Abstract
Long-term cigarette smoking (LTCS) represents an important risk factor for cardiac infarction and stroke and the central risk factor for the development of a bronchial carcinoma, smoking-associated interstitial lung fibrosis, and chronic obstructive pulmonary disease. The pathophysiologic development of these diseases is suggested to be promoted by chronic and progressive inflammation. Cigarette smoking induces repetitive inflammatory insults followed by a chronic and progressive activation of the immune system. In the pulmonary system of cigarette smokers, oxidative stress, cellular damage, and a chronic activation of pattern recognition receptors are described which are followed by the translocation of the NF-kB, the release of pro-inflammatory cytokines, chemokines, matrix metalloproteases, and damage-associated molecular patterns. In parallel, smoke pollutants cross directly through the alveolus–capillary interface and spread through the systemic bloodstream targeting different organs. Consequently, LTCS induces a systemic low-grade inflammation and increased oxidative stress in the vascular system. In blood, these processes promote an increased coagulation and endothelial dysfunction. In muscle tissue, inflammatory processes activate catabolic signaling pathways followed by muscle wasting and sarcopenia. In brain, several characteristics of neuroinflammation were described. Regular exercise training has been shown to be an effective nonpharmacological treatment strategy in smoke-induced pulmonary diseases. It is well established that exercise training exerts immune-regulating effects by activating anti-inflammatory signaling pathways. In this regard, the release of myokines from contracting skeletal muscle, the elevations of cortisol and adrenalin, the reduced expression of Toll-like receptors, and the increased mobilization of immune-regulating leukocyte subtypes might be of vital importance. Exercise training also increases the local and systemic antioxidative capacity and several compensatory mechanisms in tissues such as an increased anabolic signaling in muscle or an increased compliance of the vascular system. Accordingly, regular exercise training seems to protect long-term smokers against some important negative local and systemic consequences of smoking. Data suggest that it seems to be important to start exercise training as early as possible.
Introduction
Tobacco use is the most significant preventable cause of morbidity and mortality, with ~5 million deaths caused by direct tobacco use and >600,000 deaths due to secondhand smoke worldwide every year. Cigarette smoking (CS) is the most common form of tobacco consumption in most countries.Citation1 Due to the well-known detrimental effects of long-term cigarette smoking (LTCS) on health, many countries have implemented intensified tobacco control efforts which resulted in a reduced prevalence of daily smoking since 1980. However, in many countries, the number of smokers is actually increasing, and there are preliminary indications that global prevalence among men will increase further in the next years.Citation1,Citation2
LTCS represents an important risk factor for cardiac infarction and stroke and the central risk factor for the development of a bronchial carcinoma, smoking-associated interstitial lung fibrosis, and chronic obstructive pulmonary disease (COPD). About 20% of smokers develop a COPD which is actually ranking as the fifth most common cause of mortality worldwide.Citation2 Also, smoking cessation does not reverse the progression of COPD in patients, indicating that smoking is an important cause, but not the only driver of disease progression in COPD patients. COPD is characterized not only by the destruction of lung tissue but also by a systemic inflammation. It is suggested that a sustained systemic inflammation develops during LTCS, resulting in COPD and its comorbidities such as muscle wasting, vascular diseases, heart diseases, and stroke.Citation3,Citation4 The purpose of this review was to summarize the current knowledge about cigarette smoke-induced inflammation. Studies about the immunological effects of acute smoking, LTCS, secondhand CS, and COPD patients were included. In order to describe the molecular mechanisms of smoke-induced inflammation, in vitro studies and animal studies of smoke exposure were also included. The purpose of the second part of the review was to describe the current knowledge of the immune-regulating systemic and local potentials of regular exercise training after smoke-induced inflammation.
Methods
We searched various electronic databases such as PubMed, Web of Sciences, and Cochrane Library for English language articles without any date restriction. Our review focused on the effects of CS-induced inflammation on different organs (such as brain, lung, heart, muscle, etc) as well as on immune-regulating effects of exercise which may counteract CS-induced inflammation. Search terms on PubMed (abstract and/or title) as shown in were used. After careful review of titles and abstracts, it was decided whether the full-text will further be analyzed and consequently considered in this review.
Table 1 Search terms on PubMed
From CS to inflammation
The mechanisms of initiation and persistence of cigarette smoke (CS)-induced inflammation are not completely understood. CS comprises about 4,000 chemicals, including several carcinogens. Toxicologic studies have revealed a multitude of immunomodulatory chemicals and gas.Citation5 Consequently, LTCS results in repetitive inflammatory insults leading to a chronic and progressive activation of the immune system accompanied by an abnormal inflammatory response of the airways to various noxious gases and particles.Citation6,Citation7 On the one hand, smoke pollutants cross through the alveolus–capillary interface and spread directly through the systemic bloodstream targeting different organs.Citation7 At this point, they might be recognized by receptors of the innate immune system which initiate inflammatory signaling cascades via NF-κB activation.Citation5 On the other hand, inflammatory processes are suggested to originate in the pulmonary system. Here, toxic substances disturb the barrier function of the respiratory epithelium and impact both innate and adaptive host defense mechanisms. This primarily local inflammatory processes spillover into the circulation leading to inflammatory and degenerative processes in other organs and tissues. Thus, inflammation is suggested to be the main driver of the central comorbidities.Citation7,Citation8
Effects of CS on inflammatory processes in respiratory tract and lungs
CS comprises various components that damage the pulmonary epithelium. LTCS has been shown to injure the cell membranes and alter the mucosal permeability.Citation9 Cellular damage is followed by a compromised immune status, allowing opportunistic pathogens to cause infections that might amplify the inflammatory processes. Furthermore, components of the innate and adaptive immune system are chronically activated. Analysis of bronchoalveolar lavage fluid and breath condensate provides evidence that even acute exposure to cigarette smoke results in oxidative stress and tissue damage as suggested by increased products of lipid peroxidation and degradation products of extracellular matrix proteins.Citation5 In bronchoalveolar lavage (BAL) of long-term smokers, an increase of interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α, regulated on activation, normal T-cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, IL-12 (p40), and IL-17 was found.Citation10,Citation11 Recent data suggest that airway epithelial cells (AECs) which represent a first line of defense against inhaled toxicants have altered inflammatory signaling in response to CS exposure. These cells were shown to upregulate cytokine expression and expression of matrix metalloproteases (MMPs) via extracellular signal-regulated kinase (ERK) signaling and increased p38 activation. Furthermore, AECs show characteristics of cellular damage and cell death consequently leading to the release of damage-associated molecular patterns (DAMPs) into the extracellular space. DAMPs target pattern recognition receptors such as Toll-like receptor (TLR). TLRs are found on both immune and epithelial cells throughout the pulmonary system. TLRs recognize patterns of bacteria, fungi, and viruses, and the levels of TLR4 are elevated in cigarette smokers with COPD. After TLR activation, the NF-kB pathway is induced followed by the secretion of a variety of pro-inflammatory cytokines.Citation12 In particular, MMP-9 and -12, surfactant protein D, and IL-1, IL-6, IL-8, and IL-17 have been found in higher quantities in the lungs of long-term smokers with the ongoing inflammation.Citation10 In parallel, immune cells, macrophages, neutrophils, dendritic cells, and lymphocytes migrate into the pulmonary system.Citation11–Citation13 Alveolar macrophages might play a key role in the pathogenesis of inflammation in lungs. These cells produce increased levels of MMPs, such as MMP-1, MMP-2, MMP-9, MMP-12, and MMP-14, after smoke exposure ().Citation10
Figure 1 Illustration about cigarette-induced induction of oxidative stress and inflammation in AECs.
