Abstract
Although acupuncture therapy is increasingly used to treat diverse symptoms and disorders in humans, its underlying mechanism is not known well. Only recently have experimental studies begun to provide insights into how acupuncture stimulation generates and relates to pathophysiological responsiveness. Acupuncture intervention is frequently used to control pathologic symptoms in several visceral organs, and a growing number of studies using experimental animal models suggest that acupuncture stimulation may be involved in inducing anti-inflammatory responses. The vagus nerve, a principal parasympathetic nerve connecting neurons in the central nervous system to cardiovascular systems and a majority of visceral organs, is known to modulate neuroimmune communication and anti-inflammatory responses in target organs. Here, we review a broad range of experimental studies demonstrating anti-inflammatory effects of electroacupuncture in pathologic animal models of cardiovascular and visceral organs and also ischemic brains. Then, we provide recent progress on the role of autonomic nerve activity in anti-inflammation mediated by electroacupuncture. We also discuss a perspective on the role of sensory signals generated by acupuncture stimulation, which may induce a neural code unique to acupuncture in the central nervous system.
Introduction
Acupuncture has been used as a traditional medical treatment in East Asia for over 2,000 years,Citation1 and is becoming a popular therapy worldwide for treating various diseases.Citation2,Citation3 Acupuncture is a medical intervention in which fine needles are applied to specific parts of the body, called acupuncture points (or acupoints) and penetrated through the muscular or other subcutaneous layers. According to traditional medical theory, acupuncture stimulation facilitates the flow of qi, a life force that is supposedly circulating through the channels called meridians.Citation4,Citation5 Acupoints are presumed to be pathophysiologically associated with and possibly reflect the status of visceral organs and systemic conditions, and thus the stimulation of specific acupoints may evoke the responsiveness that controls the unbalanced internal milieu and improves body symptoms. Acupuncture stimulation is given right on the acupoint or a nearby affected area (“ashi point”) for the treatment of local symptoms, such as knee pain or muscle rigidity, whereas distal acupuncture stimulation is applied to treat diseases in the internal organs and systemic abnormalities.
There are two main types of acupuncture stimulation: manual acupuncture (MA) and electroacupuncture (EA). In MA, an acupuncturist penetrates the skin with a metallic needle and manipulates it by rotating in one or both directions or lifting and thrusting.Citation6 It is known that during acupuncture practice, acupuncturists experience a special touch sensation perceived as heaviness, tenseness, or terseness, and patients perceive feelings of numbness, heaviness, soreness, and distention around the site of needle stimulation. These are called deqi sensations. Clinical data further indicate that patients frequently feel deqi sensations spreading to other parts of the body,Citation7–Citation9 which is considered a useful criterion to evaluate the therapeutic efficacy of acupuncture.Citation7,Citation10–Citation12 In EA, a small electric current is applied to pairs of acupuncture needles, and studies have indicated that the therapeutic efficacy of EA can be modulated by varying the frequency, intensity, and duration of electrical stimulation.Citation6,Citation13 For instance, EA at low and high frequencies of electrical stimulation can activate different types of opioid receptors and different analgesic effects.Citation14,Citation15 To maximize therapeutic effects, acupuncture is usually practiced first by applying MA to evoke deqi sensation and followed by electrical stimulation for 15–20 minutes.Citation7
A growing number of recent reports have indicated that acupuncture may be effective in treating many types of diseases by regulating inflammatory responses. In this review, we highlight important findings that demonstrate how acupuncture stimulation, particularly EA, can improve inflammatory responses in pathological animal models. First, we discuss recent advances in understanding of neuroimmune communication, and then address how it has contributed to establishing experimental approaches to investigate a mechanistic basis of EA. In the last part of the review, we briefly discuss a perspective on the role of a neural code that may transmit sensory information unique to acupuncture in regulating inflammation.
Neuroimmune communication
When an organism is exposed to external pathogens, host-defense response begins with innate immunity, which is critical to induce inflammation as a defense mechanism against pathogenic infections. Pathogen-associated molecular patterns trigger inflammatory reactions in the host via interaction with membranous or cytoplasmic molecules, such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), Retinoic acid-inducible gene 1-like receptors (RLRs), and C-type lectin receptors (CLRs), collectively termed “pattern-recognition receptors”.Citation16 Activation of these receptors in target cells induces downstream-signaling pathways, including activation of the MAPK pathway and NFκB transcription factor, and induces the expression of inflammatory cytokines, including TNFα and several types of interleukins and chemokines. Chemokines recruit leukocytes into the inflammation area, and interleukins and IFNγ activate lymphocytes and macrophages.
