identified in adipocytes. Additionally, PPAR-γ1 is present in virtually all other tissues, including smooth muscle and splenic tissue. PPAR-γ3 is expressed in macrophages and in the colon.Citation19
Whole body deletion of PPAR-γ causes embryonic lethality, but several conditional knockout mouse models have been developed using homozygous floxed PPAR-γ mice that result in epithelial and hematopoietic cells lacking PPAR- γ.Citation20–Citation23 PPAR-γ conditional knockout peritoneal macrophages have markedly reduced expression of ABCG1, reduced cholesterol efflux, and more atherosclerosis when crossed to a proatherogenic mouse model, such as a low-density lipoprotein receptor knockout.Citation19 In our current studies, we sought to determine if the deletion of PPAR-γ from hematopoietic cells would result in more severe development of disease in the anti-GBM mouse model, given the anti-inflammatory and immunomodulatory effects of PPAR-γ expressed in immune cells.
Materials and methods
Mice
In this project we used PPAR-γ floxed mice expressing the Cre transgene ((PPAR-γ fl/fl; MMTV-Cre+ or PPAR-γ−/−) and PPAR-γ fl/fl; MMTV-Cre− littermate mice (PPAR-γ+/+). These mice undergo premature termination of translation due to the enzymatic activity of recombinase in genomic DNA on hematopoietic and epithelial cells.Citation20,Citation24,Citation25 We have previously used these mice to cause conditional deletion of PPAR-γ in mouse models of irritable bowel disease, obesity and diabetes, and inflammation-driven colorectal cancer.Citation25–Citation28 All experimental procedures were approved by the Institutional Animal Care and Use Committee of Virginia Polytechnic Institute and State University, and met or exceeded requirements of the Public Health Service/ National Institutes of Health and the United Stated Animal Welfare Act as amended.
Anti-GBM sera
Anti-GBM serum was provided by John Zhang (Medical University of South Carolina, Charleston, SC). Essentially, glomeruli from C57BL/6 mice were isolated with a series of grading sieves (150, 106, and 63 μm mesh) and sonicated for seven minutes. Rabbits were next immunized with glomerular sonicates in complete Freund’s adjuvant, followed by two injections of antigen in incomplete Freund’s adjuvant (2 mg antigen per rabbit per injection), three weeks apart. Sera harvested from these rabbits seven weeks after the initial injection were tested by direct immunofluorescence to demonstrate strong glomerular binding.
Induction of lupus-like disease
Six- to eight-week-old mice were injected with a single dose of 100 μg lipopolys aride (Sigma-Aldrich, St Louis, MO) in phosphate-buffered saline intraperitoneally and administered 250 μg anti-GBM sera in phosphate-buffered saline intravenously on day 0 to induce disease. At day 14, the mice were euthanized and the tissues were collected and analyzed for pathology.Citation5
Measurement of proteinuria
As a measure of renal function, urine was collected at days 0, 7, and 14 and tested for proteinuria by a standard semi-quantitative test using Bayer Multistix dipsticks (Bayer, Fernwald, Germany). Results were graded according to the manufacturer’s instructions as negative, trace (15–20 mg/dL of albumin), 1+ (30 mg/dL), 2+ (100 mg/dL), 3+ (300 mg/dL), or 4+ (>500 mg/dL).
Cytokine enzyme-linked immunosorbent assay
Interleukin-6 (IL-6) levels in the sera were quantified by an enzyme-linked immunosorbent assay according to the manufacturer’s instructions (R&D Systems Inc., Minneapolis, MN, USA).
Flow cytometric analysis
Flow cytometric analysis was performed using various combinations of antibodies, including fluorescein isothiocyanate (FITC)-conjugated CD44, R-phycoerythrin-conjugated CD4, FITC-conjugated CD8, and PerCP-CY5.5-conjugated CD25 rat antimouse monoclonal antibodies (BD Pharmingen, San Diego, CA). Splenic cells were isolated as previously described.Citation29 Briefly, spleen lymphocytes from PPAR-γ+/+ and PPAR-γ−/− mice were aseptically dissociated, treated with Tris-ammonium chloride lysis buffer (pH 7.2) to remove erythrocytes, washed, and placed in complete medium consisting of 10% heat-inactivated fetal bovine serum, 200 mM L-glutamine, 5000 IU/mL penicillin, 5000 μg/mL streptomycin, and 100 × nonessential amino acids. Cell numbers were adjusted to 5 × 106 cells/mL, stained with monoclonal antibodies or appropriate fluorochrome-tagged isotype antirat IgG2a control antibodies, and analyzed on a Coulter Epics XL/MXL flow cytometer (Hialeah, FL).