Abbreviations: AEC, airway epithelial cell; TLR4, Toll-like receptor-4; DAMP, damage-associated molecular pattern; MMP, matrix metalloprotease; ROS, reactive oxygen species; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon.
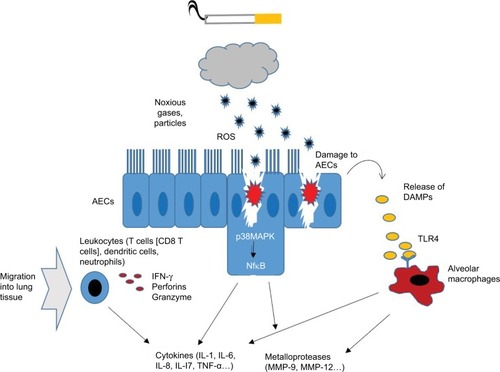
Specific role of lymphocytes
Currently, it is discussed that lymphocytes might play a crucial role in inflammatory pathogenesis. Specifically, CD8+ T lymphocytes have been shown to be dramatically increased in the lungs of heavy smokers accompanied by a shift toward a type 1 profile. This immune cell subtype produces large amounts of interferon-γ and releases perforins and granzyme. Also B lymphocytes are activated, and it is suggested that their antigen-specific responses could turn against self-epitopes, partly because of impaired tolerance. In parallel, smoke exposure led to an accumulation of forkhead-box-protein (FOX)P31 T-regulatory cells (Tregs) in lungs of mice which might participate in controlling inflammatory processes.Citation14 Accordingly, LTCS seems to alter the pulmonary immune equilibrium which turns into a chronic activated, immunosuppressed condition.Citation15
Effects of LTCS on systemic inflammation
Every smoked cigarette seems to elicit a slight increase of oxidative stress and inflammation in blood indicated by an increase of thiobarbituric acid-reactive substances, neutrophil elastase, leukotrienes, and neutrophils after acute CS in humans.Citation5 Chronically, most studies agree that LCTS induces an increase in the numbers of circulating neutrophils, macrophages, and lymphocytes. These cells show several inflammatory characteristics such as expression of activation markers and adhesion molecules which might mediate the migration into the bronchoalveolar system or other tissues.Citation8,Citation16 On the molecular levels, LTCS induces a systemic low-grade inflammation characterized by chronically elevated levels of various markers for inflammation, tissue deterioration, and coagulation, such as C-reactive protein (CRP), TNF-α, von Willebrand factor (vWF), tissue inhibitor of metalloproteinases-1, factor VII, and fibrinogen. Blood is suggested to be a transit way for transfer and spreading these molecules which target other organs and tissues.Citation17,Citation18
Effects of CS on the vascular endothelium
The particulate phase of CS consists of lipophilic components, which can pass the lipid bilayer of respiratory membranes; therefore, the damage is not limited to the lung tissue as it can also affect the vascular system.Citation19 The integrity of endothelial cells (ECs) is essential, since it preserves vascular homeostasis, allows continuous adjustment of vascular tone and maintenance of blood fluidity, and regulates leukocyte traffic.Citation20 Components of CS are toxic for ECs, and LTCS can lead to dysfunction of ECs,Citation21 an early hallmark of atherosclerosis.Citation22 Endothelial dysfunction is characterized by an imbalance of vasoconstrictors and vasodilators, aberrant interaction between endothelial and immune cells, and higher expression of adhesion molecules.Citation23 Dysfunctional ECs express lower levels of prostacyclin, thrombomodulin, tissue plasminogen activator (tPA), and NO while expression levels of endothelin-1, angiotensin II, plasminogen activator inhibitor-1 (PAI-1), and vWF are increased.Citation20 Therefore, CS favors inflammatory processes in ECs and is a huge risk factor for the development of atherosclerosis and cardiovascular diseases (CVDs).Citation22 In vitro studies in ECs demonstrate that CS induces cell injury in a dose- and time-dependent mannerCitation24 which can lead to apoptosis,Citation25 autophagic cell death,Citation26 and necrosis.Citation27 Different mechanisms are responsible for the induction of apoptosis in ECs induced by CS. One study showed that aqueous filtrates of CS lead to mitochondrial membrane depolarization, representing an early step in the apoptotic pathway.Citation22 The negative influence of CS on apoptotic-related genes has also been reported. For example, CS decreases p53 and Bcl-2 expression,Citation28 disrupts the vascular endothelial growth factor (VEGF), and fluid shear stress-mediated VEGFR2/phosphoinositide 3-kinase (PI3K) signaling pathwayCitation29 and reduces the cytochrome-c oxidase II expression through aberrant DNA methylation.Citation25 Vascular damage through excessive apoptosis was also shown to be initiated by a p53-independent caspase-3 activating pathway.Citation30 EC injury may also be mediated through protein carbonylation which is caused by reactive species in CS.Citation31 Recruitment of leukocytes to the inflammation site happens by cytokine signaling, MMP-1 and MMP-9 upregulation, and through cell adhesion of immune cells to ECs.Citation32
Potential mechanisms of atherogenesis
The underlying mechanism of atherogenesis of ECs induced by CS is not fully understood yet.Citation33 So far, CS-induced inflammation-related responses have been described in experimental studies in vitro. CS led to phosphorylation of various mitogen-activated protein kinases (MAPK), like p38,Citation34 c-Jun N-terminal kinase (JNK), and ERK.Citation34,Citation35 The expression levels of osteopontin,Citation35 E-selectin, intercellular cell adhesion molecule-1 (ICAM-1),Citation36 and IL-8Citation37 were also induced by CS. Furthermore, CS induced nicotinamide adenine dinucleotide phosphate (NAD(P)H)-oxidase-derived H2O2 generation,Citation38 upregulated fractalkine (CX3CL1), increased IL-13Rα2 through the activation of protein kinase A-cAMP response element-binding protein (PKA-CREB) pathway,Citation39 increased cyclooxygenase-2 (COX-2) expression through nuclear β-catenin accumulation due to the activation of epidermal growth factor receptor (EGFR)/Akt/glycogen synthase kinase-3β pathways.Citation40 One cell culture study demonstrated that ECs treated with CS for 72 hours expressed only minor differences in various cytokines on mRNA level.Citation41 CS promotes endothelial dysfunction as well by impairing endothelium-dependent relaxation, presumably through suppression of NO productionCitation42 and CS-low-density lipoprotein (CS-LDL).Citation43 Cell culture studies proved that exposure of ECs to CS impaired the VEGF-induced EC migrationCitation44,Citation45 and tube formation, explaining the negative effect of CS on vessel growth and endothelial function.Citation45
Effect of smoking on the cardiac tissue
Persistent inflammation is an important factor in the development of CVD.Citation46 Since CS promotes inflammationCitation47 and injures the cardiovascular system chronically, it is not surprising that the risk for CVD is twice as high in smokers than in nonsmokers.Citation48 Toxic effects of CS on the myocardium have been proved experimentally as well as clinically,Citation15 but whether smoking is a direct or indirect cause of CVD still needs to be proved.Citation49 It is somehow remarkable that even secondhand smoke has the ability to increase the risk for CVD to as high as 30%.Citation48,Citation50–Citation54 Secondhand smoking combined with an unhealthy lifestyle was shown to reduce the ability of the heart adapt sensitively to sidestream smoke in a murine model.Citation48 Furthermore, in nonsmoking humans, secondhand smoking increased WBC count immediately as well as CRP levels 18 h after exposure. Both of these are markers for inflammation and have been linked to a higher incidence of CVD.Citation50
The situation for smokers is worse. Active smoking increases cardiac afterload, promotes a pro-thrombotic status, reduces fibrinolysis, changes the profile of circulating lipids,Citation48 promotes neutrophil infiltration in the myocardium,Citation55 alters T-cell function,Citation56 and causes DNA adducts in the myocardium.Citation57 CS leads to the production of reactive oxygen species (ROS) which initiate ROS-sensitive signal transduction pathways, such as MAPKs, and various transcription factors, including NF-kBCitation15 resulting in an aberrant cytokine profile.Citation58–Citation60 Gene analysis of the hearts of mice revealed an upregulation of the xenobiotic-metabolizing enzyme cytochrome P-450 1A1 and a downregulation of PAI-1, representing a key gene involved in fibrinolysis.Citation61 Taken together, all mentioned factors are suggested to increase the risk for several diseases of the cardiovascular system also in human smokers.Citation62
Effects of LTCS on muscle tissue