Cholinergic anti-inflammatory reflex
In the early 2000s, Borovikova et al reported on their seminal work on the regulation of inflammatory responses by vagus nerve activity. They found that the electrical stimulation of the vagus nerve in vivo decreased the production of TNFα in the spleen of lipopolysaccharide (LPS)-injected animals, and treatment with acetylcholine of cultured macrophages attenuated levels of inflammatory cytokines as well.Citation17 They further demonstrated that the suppression of TNFα production by acetylcholine treatment was mediated by the activation of α7-nicotinic acetylcholine receptors, which subsequently inhibited NFκB activation while stimulating the STAT3 pathway.Citation18–Citation20 The vagus nerve is known to account for 70% of the parasympathetic, visceral regulation of internal organs, thus acting as a functional bridge connecting the brain to internal organs. Inflammatory cytokines produced from peripheral organs can activate an afferent part of the vagus nerve, stimulate vagal efferent nerves through the synaptic transmission from the solitary nucleus to the dorsal vagal nucleus in the brain stem, and downregulate the production of inflammatory cytokines, thereby completing the cholinergic anti-inflammatory reflex.Citation21 Rosas-Ballina et al reported that the vagal efferent nerves are connected to adrenergic post-ganglionic neurons in the celiac ganglion, which primarily receive splanchnic preganglionic sympathetic inputs from the spinal cord,Citation22 and further showed that norepinephrine released from celiac ganglion neurons stimulated the secretion of acetylcholine from choline acetyltransferase-expressing T cells in the spleen that binds to nicotinic acetylcholine receptors in macrophages.Citation23 Similar experimental strategies investigating the effects of vagus nerve activity on the pathologic regulation of several conditions, such as arthritis, obesity, and head trauma, have been considered for clinical application, and the anti-inflammatory reflex is becoming accepted as a concept explaining neuroimmunogenic control of diseases.Citation24
Neuroimmunogenic regulation of inflammation further provides insights into studies on how brain activity modulates pathological responsiveness in major internal organs. For instance, mental activities, such as stress, biofeedback therapy, and meditation, have been reported to be positively related to increased vagus nerve activity.Citation23 It has also been reported that impaired vagal activity increased the vulnerability of inflammatory bowel disease in an animal model of depression.Citation25
It should however be noted that the connectivity between the terminals of autonomic fiber and the target immune cells has not been clearly demonstrated in several organs. While increasing numbers of publications have reported on the role of vagal activity in hepatic hypertension and inflammation based on the concept of cholinergic anti-inflammatory reflex,Citation17,Citation26,Citation27 hepatic target cells of vagal efferent fibers have not been identified.Citation28,Citation29 Similarly, autonomic connections to the spleen are unclear and controversial. In this regard, it is worthwhile to note one recent report showing that the electrophysiological and histological identification of serotonin-secreting enterochromaffin cells in the intestine that modulate synaptically connected afferent nerve fibers can fulfill the minimal requirement of brain–gut communication.Citation30
Functional intervention of sympathetic activity
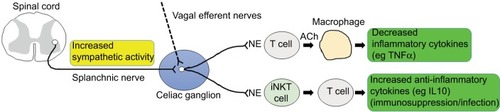
While the vagus nerve has been a primary target mediating neuroimmune reaction in many studies, a potential role of sympathetic nerve activity has also been proposed. Martelli et al claimed that neurons from celiac ganglia that are primarily innervated by sympathetic splanchnic nerves were responsible for anti-inflammatory reflexes.Citation31,Citation32 Adrenergic inputs activated hepatic invariant natural killer T (iNKT) cells and elevated the production of anti-inflammatory cytokines from T-helper 2 cells, rendering the organ more susceptible to infection via immunosuppression.Citation33 Possible connections of sympathetic nerve activity to immune cells in target organs, such as liver and spleen, for the regulation of inflammatory reactions are depicted in .
Figure 1 Sympathetic connection to immune cells in target organs.
Notes: Splanchnic nerve activity transmitted to the celiac ganglion may increase the release of norepinephrine (NE) of adrenergic postganglionic neurons. This in turn would activate immune-cell responses in target organs, such as macrophages in the spleen and TH2 cells in the liver, and these cells regulate the production of pro- and anti-inflammatory cytokines. Vagal input, which has not been clearly identified, may have similar effects on the regulation of inflammation as sympathetic activity.
Abbreviations: TH, T helper; ACh, acetylcholine; iNKT, invariant natural killer T.
Experimental studies on EA using pathologic animal models
As a modified version of traditional MA, EA is manipulated at the same acupuncture points as MA, but electric current is additionally applied. Needle rotation, which is performed routinely during MA, can result in mechanical deformation of dermal tissue and may activate special types of mechanosensory receptors (eg, Ruffini corpuscles). Previous studies have suggested that acupuncture-specific responses, such as the production of mechanical torque and induction of specific types of integrin proteins, are related to needle rotation.Citation34,Citation35 In contrast, electric current, which is applied in EA, would spread to a nearby area and affect the peripheral nerve pulses (ie, action potential) more intensely, which may act as a possible reason to explain a certain level of therapeutic effects caused by sham EA, as reported in human subjects,Citation36 yet in other studies, sham EA stimulation (EAS) was less effective than a combined manipulation of MA and EA.Citation37,Citation38
It has been reported that MA can attenuate inflammatory responses by regulating the production of IL10 in macrophages, hypothalamic expression of IL1β and IL6 mRNA, and serum TNFα production in LPS-injected animals.Citation39–Citation41 However, edema response by capsaicin injection is reduced by EA, but not by MA.Citation42 Since the vast majority of studies on the regulation of inflammation have been conducted using EA, here we focus primarily on EA studies unless otherwise indicated.