Kidney pathology
As we previously described, renal pathology was assessed by a veterinary pathologist who was blinded to the treatment groups.Citation30 At day 14, the mice were euthanized for pathologic evaluation. At the time of euthanasia, the mice were weighed and the kidneys were removed and divided into sections. One portion was placed in neutral buffered formalin for subsequent embedding in paraffin, sectioning, and hematoxilyn and eosin and periodic acid-Schiff staining. Sections were assessed via light microscopy for glomerular proliferation, glomerular inflammation, glomerular size, number of nuclei per glomerulus, crescents, necrosis, and fibrosis. Each of these parameters was graded as 0 (normal), 1 (mild increase in mesangial matrix and cellularity), 2 (moderate increase in mesangial matrix and cellularity), 3 (focal endocapillary hypercellularity, obliterated capillary lumen, and marked thickening of glomerular basement membrane), or 4 (crescent formation, segmental necrosis, marked hypercellularity, and hyalinized glomeruli), and an overall glomerular score was derived.Citation31 One portion of the kidney was frozen in optimal cutting temperature media and cut into 5 μm sections and stained with FITC-conjugated antibodies (goat antimouse IgG diluted 1:100, Pierce, Rockford, IL).
Statistical analysis
Statistical analysis was performed by Student’s t-test. A P value < 0.05 was considered statistically significant.
Results
Rabbit IgG deposition in kidneys after anti-GBM antibody injection
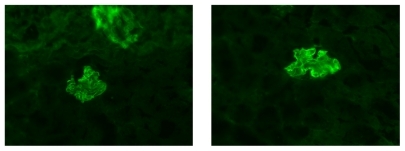
Before assessing the impact of PPAR-γ gene deletion on susceptibility to immune-mediated glomerular basement nephritis, we first sought to determine if the kidneys from the PPAR-γ+/+ and PPAR-γ−/− mice showed similar glomerular staining patterns to anti-GBM treatment. Fourteen days after the induction of disease, the anti-GBM antibodies were observed to bind exclusively to the glomerular capillary wall in a linear pattern (). Additionally, the kidneys from both the PPAR-γ+/+ and PPAR-γ−/− mice showed similar amounts of rabbit antimouse IgG antibodies in their glomeruli.
Determination of proteinuria
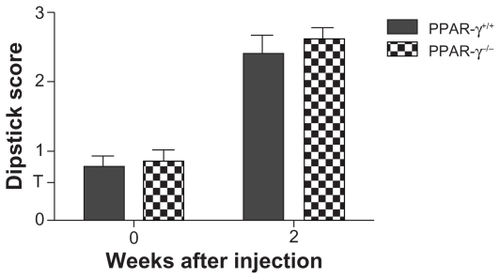
Prior to the induction of disease (day 0), both the PPAR-γ+/+ and PPAR-γ−/− mice showed minimal proteinuria (). At day 14, both the PPAR-γ+/+ and PPAR-γ−/− animals had significantly greater amounts of proteinuria by dipstick analysis compared with the baseline. The differences were not statistically significant between the PPAR-γ+/+ and PPAR-γ−/− mice at day 14.