Human smokers tend to have a lower BMI while central or abdominal obesity seems to be increased. Thus, the weight loss associated with tobacco smoking may be due to loss of lean mass rather than fat.Citation63 It is suggested that inflammation and oxidation of proteins are two main contributors to the development of skeletal muscle loss and dysfunction observed in LTCS and COPD patients.Citation63 Structurally, LTCS leads to a reduced percentage of type I fiber, a lower muscle fiber cross-sectional area, an increased glycolytic enzymatic activity, and decreased muscle oxidative activity.Citation8,Citation63,Citation64 Mice chronically exposed to cigarette smoke tend to a reduced muscle capillary to fiber ratio along with decreased VEGF, lowered endothelial and neuronal nitrite oxide synthase activities in muscle vessels, and increased inflammatory activity indicated by an increased mRNA expression of TNF-α and IL-1β.Citation8,Citation64,Citation65 The role of numerous cell signaling pathways in the development of skeletal muscle atrophy, a key element of muscle dysfunction in long-term cigarette smokers and COPD patients, has been investigated.Citation65 In general, atrophy occurs when protein degradation exceeds protein synthesis. With regard to protein degradation, the ubiquitin proteasome system (UPS) seems to have an important role during LTCS. In smoke-exposed mice, an increased ubiquitination of target proteins was demonstrated, which was indicated by the increased activities of the E3 ubiquitin ligases atrogin-1 and muscle RING finger protein-1 (MuRF1).Citation66 In parallel, key factors that induce protein synthesis such as insulin-like growth factor 1 (IGF-1), are reduced followed by a lower activation of anabolic signaling pathways such as protein kinase B (Akt) and rapamycin (mTOR) pathways. All the mentioned pathways interact with inflammatory signaling molecules such as TNF-α turning protein balance toward an enhanced degradation leading to muscle wasting.Citation63,Citation66
Effects of LTCS on brain inflammation
Atherosclerosis and vascular brain lesions share similar pathological features such as oxidative stress and increased inflammation.Citation67 Oxidative stress, for example, plays a decisive role in the pathogenesis of ischemic brain injury.Citation68 It is not surprising that direct and secondhand CS are associated with various cerebrovascular-related diseases,Citation32 in particular, smoking is a risk factor for stroke.Citation69
Likewise to the effects of CS on endothelial cells,Citation70 higher expression of VEGF, ICAM-1, IL-8, and nuclear factor (erythroid-derived 2)-like 2 was also observed in cultured brain ECs.Citation71 Furthermore, it was shown that CS extracts induced heme oxygenase-1 (HO-1) expression mediated by phosphatidylcholine phospholipase C/protein kinase Cδ/NADPH oxidase-dependent platelet-derived growth factor receptor (PDGFR)/PI3K/Akt pathway.Citation19 Higher HO-1 expression was shown to exacerbate early brain injury during intracerebral hemorrhagic stroke.Citation72 Endothelin-1 levels decreased in rat brains exposed to CS, implying that endothelin-1 may contribute to the hemodynamic response to chronic CS.Citation73
Effects on brain ECs
Moreover, animal experiments proved that CS negatively affected endothelial tight junctionsCitation71 and downregulated the activity of Na-K-2Cl cotransporter in brain ECs. The latter could possibly contribute to an increase in extracellular K+. Therefore, CS may exacerbate ischemic cellular damage and hinder recovery from ischemic damage. In addition, accumulation of extracellular fluid K+ is a risk factor for cellular edema in astrocytes and neurons and could impair neuronal conduction after stroke.Citation74 Increased blood viscosity due to CS impairs the blood flow and risks the integrity of the brain microvasculature.Citation32 On top of everything, CS negatively affects the viability of the blood–brain barrier (BBB). Taken together, CS and hemodynamic impairments contribute synergistically to vascular inflammation and BBB damage.Citation32 Inflammation of brain cells due to CS was also confirmed in various in vivo studies using mouse and rat models. Inflammation and cell death processes in the brain are often characterized by alterations of the neuroproteome.Citation73–Citation75 Mice exposed to secondhand CS, showed higher levels of ROS, induction of lipid peroxidation, activation of the transcription factors NF-kB and activator protein-1, as well as activation of MAPK, including JNK, ERK, and p38, and COX-2 in various regions of the brain.Citation69 Furthermore, secondhand CS altered enzymatic antioxidant defenses by reducing superoxide dismutase as well as catalase and increasing glutathione S-transferase activity in rat brains. Moreover, rats exposed to secondhand CS showed increased proteolytic degradation of αII-spectrin through caspase-3 and dephosphorylation of phosphoprotein enriched in astrocytes-15, both indicating apoptotic cell death.Citation75
Immune-regulating effects of exercise training
Exercise training has been shown to be an effective nonpharmacological treatment strategy in pulmonary diseases and systemic lung diseases. Furthermore, regular exercise has been shown to increase patients’ strength, endurance capacity, quality-of-life scores, and symptoms of fatigue and dyspnea.Citation76 Thus, the beneficial effects of exercise training in pulmonary rehabilitation are well established. Recent studies provided evidence that regular and moderate exercise exerts protective effects against smoke-induced lung disease due to its anti-inflammatory effects.Citation64,Citation77
Anti-inflammatory effects of exercise
Exercise training exerts its immune-regulating effects by activating anti-inflammatory signaling pathways.Citation78,Citation79 Contracting skeletal muscle produces and secretes the anti-inflammatory myokine IL-6 during an acute bout of exercise, which evokes a subsequent rise in circulating levels of IL-6 followed by an ensuing increase in systemic levels of the anti-inflammatory cytokines IL-10 and IL-1RA.Citation77–Citation79 IL-10, which is mainly produced by Tregs, reduces tissue damage caused by inflammation and is known to diminish the adaptive immune response.Citation80–Citation82 Complementarily, IL-1RA is capable to limit the effects of the pro-inflammatory cytokine IL-1β and therefore serves as an important contributor to exercise-induced anti-inflammatory state.Citation79 Besides, exercise-induced systemic elevations of cortisol, adrenalin, and IL-6 inhibit the secretion of pro-inflammatory TNF-α by monocytes.Citation78,Citation82,Citation83 Moreover, after an acute bout of strenuous prolonged exercise, a reduced expression of TLRs on monocytes can be observed, which results in subsequent inhibition of pro-inflammatory cytokines and promotes the expression of costimulatory molecules and major histocompatibility complex.Citation83,Citation84 CD14lowCD16+ monocytes are characterized by heightened TLR-4 expression and thereby associated with pro-inflammatory properties.Citation85 Regular exercise lowers the ratio of pro-inflammatory monocytes (CD14lowCD16+) to classical monocytes (CD14hiCD16−).Citation86 Chronic exercise training also increases Treg cell numbers in circulation. In detail, athletes participating in sports where aerobic capacity is a prominent factor for performance outcome seem to have increased Treg counts.Citation87,Citation88
Specific role of exercise during obesity
In case of obesity, exercise training stimulates anti-inflammatory signaling via a reduction in visceral fat mass, which is accompanied by a decrease in the production of several pro-inflammatory adipokines (eg, TNF-α, leptin, retinol-binding protein) and higher levels of adiponectin, which has anti-inflammatory effects and functions as an insulin sensitizer.Citation89 Current mouse and rat model studies indicate that acute bouts of exercise and exercise training stimulate phenotypic switching from M1-type macrophages producing TNF, IL-6, and nitric oxide toward M2-type macrophages, which release arginase and anti-inflammatory cytokines.Citation90,Citation91 In addition, after exercise training, a reduced tissue expression of ICAM-1, which is involved in the adhesion of inflammatory cells to endothelium and conveys interactions of T cells with target cells, and an inhibition of pro-inflammatory M1-type macrophage migration in adipose tissue occur.Citation91
Effects of exercise on pulmonary system after LTCS
Experimental animal studies demonstrated that aerobic exercise after CS exposure or asthma induction reduces lung inflammationCitation92 and remodeling.Citation93,Citation94
In particular, exercise was shown to increase Th1 response and suppress Th2 cytokine levels in lungs of smoke-exposed mice.Citation95 In parallel, exercise increased antioxidant defense and reduced oxidative stress markers.Citation96,Citation97 In CS-exposed mice, it was shown that prior exercise training significantly reduced bronchoalveolar capillary permeability, inflammatory cell infiltration, epithelial thickening, expression of proliferating cell nuclear antigen, mucin 2, cytokines, chemokines, adhesion molecules, and activation of NF-κB.Citation98 These data proved an important preventive effect of exercise training for smoke-induced inflammation in lung tissue.
Effects of exercise on CS-induced inflammation in blood
Regular exercise training has been shown to lower the levels of several inflammatory, chemoattractive, and coagulative factors in the blood of smoke-exposed mice. However, some human studies and clinical trials also demonstrated that due to dyspnea, COPD patients have restricted activity levels and muscle wasting, a markedly impaired exercise capacity.Citation99,Citation100 Therefore, some of these patients develop a kind of exercise intolerance. In this regard, it seems to be important to start exercise programs carefully, because acute and intensive bouts of exercise are known to induce a systemic immunologic response and oxidative stress, which might force inflammation in patients. However, a pro-inflammatory effect of exercise was only shown in muscle-wasted COPD patients after acute and intensive bouts of exercise. Interestingly, this effect was partially blunted by short-term supplementary oxygen.Citation100,Citation101 In general, longer periods of regular exercise training show a decrease of many inflammatory cytokines such as TNF-α, IL-2, IL-4, and CRP in COPD patients.Citation102 Similarly, in murine models, a reduced expression of cell surface markers on circulating immune cells such as vascular adhesion molecule-1 (VCAM-1), ICAM-1, and CD62L was shown after regular treadmill running. Also several other inflammatory cytokines such as IL-1α, MCP-3, MIP-1β, MIP-1α, and CD40L were shown to decrease in smoke-exposed mice after training.Citation64 In addition, regular endurance exercise has been shown to have favorable effects on blood coagulation by affecting fibrinolysis via decreasing vWF and factor VII.Citation103
Effects of exercise on endothelium after LTCS
The effects of CS and exercise on the inflammation of ECs have been well established in the literature. However, to our knowledge, no study has investigated the effects of exercise toward CS-induced inflammation in blood vessels. Therefore, it can only be hypothesized how exercise possibly ameliorates CS-induced inflammation in vessel walls by its effects on inflammation in general.
Endurance exercise training promotes endothelium-dependent vasodilationCitation104–Citation108 which is related to a shear stress-induced and Akt-dependent phosphorylation of endothelial NOS, resulting in NO activation.Citation107 Furthermore, regular physical activity reduces oxidative stress,Citation108 inflammation,Citation109,Citation110 and promotes LDL oxidation.Citation19 It has also been demonstrated that exercise training has a positive impact on inflammatory markers. Regular physical activity reduced the levels of circulating adhesion moleculesCitation111 like soluble intercellular adhesion molecule-1,Citation112,Citation113 soluble vascular adhesion molecule-1 (sVCAM-1),Citation112 soluble P-selectin, and circulating CRP.Citation109 Prolonged exercise sessions may increase cell adhesion molecules like P-selectin,Citation113 E-selectin, ICAM-1, and VCAM-1 first, but the endothelium recovers rapidly afterwards.Citation114 It is noteworthy that the potential influence of exercise training on inflammation, circulating biomarkers, and anti-oxidative capacity depends on exercise capacity.Citation115–Citation117 For example, heart failure patients showed reduced endothelial response toward exercise. Indeed, plasma levels of vWF and tPA remained unaffected after exercise while their values increased in healthy subjects.Citation118,Citation119 Therefore, endothelial dysfunction and chronic inflammation probably impair exercise capacity.Citation120 It is therefore crucial that exercise interventions in smokers should be considered as soon as possible since its benefits may decline with the progression of possible diseases.
Effects of exercise on cardiac tissue of smokers
Smoking and physical inactivity are two avoidable risk factors for CVD.Citation121 As endothelial dysfunction can lead to cardiac dysfunction,Citation122,Citation123 it is reasonable to assume that protective effects of exercise toward endothelium in (non-) smokers, which are mentioned above, might also be cardioprotective. The decreasing risks for CVDs through moderate exercise training are in part mediated through inducing anti-inflammatory factors.Citation124–Citation127 Nonetheless, as it was mentioned for vessel walls, studies examining anti-inflammatory effects of exercise in CS-induced inflammation of the myocardium remain poor.