Effect of anti-GBM sera on splenic tissue
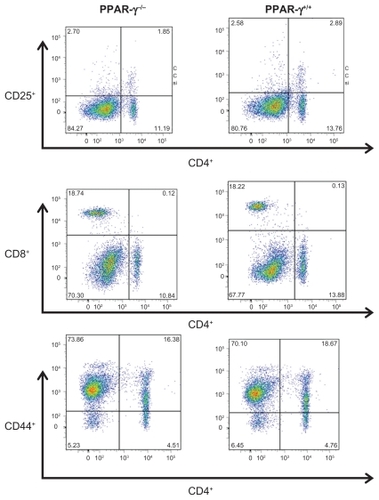
At day 14, the animals were sacrificed and the spleen weights were measured. The spleen weights and spleen to body weights were not statistically different between PPAR-γ+/+ and PPAR-γ−/− mice (0.056 ± 0.14 g versus 0.054 ± 0.014 g for spleen and 22.5 ± 2.3 g versus 23.4 ± 3.2 g body weight, respectively). To characterize the splenic phenotype, we isolated the dissociated splenocytes. There was no difference in total splenocyte numbers between the PPAR-γ+/+ and PPAR-γ−/− mice (data not shown). Next, we assessed the T cell populations by flow cytometry. We examined the CD4+:CD8+ ratio, CD4+CD25+ (T regulatory cells), and CD4+CD44+ (activated memory T cells) due to prior reports showing PPAR-γ expression or activation modulates T cell profiles by altering the CD4+:CD8+ ratio and the T regulatory cell population.Citation32,Citation33 Our results showed that there was no difference in the CD4+CD44+ expression profiles in PPAR-γ+/+ mice compared with PPAR-γ−/− mice. Intriguingly, we found that the PPAR-γ−/− mice exhibited a significant decrease in the CD4+:CD8+ T cell ratio, as well as a decrease in CD4+CD25+ cells compared with the PPAR-γ+/+ mice ( and ).
Table 1 Percentage of T cell markers in the spleens of mice with autoimmune anti-GBM glomerulonephritis (n = 10)
Figure 3 Representative histograms of flow cytometric staining of freshly isolated splenocytes from PPAR-γ+/+ and PPAR-γ−/− mice 14 days after stimulation with anti-GBM sera/LPS administration.
Abbreviations: GBM, glomerular basement membrane; PPAR-γ, peroxisome proliferator-activated receptor gamma; LPS, lipopolysaccharide.
Kidney pathology
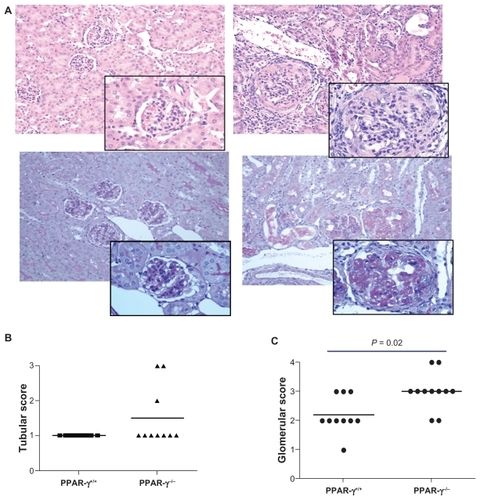
Both PPAR-γ+/+ and PPAR-γ−/− mice developed renal disease with anti-GBM antibody administration characterized by relatively mild glomerular inflammation. However, the PPAR-γ−/− mice showed significantly more severe disease compared with the PPAR-γ+/+ mice (). In addition to increased glomerular inflammation, the PPAR-γ−/− mice had increased mesangial matrix that obliterated the glomerular architecture (as shown in the periodic acid-Schiff-stained sections of the kidney). Furthermore, several of the PPAR-γ−/− mice showed severe interstitial abnormalities.
Figure 4 Renal pathology of PPAR-γ+/+ and PPAR-γ−/− two weeks after anti-GBM administration. A Representative photomicrograph of a kidney from a PPAR-γ+/+ (upper left) and PPAR-γ−/− (upper right) with hematoxylin and eosin stain (insert 25 × magnification of glomeruli). Note the severe cellular infiltrate and crescent formation in the PPAR-γ−/− mouse kidney. Representative photomicrograph of a kidney from a PPAR-γ+/+ (lower left) and PPAR-γ−/− (lower right) with periodic acid-Schiff stain. Note the increased deposition of periodic acid-Schiff-positive mesangial matrix in the PPAR-γ−/− mouse kidney. B Tabulation of renal tubular and C glomerular interstitium.
Abbreviations: GBM, glomerular basement membrane; PPAR-γ, peroxisome proliferator-activated receptor gamma.