Short-time swimming exercise in CS-exposed Wistar rat could attenuate the impact of CS to the cardiovascular system compared to the control group.Citation47 Another study with young women demonstrated that even secondhand smoking had a negative influence on exercise capacity due to reduced values of VO2max and exercise duration and an increased R-to-R value.Citation129
Exercise intensity rather than duration has a more powerful impact on physiological adaptations regarding inflammation and oxidative stress,Citation128 like it was described for vessel walls. Even if high-risk patients with severe coronary artery disease or heart failure could benefit the most from more intensive exercise training like high-intensity interval training, its safety has not been properly established.Citation130 For example, exercise intensity might be a critical factor for the development of exercise-induced hypertension. Increased exercise intensity could trigger more endothelial responses in the absence of inflammatory markers. Therefore, exercise intervention plans should always have to be appropriate to each condition.Citation23
Effects of exercise on muscle wasting after LTCS
Exercise is able to reverse sarcopenia and muscle wasting in LTCS by different pathways. On the one hand, a decrease of systemic inflammation and inflammatory mediators in muscle such as TNF-α and IL-1β might indirectly reduce the activation of catabolic pathways and increase anabolic signals.Citation64 In this regard, exercise has been shown to decrease for FoxO1 phosphorylation and reduce the expression of atrogin-1 and MuRF-1 in skeletal muscle of smoke-exposed mice. Consequently, exercise training abrogates the expression of protein catabolic E3 ligases, which are considered key factors in myofibrillar protein breakdown via the UPS. On the other hand, in particular, resistance training is also known to directly increase IGF-1 signaling followed by the activation of the Akt–mTOR–pathway.Citation131,Citation132 The reduction of catabolic and stimulation of anabolic signaling attenuate or reverse muscle wasting after smoke exposure. Endurance training was also shown to increase metabolic capacities of muscles by increased expression of genes involved in fatty acid transport into the mitochondrial matrix. Similarly, glucose uptake was optimized after regular exercise training.Citation64 The differentiated effects of exercise training on muscle tissue might also depend on the mode of exercise. While endurance training more efficiently addresses type I fibers and oxidative metabolism, strength or resistance training mainly affects type II fibers, induces hypertrophy, and increases strength capabilities. However, most pulmonary rehabilitation programs include both endurance and resistance exercise to maximize gains from both modalities. Alternatively, it has been shown that combined training program which includes both resistance and endurance exercise modalities increases strength and endurance in COPD patients.Citation133
Effects of exercise on brain after LCTS
Inflammation and vascular-induced abnormalities in the brain are two conditions associated with stroke and other neurovascular diseases, which can be protected by regular physical activity in humans.Citation134 In humans and animals, exercise upregulates brain neurotrophin and brain-derived neurotophin factor (BDNF), which is an important factor of neuronal function, growth, and survival. BDNF increases the brain’s resistance to damage and degeneration.Citation135 The immediate response of the brain to acute exercise produces only marginal changes of inflammatory mediators.Citation136 On the other hand, regular physical activity improves the overall immune condition in the brain.Citation137 Murine studies proved that pro-inflammatory cytokines impair the IGF-1 signal transduction in neurons. Peripheral IGF-1 is essential in glucose metabolism and cerebrovascular function. One mechanism by which the negative effects of inflammation are counteracted by exercise is the restoration of IGF-1 signaling.Citation137 In a study with mice, endurance and strength training decreased most of the inflammatory factors, such as IL-1α, IL-2, and IL-18. Interestingly, NF-κB and COX-2 protein levels were significantly increased probably due to circulating IL-6 after training. The increased expression of COX-2 and microsomal prostaglandin E synthase, an enzyme downstream of COX-2, were independent of peripheral inflammation.Citation138 Exercise improved oxidative stress and inflammation directly at the brain of old high-fat-fed ApoE−/− mice, reaffirming the neuroprotective effects of exercise in a model of mice with vascular brain lesions.Citation138 On the other hand, aged mice, training above the lactate threshold showed increased levels of brain PGC-1α, mTOR, and phospho-mTOR protein levels, as well as citrate synthase mRNA levels.Citation139 A similar relationship has been confirmed in young mice.Citation140 In addition, for FOXO-3 translocated from the nucleus to the cytoplasm, predicting an increased and facilitated VEGF-A expression.Citation141 Another study using rats in a traumatic brain injury model showed that aerobic exercise training enhances the endogenous anti-inflammatory response (IL-10), inhibits the infiltration of neutrophils, and attenuates BBB breakdown as well as pro-inflammatory cytokines (IL-1β, TNFα).Citation141
Neuroprotective effects of exercise
Neuroprotective and anti-inflammatory effects of exercise on the brain have also been shown in humans. Moderate intensity interval training in Parkinson’s disease patients attenuated inflammation by decreasing circulating sVCAM-1 and serum TNF-α and increasing serum BDNF levels.Citation142
Accordingly, exercise training has the potential to be a new therapeutic approach to control acute inflammation.Citation143 These effects remain to be proven in response to CS-induced inflammation. So far, only one study has investigated the effects of exercise training on CS-exposed brain oxidative stress.Citation144 Mice exposed to CS showed decreased levels of BDNF and higher immobility in a forced swim test. Exercise was able to prevent oxidative damage, but surprisingly, it could neither reverse the decrease of BDNF nor it was able to prevent CS-induced depressive-like behavior.Citation144 These results clearly show that molecular effects of exercise on CS-induced inflammation at the brain needs to be investigated in future research projects.
Conclusion
Taken together, LTCS induces local and systemic inflammatory processes which might be mediated directly by pollutant particles and a spillover of inflammatory signals to other tissues. These inflammatory processes might induce or amplify signals of tissue degradation and catabolic processes. Exercise training has been shown to prevent and even reverse inflammatory processes leading to reduced tissue degradation and catabolic processes. In parallel, exercise elicits anabolic signals leading to an increased functional capacity. Accordingly, regular exercise training seems to protect long-term smokers against some important negative local and systemic consequences of smoking. In this regard, the immune-regulating properties of exercise might have relevance (). It has to be considered that many molecular findings from smoking or exercise effects on tissues were obtained from animal studies, and this knowledge has to be only carefully transferred to humans.
Figure 2 Overview about the distribution of inflammatory signals induced by tobacco smoking from the pulmonary system to blood, brain, cardiac tissue, and muscle and the immune-regulating effects of regular exercise training.
Abbreviations: BBB, blood–brain barrier; TLR, Toll-like receptor; DAMP, damage-associated molecular pattern.
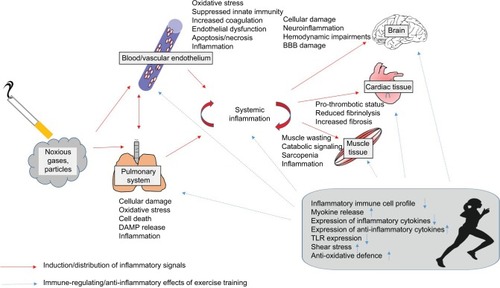
More studies are needed to discover the mechanisms how exercise affects inflammation in smokers. Specifically, the effects of exercise on inflammation in various organs in smokers have to be confirmed. From a clinical point of view, data suggest that it seems to be important to start exercise training as early as possible for smokers. In this regard, the progressive increase of certain inflammatory signals might be important predictors of the necessity of starting a regular exercise training program.
Disclosure
The authors report no conflicts of interest in this work.
References
- MarieNgFreemanMKFlemingTDSmoking prevalence and cigarette consumption in 187 countries, 1980–2012JAMA2014311218319224399557
- WakefieldMAGermainDDurkinSJHow does increasingly plainer cigarette packaging influence adult smokers’ perceptions about brand image? An experimental studyTob Control200817641642118827035
- EdwardsRThe problem of tobacco smokingBMJ200432821721914739193
- Foschino BarbaroMPCarpagnanoGESpanevelloACagnazzoMGBarnesPJInflammation, oxidative stress and systemic effects in mild chronic obstructive pulmonary diseaseInt J Immunopathol Pharmacol200720475376318179748
- van der VaartHPostmaDSTimensWTen HackenNHAcute effects of cigarette smoke on inflammation and oxidative stress: a review200459713721
- PauwelsRABuistASMaPJenkinsCRHurdSSGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summaryRespir Care200146879882511463370
- ZhangJLiuYShiJLarsonDFWatsonRRSide-stream cigarette smoke induces dose-response in systemic inflammatory cytokine production and oxidative stressExp Biol Med20022279823829
- KrügerKDischereitGSeimetzMWilhelmJWeissmannNMoorenFCTime course of cigarette smoke-induced changes of systemic inflammation and muscle structureAm J Physiol Lung Cell Mol Physiol20153092L119L12826001775
- RusznakCMillsPRDevaliaJLSapsfordRJDaviesRKLoze-wiczSEffect of cigarette smoke on the permeability and IL-1b and sICAM-1 release from cultured human bronchial epithelial cells of never-smokers, smokers, and patients with chronic obstructive pulmonary diseaseAm J Respir Cell Mol Biol20002353053611017919
- Crotty AlexanderLEShinSHwangJHInflammatory diseases of the lung induced by conventional cigarette smoke: a reviewChest201514851307132226135024
- KouYRKwongKLeeLYAirway inflammation and hypersensitivity induced by chronic smokingRespir Physiol Neurobiol201117839540521397052
- PouwelsSDHesseLFaizASusceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPDAm J Physiol Lung Cell Mol Physiol20163115L881L89227612964
- TangGJWangHYWangJYNovel role of AMP-activated protein kinase signaling in cigarette smoke induction of IL-8 in human lung epithelial cells and lung inflammation in miceFree Radic Biol Med201150111492150221376115
- BotelhoFMGaschlerGJKianpourSInnate immune processes are sufficient for driving cigarette smoke-induced inflammation in miceAm J Respir Cell Mol Biol201042439440319502389
- KaplanAAbidiEGhaliRBoozGWKobeissyFZoueinFAFunctional, cellular, and molecular remodeling of the heart under influence of oxidative cigarette tobacco smokeOxid Med Cell Longev20172017375918628808498
- MajoJGhezzoHCosioMGLymphocyte population and apoptosis in the lungs of smokers and their relation to emphysemaEur Respir J20011794695311488331
- WannametheeSGLoweGDShaperAGRumleyALennonLWhincupPHAssociations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular diseaseEur Heart J200526171765177315817606
- LiuJLiangQFrost-PinedaKRelationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress, and platelet activation in adult cigarette smokersCancer Epidemiol Biomarkers Prev20112081760176921708936
- ShihRHChengSEHsiaoLDKouYRYangCMCigarette smoke extract upregulates heme oxygenase-1 via PKC/NADPH oxidase/ ROS/PDGFR/PI3K/Akt pathway in mouse brain endothelial cellsJ Neuroinflammation2011810421861928
- RibeiroFAlvesAJDuarteJAOliveiraJIs exercise training an effective therapy targeting endothelial dysfunction and vascular wall inflammation?Int J Cardiol2010141321422119896741
- UnverdorbenMder BijlAPotgieterLLiangQMeyerBHRoethigHJEffects of levels of cigarette smoke exposure on symptom-limited spiroergometryPrev Cardiol2007102839117396059
- Vayssier-TaussatMCamilliTAronYEffects of tobacco smoke and benzo[a]pyrene on human endothelial cell and monocyte stress responsesAm J Physiol Heart Circ Physiol20012803H1293H130011179076
- JeeHParkJOhJGLeeYHShinKAKimYJEffect of a prolonged endurance marathon on vascular endothelial and inflammation markers in runners with exercise-induced hypertensionAm J Phys Med Rehabil201392651352223685440
- HoshinoSYoshidaMInoueKCigarette smoke extract induces endothelial cell injury via JNK pathwayBiochem Biophys Res Commun20053291586315721273
- YangMChenPPengHCigarette smoke extract induces aberrant cytochrome-c oxidase subunit II methylation and apoptosis in human umbilical vascular endothelial cellsAm J Physiol Cell Physiol20153085C378C38425500741
- CsordasAKreutmayerSPlonerCCigarette smoke extract induces prolonged endoplasmic reticulum stress and autophagic cell death in human umbilical vein endothelial cellsCardiovasc Res201192114114821676957
- MessnerBFrotschnigSSteinacher-NigischAApoptosis and necrosis: two different outcomes of cigarette smoke condensate-induced endothelial cell deathCell Death Dis2012311e42423152060
- YangYMLiuGTDamaging effect of cigarette smoke extract on primary cultured human umbilical vein endothelial cells and its mechanismBiomed Environ Sci200417212113415386938
- EdirisingheIRahmanICigarette smoke-mediated oxidative stress, shear stress, and endothelial dysfunction: role of VEGFR2Ann N Y Acad Sci20101203667220716285
- WangJWilckenDEWangXLCigarette smoke activates caspase-3 to induce apoptosis of human umbilical venous endothelial cellsMol Genet Metab2001721828811161833
- GornatiRColomboGClericiMProtein carbonylation in human endothelial cells exposed to cigarette smoke extractToxicol Lett2013218211812823396223
- MazzonePTierneyWHossainMPuvennaVJanigroDCuculloLPathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated areaInt J Environ Res Public Health20107124111412621317997
- GiunzioniIBonomoABishopECastiglioniSCorsiniABellostaSCigarette smoke condensate affects monocyte interaction with endotheliumAtherosclerosis2014234238339024747113
- LowBLiangMFuJp38 mitogen-activated protein kinase mediates sidestream cigarette smoke-induced endothelial permeabilityJ Pharmacol Sci2007104322523117652909
- BishopETheophilusEHFearonIMIn vitro and clinical studies examining the expression of osteopontin in cigarette smoke-exposed endothelial cells and cigarette smokersBMC Cardiovasc Disord2012127522978720
- ChenHWLiiCKKuHJWangTSCigarette smoke extract induces expression of cell adhesion molecules in HUVEC via actin filament reorganizationEnviron Mol Mutagen20095029610419107907
- WangHYeYZhuMChoCIncreased interleukin-8 expression by cigarette smoke extract in endothelial cellsEnviron Toxicol Pharmacol200091–2192311137464
- OroszZCsiszarALabinskyyNCigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activationAm J Physiol Heart Circ Physiol20072921H130H13917213480
- RiusCCompanyCPiquerasLCritical role of fractalkine (CX3CL1) in cigarette smoke-induced mononuclear cell adhesion to the arterial endotheliumThorax201368217718623143793
- MengMLiaoHZhangBCigarette smoke extracts induce overexpression of the proto-oncogenic gene interleukin-13 receptor alpha2 through activation of the PKA-CREB signaling pathway to trigger malignant transformation of lung vascular endothelial cells and angiogenesisCell Signal201731152527986643
- BarbieriSSWekslerBBTobacco smoke cooperates with interleukin-1beta to alter beta-catenin trafficking in vascular endothelium resulting in increased permeability and induction of cyclooxygenase-2 expression in vitro and in vivoFASEB J20072181831184317317723
- AllamEDelacruzKGhoneimaASunJWindsorLJEffects of tobacco on cytokine expression from human endothelial cellsOral Dis201319766066523279317
- OtaYKugiyamaKSugiyamaSImpairment of endothelium-dependent relaxation of rabbit aortas by cigarette smoke extract – role of free radicals and attenuation by captoprilAtherosclerosis199713121952029199272
- KagotaSYamaguchiYShinozukaKKwonYMKunitomoMCigarette smoke-modified low density lipoprotein impairs endothelium-dependent relaxation in isolated rabbit arteriesGen Pharmacol19962734774818723530
- SnajdarRMBusuttilSJAverbookAGrahamDJInhibition of endothelial cell migration by cigarette smoke condensateJ Surg Res2001961101611180990