Evaluation of cytokine levels
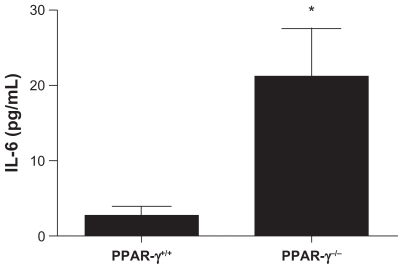
We measured serum levels of the proinflammatory cytokine IL-6 as a measure of inflammation (). Prior to induction of anti-GBM disease, IL-6 levels were not detectable. Two weeks after anti-GBM antibody administration, IL-6 levels in the PPAR-γ−/− mice were significantly elevated compared with PPAR-γ+/+ mice.
Figure 5 Serum interleukin-6 levels in PPAR-γ+/+ and PPAR-γ−/− mice with induction of anti-GBM disease. Sera were assayed by enzyme-linked immunosorbent assay14 days after induction of disease. The levels of interleukin-6 in the PPAR-γ−/− mice were significantly greater compared with the PPAR-γ+/+ mice (n = 10). *P < 0.05.
Abbreviations: GBM, glomerular basement membrane; PPAR-γ, peroxisome proliferator-activated receptor gamma.
Discussion
The activation of PPAR-γ was originally shown to block the proinflammatory effects of lipopolysaccharide and various cytokines in both monocytes and macrophages by antagonizing the activities of AP-1, STAT, and NF-κB.Citation34,Citation35 Specifically, activation of PPAR-γ decreased the production of nitric oxide, IL-6, and tumor necrosis factor-alpha. Since these initial observations, numerous studies have further characterized the expression, regulation, and mechanism of PPAR-γ’s anti-inflammatory activity.
In our current studies, we sought to determine the effects of PPAR-γ gene deletion in hematopoietic and epithelial cells using a mouse model of induced glomerulonephritis. The administration of anti-GBM antibodies and lipopolysaccharide stimulation serves as an acceptable model for murine glomerulonephritis with characteristics of Goodpasture’s syndrome as well as lupus nephritis.Citation4,Citation36 Our studies demonstrate that PPAR-γ has a role in the maintenance of regulatory T cell numbers. We found that PPAR-γ gene deletion resulted in a decreased CD4+CD25+ T cell population, along with an increase in disease in mice challenged with lipopolysaccharide and anti-GBM antibodies. This coincides with previous reports demonstrating that the deletion of PPAR-γ in T regulatory cells abrogates their ability to prevent CD4+ T cell-induced colitis in adoptive transfer studies and ameliorate graft versus host disease.Citation26,Citation33 We have previously shown the importance of T regulatory cells in decreasing disease severity in lupus mice.Citation37 Together, these studies indicate that a decrease in T regulatory cell numbers and/or function contributes to the development of autoimmune diseases.
Several lines of evidence suggest that PPAR-γ exerts anti-inflammatory effects by negatively regulating the expression of proinflammatory genes induced during macrophage differentiation/activation and the production of proinflammatory cytokines.Citation38 We observed a significant increase in the production of Th1/Th17 cytokine IL-6 after PPAR-γ gene deletion. IL-6 is not only important for the differentiation of Th1/Th17 phenotypes, but is also critical for inhibiting the differentiation of T regulatory cells.Citation39 This is consistent with our flow cytometry analyses showing a decreased T regulatory cell population of mice deficient in PPAR-γ. The general skewing of immune cell phenotypes may play a significant role during the pathogenesis of renal nephritis and lupus in mice and humans. Moreover, the deletion of PPAR-γ in CD4+ T cells results in enhanced antigen-specific proliferation and overproduction of interferon-γ in response to IL-12, indicating that adequate expression of PPAR-γ in CD4+ T cells is required to downregulate excessive Th1 responses.Citation26
Perhaps the most striking difference we observed in the PPAR-γ−/− mice was the more severe development of glomerular and interstitial lesions compared with the PPAR-γ+/+ mice, demonstrating the role for PPAR-γ gene expression in modulating renal disease. Direct binding of antiglomerular antibodies to glomerular antigens plays a key role in disease pathogenesis in the GBM model. All specimens of the anti- GBM antibody-treated mice showed linear deposition of IgG along the GBM accompanied by complement C3 deposits. Our observations by light microscopy revealed a significant overall increase in glomerular pathology, including crescent formation in the PPAR-γ−/− mice, while the PPAR-γ+/+ mice showed less pathology. Interestingly, we found that proteinuria was similar in both the PPAR-γ−/− and PPAR-γ+/+ mice. This finding could be due to the single time point at which we collected urine and our method of determination of proteinuria. A 24-hour urine collection may have shown a better representation of urine output with respect to proteinuria.
In lupus nephritis mouse models, PPAR-γ activation has shown therapeutic effects, leading to the reduction of disease. Recently Venegas-Pont et al reported that PPAR-γ agonists showed beneficial effects on renal function in the NZB/W lupus mouse.Citation12 Others have suggested that PPAR-γ modulates renal disease in lupus nephritis through induction of adiponectin.Citation13 These studies support our findings for a therapeutic benefit of PPAR-γ in autoimmune nephritis. We have previously demonstrated that mesangial cells produce endogenous PPAR-γ ligands.Citation40 The results presented in this paper are in line with the effect of endogenous PPAR-γ ligands on PPAR-γ expressed in immune cells, because the deletion of PPAR-γ resulted in increased kidney pathology in autoimmune mice. Additionally, endogenously generated lipid molecules can also activate PPAR-γ, suggesting pharmacologic or dietary intervention may not be required for PPAR-γ modulation of disease.Citation41,Citation42 Taken together, these observations support a critical role for PPAR-γ expression in the maintenance of kidney homeostasis and support the notion that PPAR-γ may be a therapeutic avenue to target for inhibition of autoimmune inflammatory kidney diseases.
Disclosure
The authors report no conflicts of interest in this work.
References
- AbbateMKalluriRCornaDExperimental Goodpasture’s syndrome in Wistar-Kyoto rats immunized with alpha3 chain of type IV collagenKidney Int199854155015619844131
- KettritzRAutoimmunity in kidney diseasesScand J Clin Lab Invest Suppl20082419910318569975
- BoschXFontJThe pulmonary-renal syndrome: A poorly understood clinicopathologic conditionLupus1999825826210413202
- DuYFuYMohanCExperimental anti-GBM nephritis as an analytical tool for studying spontaneous lupus nephritisArch Immunol Ther Exp (Warsz)200856314018250969
- XieCRahmanZSXieSStrain distribution pattern of immune nephritis – a follow-up studyInt Immunol20082071972818381352
- LiQZZhouJYangRThe lupus-susceptibility gene kallikrein downmodulates antibody-mediated glomerulonephritisGenes Immun20091050350819262577
- DesvergneBWahliWPeroxisome proliferator-activated receptors: Nuclear control of metabolismEndocr Rev19992064968810529898
- NestoRWBellDBonowROThiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association107Circulation200310829412948
- YangMParizaMWCookMEDietary conjugated linoleic acid protects against end stage disease of systemic lupus erythematosus in the NZB/W F1 mouseImmunopharmacol Immunotoxicol20002243344910946824
- YangXYWangLHChenTActivation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFATJ Biol Chem20002754541454410671476
- BergamoPLuongoDMauranoFMazzarellaGStefanileRRossiMConjugated linoleic acid enhances glutathione synthesis and attenuates pathological signs in MRL/MpJ-Fas(lpr) miceJ Lipid Res2006472382239116877747
- Venegas-PontMSartori-ValinottiJCMaricCRosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosusAm J Physiol Regul Integr Comp Physiol2009296R1282128919193937
- AprahamianTBonegioRGRichezCThe peroxisome proliferator- activated receptor gamma agonist rosiglitazone ameliorates murine lupus by induction of adiponectinJ Immunol200918234034619109165
- MangelsdorfDJEvansRMThe RXR heterodimers and orphan receptorsCell1995838418508521508
- SpiegelmanBMPPAR gamma in monocytes: Less pain, any gainCell1998931531559568708
- MansenAGuardiola-DiazHRafterJBrantingCGustafssonJAExpression of the peroxisome proliferator-activated receptor (PPAR) in the mouse colonic mucosaBiochem Biophys Res Commun19962228448518651933
- SchoonjansKMartinGStaelsBAuwerxJPeroxisome proliferator-activated receptors, orphans with ligands and functionsCurr Opin Lipidol199781591669211064
- FajasLFruchartJCAuwerxJPPAR gamma3 mRNA: A distinct PPARgamma mRNA subtype transcribed from an independent promoterFEBS Lett199843855609821958