- BjornstadHHBruvikJBjornstadABHjellestadBLDamasJKAukrustPExercise training decreases plasma levels of soluble CD40 ligand and P-selectin in patients with chronic heart failureEur J Cardiovasc Prev Rehabil2008151434818277184
- de SaFGda MotaGRSant’AnaPGda CunhaMRMarocoloMCastardeliETraining and cardiovascular responses from cigarette smoke exposureChin J Physiol201457631531925575519
- TiltonSCKarinNJWebb-RobertsonBJImpaired transcriptional response of the murine heart to cigarette smoke in the setting of high fat diet and obesityChem Res Toxicol20132671034104223786483
- StallonesRAThe association between tobacco smoking and coronary heart diseaseInt J Epidemiol201544373574326174518
- ZhangJFangSCMittlemanMAChristianiDCCavallariJMSecondhand tobacco smoke exposure and heart rate variability and inflammation among non-smoking construction workers: a repeated measures studyEnviron Health20131218324083379
- BlackburnHEnvironmental tobacco smoke exposure was associated with an increased risk of ischemic heart diseaseEvid Based Cardiovasc Med199822434416379803
- JapuntichSJEilersMAShenhavSSecondhand tobacco smoke exposure among hospitalized nonsmokers with coronary heart diseaseJAMA Intern Med2015175113313625383761
- VandivierRWLearning to act on secondhand tobacco smoke exposure to limit risk for coronary heart diseaseJAMA Intern Med2015175113625383686
- EnstromJEKabatGCEnvironmental tobacco smoke and coronary heart disease mortality in the United States – a meta-analysis and critiqueInhal Toxicol200618319921016399662
- DasADeyNGhoshADasSChattopadhyayDJChatterjeeIBMolecular and cellular mechanisms of cigarette smoke-induced myocardial injury: prevention by vitamin CPLoS One201279e4415122970172
- LeoneABiochemical markers of cardiovascular damage from tobacco smokeCurr Pharm Des200511172199220816026289
- IzzottiAD’AgostiniFBalanskyRExposure of mice to cigarette smoke and/or light causes DNA alterations in heart and aortaMutat Res20086441–2384218640134
- ZhouXLiCXuWChenJTrimetazidine protects against smoking-induced left ventricular remodeling via attenuating oxidative stress, apoptosis, and inflammationPLoS One201277e4042422792312
- ZhouXLiCXuWChenJProtective effects of valsartan against cigarette smoke-induced left ventricular systolic dysfunction in ratsInt J Cardiol2013167367768022464480
- KhannaAKXuJMehraMRAntioxidant N-acetyl cysteine reverses cigarette smoke-induced myocardial infarction by inhibiting inflammation and oxidative stress in a rat modelLab Investig20129222423521968809
- HalappanavarSStampfliMRBerndt-WeisLWilliamsADouglasGRYaukCLToxicogenomic analysis of mainstream tobacco smoke-exposed mice reveals repression of plasminogen activator inhibitor-1 gene in heartInhal Toxicol2009211788518925475
- McCallMRvan den BergJJKuypersFAModification of LCAT activity and HDL structure. New links between cigarette smoke and coronary heart disease riskArterioscler Thromb19941422482538305416
- GeaJAgustíARocaJPathophysiology of muscle dysfunction in COPDJ Appl Physiol (1985)201311491222123423519228
- KrügerKSeimetzSRingseisRExercise training reverses inflammation and muscle wasting after smoke exposureAm J Physiol Regul Integr Comp Physiol2017 Epub111
- BasicVTTadeleEElmabsoutAAExposure to cigarette smoke induces overexpression of von Hippel-Lindau tumor suppressor in mouse skeletal muscleAm J Physiol Lung Cell Mol Physiol20123036L519L52722842216
- GomesMDLeckerSHJagoeRTNavonAGoldbergALAtrogin-1, a muscle531 specific F-box protein highly expressed during muscle atrophyProc Natl Acad Sci U S A20019825144401444511717410
- DuttaPCourtiesGWeiYMyocardial infarction accelerates atherosclerosisNature2012487740732532922763456
- Hafezi-MoghadamAThomasKLWagnerDDApoE deficiency leads to a progressive age-dependent blood-brain barrier leakageAm J Physiol Cell Physiol20072924C1256C126216870825
- MannaSKRangasamyTWiseKLong term environmental tobacco smoke activates nuclear transcription factor-kappa B, activator protein-1, and stress responsive kinases in mouse brainBiochem Pharmacol200671111602160916569398
- SeoSBChoeESKimKSShimSMThe effect of tobacco smoke exposure on the generation of reactive oxygen species and cellular membrane damage using co-culture model of blood brain barrier with astrocytesToxicol Ind Health201733653053628125953
- PrasadSSajjaRKParkJHNaikPKaisarMACuculloLImpact of cigarette smoke extract and hyperglycemic conditions on blood-brain barrier endothelial cellsFluids Barriers CNS2015121826206552
- WangJDoreSHeme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhageBrain2007130Pt 61643165217525142
- OhnoNTanakaTKitaTChanges of brain endothelin levels and peripheral endothelin receptors by chronic cigarette smoke in spontaneously hypertensive ratsJ Pharmacol Sci200494328729615037814
- PaulsonJRRoderKEMcAfeeGAllenDDVan der SchyfCJAbbruscatoTJTobacco smoke chemicals attenuate brain-to-blood potassium transport mediated by the Na,K,2Cl-cotransporter during hypoxia-reoxygenationJ Pharmacol Exp Ther2006316124825416174793
- FullerBFGoldMSWangKKOttensAKEffects of environmental tobacco smoke on adult rat brain biochemistryJ Mol Neurosci201041116517119960371
- McCarthyBCaseyDDevaneDMurphyKMurphyELacasseYPulmonary rehabilitation for chronic obstructive pulmonary diseaseCochrane Database Syst Rev20152CD003793
- PedersenBKFebbraioMAMuscle as an endocrine organ: focus on muscle-derived interleukin-6Physiol Rev20088841379140618923185
- GleesonMBishopNCStenselDJLindleyMRMastanaSSNimmoMAThe anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of diseaseNat Rev Immunol201111960761521818123
- PedersenBKAnti-inflammatory effects of exercise: role in diabetes and cardiovascular diseaseEur J Clin Invest201747860061128722106
- MaynardCLWeaverCTDiversity in the contribution of IL 10 to cell-mediated immune regulationImmunol Rev200822621923319161427
- MooreKWde Waal MalefytRCoffmanRLO’GarraAInterleukin 10 and the interleukin 10 receptorAnnu Rev Immunol20011968376511244051
- BergmannMGornikiewiczASautnerTAttenuation of catecholamine-induced immunosuppression in whole blood from patients with sepsisShock199912642142710588509
- GleesonMMcFarlinBKFlynnMGExercise and Toll-like receptorsExerc Immunol Rev200612345317201071
- OliveiraMGleesonMThe influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy menEur J Appl Physiol2010109225125720063104
- SkinnerNAMacIsaacCMHamiltonJAVisvanathanKRegulation of Toll-like receptor (TLR)2 and TLR4 on CD14dimCD16+ monocytes in response to sepsis-related antigensClin Exp Immunol2005141227027815996191
- TimmermanKLFlynnMGCoenPMMarkofskiMMPencePBExercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise?Leukoc Biol200884512711278
- ShawDMMerienFBraakhuisADulsonDT-cells and their cytokine production: the anti-inflammatory and immunosuppressive effects of strenuous exerciseCytokine2017 pii:S1043-4666(17)30292-2
- WeinholdMShimabukuro-VornhagenAFrankeAPhysical exercise modulates the homeostasis of human regulatory T cellsJ Allergy Clin Immunol2016137516071610.e826774657
- KrügerKInflammation during obesity – pathophysiological concepts and effects of physical activityDtsch Z Sportmed201768163169
- OliveiraAGAraujoTGCarvalhoBMGuadagniniDRochaGZBagarolliRAAcute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet-induced obese ratsObesity2013212545255623512570
- KawanishiNYanoHYokogawaYSuzukiKExercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese miceExerc Immunol Rev20101610511820839495
- LowderTDuggerKDeshaneJEstellKSchwiebertLMRepeated bouts of aerobic exercise enhance regulatory T cell responses in a murine asthma modelBrain Behav Immun201024115315919781626
- PastvaAEstellKSchoebTRAtkinsonTPSchwiebertLMAerobic exercise attenuates airway inflammatory responses in a mouse model of atopic asthmaJ Immunol200417274520452615034069
- DuggerKJChrismanTJonesBModerate aerobic exercise alters migration patterns of antigen specific T helper cells within an asthmatic lungBrain Behav Immun201334677823928286
- Lakier SmithLOvertraining, excessive exercise, and altered immunity: is this a T helper-1 versus T helper-2 lymphocyte response?Sports Med200333534736412696983
- OnurEKabaroğluCGünayOThe beneficial effects of physical exercise on antioxidant status in asthmatic childrenAllergol Immunopathol (Madr)2011392909521242022