- BraissantOFoufelleFScottoCDaucaMWahliWDifferential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha, -beta, and -gamma in the adult ratEndocrinology19961373543668536636
- CuiYMiyoshiKClaudioELoss of the peroxisome proliferation-activated receptor gamma (PPARgamma) does not affect mammary development and propensity for tumor formation but leads to reduced fertilityJ Biol Chem2002277178301783511884400
- AdachiMKurotaniRMorimuraKPeroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel diseaseGut2006551104111316547072
- GuriAJMohapatraSKHorneWT2ndHontecillasRBassaganya-RieraJThe role of T cell PPAR gamma in mice with experimental inflammatory bowel diseaseBMC Gastroenterol2010106020537136
- MohapatraSKGuriAJClimentMImmunoregulatory actions of epithelial cell PPAR gamma at the colonic mucosa of mice with experimental inflammatory bowel diseasePLoS One.20105e1021520422041
- AkiyamaTESakaiSLambertGConditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol effluxMol Cell Biol2002222607261911909955
- Bassaganya-RieraJReynoldsKMartino-CattSActivation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel diseaseGastroenterology200412777779115362034
- HontecillasRBassaganya-RieraJPeroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitisJ Immunol20071782940294917312139
- GuriAJHontecillasRFerrerGLoss of PPAR gamma in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissueJ Nutr Biochem20081921622817618105
- EvansNPMisyakSASchmelzEMGuriAJHontecillasRBassaganya-RieraJConjugated linoleic acid ameliorates inflammation-induced colorectal cancer in mice through activation of PPARgammaJ Nutr201014051552120089779
- AhmedSAGogalRMJrWalshJEA new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assayJ Immunol Methods19941702112248157999
- SekineHReillyCMMolanoIDComplement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr miceJ Immunol20011666444645111342671
- XieCSharmaRWangHZhouXJMohanCStrain distribution pattern of susceptibility to immune-mediated nephritisJ Immunol20041725047505515067087
- MalurAMcCoyAJArceSDeletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory responseJ Immunol20091825816582219380830
- WohlfertEANicholsFCNeviusEClarkRBPeroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: Enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanismsJ Immunol20071784129413517371968
- LavinskyRMJepsenKHeinzelTDiverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexesProc Natl Acad Sci U S A199895292029259501191
- RicoteMHuangJFajasLExpression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoproteinProc Natl Acad Sci U S A199895761476199636198
- FuYDuYMohanCExperimental anti-GBM disease as a tool for studying spontaneous lupus nephritisClin Immunol200712410911817640604
- ReillyCMThomasMGogalRJrThe histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 miceJ Autoimmun20083112313018650065
- AbdelrahmanMSivarajahAThiemermannCBeneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shockCardiovasc Res20056577278115721857
- WanSXiaCMorelLIL-6 produced by dendritic cells from lupusprone mice inhibits CD4+CD25+ T cell regulatory functionsJ Immunol200717827127917182564
- ReillyCMOatesJCCookJAMorrowJDHalushkaPVGilkesonGSInhibition of mesangial cell nitric oxide in MRL/lpr mice by prostaglandin J(2) and proliferator activation receptor-gamma agonistsJ Immunol20001641498150410640767
- NamgaladzeDMorbitzerDvon KnethenABruneBPhospholipase A2-modified low-density lipoprotein activates macrophage peroxisome proliferator-activated receptorsArterioscler Thromb Vasc Biol20103031332019948841
- BorniquelSJanssonEAColeMPFreemanBALundbergJONitrated oleic acid up-regulates PPARgamma and attenuates experimental inflammatory bowel diseaseFree Radic Biol Med20104849950519932165