- MenegaliBTNesiRTSouzaPSThe effects of physical exercise on the cigarette smoke-induced pulmonary oxidative responsePulm Pharmacol Ther200922656757319683592
- YuBULiaoYWSuKHPrior exercise training alleviates the lung inflammation induced by subsequent exposure to environmental cigarette smokeActa Physiol (Oxf)2012205453255422448892
- RennardSIDrummondMBEarly chronic obstructive pulmonary disease: definition, assessment, and preventionLancet201538599791778178825943942
- SaeyDDebigareRLeBlancPContractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003168442543012714348
- Van HelvoortHAHeijdraYFThijsHMVinaJWantenGJDekhuijzenPNExercise-induced systemic effects in muscle-wasted patients with COPDMed Sci Sports Exerc20063891543155216960513
- Abd El-KaderSMAl-JiffriOHAl-ShreefFMPlasma inflammatory biomarkers response to aerobic versus resisted exercise training for chronic obstructive pulmonary disease patientsAfr Health Sci201616250751527605966
- El-SayedMSSaleCJonesPGChesterMBlood hemostasis in exercise and trainingMed Sci Sports Exerc200032591892510795781
- ClarksonPMontgomeryHEMullenMJExercise training enhances endothelial function in young menJ Am Coll Cardiol19993351379138510193742
- KellyASWetzsteonRJKaiserDRSteinbergerJBankAJDengelDRInflammation, insulin, and endothelial function in overweight children and adolescents: the role of exerciseJ Pediatr2004145673173615580192
- HambrechtRWolfAGielenSEffect of exercise on coronary endothelial function in patients with coronary artery diseaseN Engl J Med2000342745446010675425
- HambrechtRAdamsVErbsSRegular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthaseCirculation2003107253152315812810615
- LaufsUWassmannSCzechTPhysical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosisArterioscler Thromb Vasc Biol200525480981415692095
- MorikawaYMizunoYHaradaEAerobic interval exercise training in the afternoon reduces attacks of coronary spastic angina in conjunction with improvement in endothelial function, oxidative stress, and inflammationCoron Artery Dis201324317718223249633
- BabbittDMDiazKMFeairhellerDLEndothelial activation microparticles and inflammation status improve with exercise training in African AmericansInt J Hypertens2013201353801723691280
- ZoppiniGTargherGZamboniCEffects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetesNutr Metab Cardiovasc Dis200616854354917126770
- WeggeJKRobertsCKNgoTHBarnardRJEffect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery diseaseMetabolism200453337738115015151
- AdamopoulosSParissisJKroupisCPhysical training reduces peripheral markers of inflammation in patients with chronic heart failureEur Heart J200122979179711350112
- WaltherCMobius-WinklerSLinkeARegular exercise training compared with percutaneous intervention leads to a reduction of inflammatory markers and cardiovascular events in patients with coronary artery diseaseEur J Cardiovasc Prev Rehabil200815110711218277195
- NielsenHGLybergTLong-distance running modulates the expression of leucocyte and endothelial adhesion moleculesScand J Immunol200460435636215379860
- BartzeliotouAIMargeliAPTsironiMCirculating levels of adhesion molecules and markers of endothelial activation in acute inflammation induced by prolonged brisk exerciseClin Biochem2007401176577017320067
- WisloffUStoylenALoennechenJPSuperior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized studyCirculation2007115243086309417548726
- GibalaMJLittleJPMacdonaldMJHawleyJAPhysiological adaptations to low-volume, high-intensity interval training in health and diseaseJ Physiol201259051077108422289907
- CugnoMAgostoniPMariDImpaired bradykinin response to ischaemia and exercise in patients with mild congestive heart failure during angiotensin-converting enzyme treatment. Relationships with endothelial function, coagulation and inflammationBr J Haematol2005130111312015982353
- JeeHJinYEffects of prolonged endurance exercise on vascular endothelial and inflammation markersJ Sports Sci Med201211471972624150084
- NelsonMRAlkhateebANRyanPPhysical activity, alcohol and tobacco use and associated cardiovascular morbidity and mortality in the Second Australian National Blood Pressure study cohortAge Ageing201039111211619903774
- ForstermannUMuggeAAlheidUHaverichAFrolichJCSelective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteriesCirc Res19886221851902448055
- TreasureCBVitaJACoxDAEndothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathyCirculation19908137727792306829
- MoraSCookNBuringJERidkerPMLeeIMPhysical activity and reduced risk of cardiovascular events: potential mediating mechanismsCirculation2007116192110211817967770
- GarberCEBlissmerBDeschenesMRAmerican College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exerciseMed Sci Sports Exerc20114371334135921694556
- O‘DonovanGBlazevichAJBorehamCThe ABC of physical activity for health: a consensus statement from the British Association of Sport and Exercise SciencesJ Sports Sci201028657359120401789
- GuaschEBenitoBNattelSExercise training, inflammation and heart failure: working out to cool downJ Physiol2010588Pt 142525252620634182
- McMurrayRGHicksLLThompsonDLThe effects of passive inhalation of cigarette smoke on exercise performanceEur J Appl Physiol Occup Physiol19855421962003930241
- Ribeiro-SamoraGARabeloLAFerreiraACCInflammation and oxidative stress in heart failure: effects of exercise intensity and durationBraz J Med Biol Res2017509e639328793058
- MeyerPGaydaMNormandinEGuiraudTJuneauMNigamAHigh-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation: a randomized controlled trial evaluating the relationship to endothelial function and inflammationAm Heart J200915873474119853690 Am Heart J20101593e2120211291
- TerzisGStratakosGMantaPGeorgiadisGThrowing performance after resistance training and detrainingJ Strength Cond Res20082241198120418545188
- WalkerDKDickinsonJMTimmermanKLExercise, amino acids, and aging in the control of human muscle protein synthesisMed Sci Sports Exerc201143122249225821606874
- Zambom-FerraresiFCebolleroPGorostiagaEMEffects of combined resistance and endurance training versus resistance training alone on strength, exercise capacity, and quality of life in patients with COPDJ Cardiopulm Rehabil Prev201535644645326252342
- ChiricoENDi CataldoVChauveauFMagnetic resonance imaging biomarkers of exercise-induced improvement of oxidative stress and inflammation in the brain of old high-fat-fed ApoE−/− miceJ Physiol2016594236969698527641234
- NeeperSAGomez-PinillaFChoiJCotmanCExercise and brain neurotrophinsNature199537365101097816089
- EnosRTDavisJMMcClellanJLMurphyEAIndomethacin in combination with exercise leads to muscle and brain inflammation in miceJ Interferon Cytokine Res201333844645123651238
- CotmanCWBerchtoldNCChristieLAExercise builds brain health: key roles of growth factor cascades and inflammationTrends Neurosci200730946447217765329
- KrügerKBredehoftJMoorenFCRummelCDifferent effects of strength and endurance exercise training on COX-2 and mPGES expression in mouse brain are independent of peripheral inflammationJ Appl Physiol (1985)2016121124825427283912
- BurnsJMSwerdlowRHEffect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammationNeurobiol Aging201435112574258325002036
- LuJSelfridgeJEBurnsJMSwerdlowRHLactate administration reproduces specific brain and liver exercise-related changesJ Neurochem201312719110023927032
- MotaBCPereiraLSouzaMAExercise pre-conditioning reduces brain inflammation and protects against toxicity induced by traumatic brain injury: behavioral and neurochemical approachNeurotox Res201221217518421735317
- ZoladzJAMajerczakJZeligowskaEModerate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patientsJ Physiol Pharmacol201465344144824930517
- MassonGSCostaTSYshiiLTime-dependent effects of training on cardiovascular control in spontaneously hypertensive rats: role for brain oxidative stress and inflammation and baroreflex sensitivityPLoS One201495e9492724788542
- TuonTValvassoriSSLopes-BorgesJEffects of moderate exercise on cigarette smoke exposure-induced hippocampal oxidative stress values and neurological behaviors in miceNeurosci Lett20104751161920303389