Anti-inflammatory effects of EA in animal models
A study using animals given radiant heat on the tail identified gene groups (neurotransmitter-related genes vs proinflammatory cytokine-related genes) showing differential regulation of expression in the spinal dorsal horn after EA,Citation43 suggesting that EA may be involved in the regulation of inflammation at gene-expression level. In order to investigate the effects of EA on the regulation of inflammation, injury models of complete Freund’s adjuvant (CFA)-induced inflammation, collagen-induced inflammation, cerebral ischemia, reperfusion injury, and others have been used. The CFA-inflammatory model is frequently used to investigate inflammation-related pain and is thus useful to investigate pain regulation by EA. EA manipulation efficiently suppresses glial cell-marker proteins and TRPV1 and attenuates pain responses.Citation44,Citation45 EA also suppresses edema in CFA-inflammation animals by activating corticotropin-releasing hormone-producing neurons in the hypothalamus and increasing levels of adrenocorticotropic hormone.Citation46 In an animal model of collagen-induced arthritis, EA attenuated inflammatory pain via the mediation of cholinergic and serotonergic receptors and also attenuated the production of inflammatory cytokines, such as IL1β, -6, and -8, TNFα, and NFκB in synovial tissue.Citation47,Citation48 Moreover, EA was effective in regulating the levels of TNFα, IL1β, IL6, and myeloperoxidase in animal models of ulcerative colitis and zymosan-induced acute arthritis,Citation13,Citation49,Citation50 increased superoxide dismutase, while reducing death-related proteins, such as caspase 3, and phosphorylation of p38 and JNK in animals with cardiopulmonary bypass-induced lung injury,Citation6 and suppressed NFκB in a rat-tissue chamber model of inflammation.Citation51 In an acute alcoholic liver-injury model, EA improved hepatic circulation and adjusted ALT and AST levels.Citation52 Finally, systemic inflammatory response and survival rate are significantly improved by EA in animals injected with a lethal dose of LPS.Citation53 Major studies on the regulation of inflammation by EA are summarized in .
Table 1 Summary of electroacupuncture (EA) studies on the regulation of inflammation in experimental animals
EA-related signaling events in the nervous system
While an increasing number of reports strongly indicate that EA can regulate inflammation and associated pathologic symptoms, underlying mechanisms remain largely elusive. Considering that acupuncture and meridian networks encompass the whole body, as does the neural network, it is not surprising that the nervous system has been a primary concern for mechanistic studies on acupuncture. Indeed, a growing number of studies have supported this notion. Cutaneous acupuncture at the zusanli acupoint has been reported to elevate levels of focal adenosine and its A1 receptor for antinociceptive actionsCitation54 and upregulate the expression of purinergic receptors in the dorsal root ganglion,Citation35,Citation55 suggesting acupuncture-mediated induction of afferent signals. It has been further reported that acupuncture stimulation induced c-Fos expression in neurons in the dorsal vagal complex area, including both nucleus tractus solitarii (NTS) and dorsal motor nucleus, rostral ventromedial medulla, and raphe nucleus, implying that the ascending neuronal signals generated from the cutaneous acupoints relay synaptic inputs into the vagal neural circuits and possibly further up to the cerebral neural circuits.Citation39,Citation56–Citation58
Evidence demonstrating the involvement of autonomic nerve activity in acupuncture action largely comes from physiological studies analyzing cardiovascular responses and gastrointestinal (GI) motility in experimental animals and humans. Physiological assessment of GI activity is useful to investigate acupuncture effects on autonomic function, because the regulation of GI disorders is one of the major realms of traditional acupuncture medicine.Citation59,Citation60 One study reported that the manipulation of EA in dogs improved gastric emptying and increased vagal activity assessed by spectral analysis of heart-rate variability while suppressing sympathetic activity.Citation61 Acupuncture effects on regulation of heart-rate variability were similarly demonstrated in a rodent model of inflammatory bowel disease and human subjects.Citation13,Citation62 Longhurst and Tjen-A-Looi reported that acupuncture stimulation regulated cardiovascular function (blood pressure), whereby neurotransmitter release and neuromodulation in the hypothalamus and several cardiovascular nuclei in the brain stem mediated acupuncture effects.Citation63
In addition to nuclei in the vagal complex, EA was reported to induce neuronal activation in the cerebrum. Functional magnetic resonance-imaging studies using human subjects revealed that analgesic effects caused by low-frequency EA (2 Hz) were positively correlated with activation in the contralateral primary motor area, supplementary motor area, and ipsilateral superior temporal gyrus, but negatively correlated in the bilateral hippocampus.Citation64 However, analgesic effects induced by high-frequency EA (100 Hz) were positively correlated with activation in other areas, such as the contralateral inferior parietal lobule, ipsilateral anterior cingulate cortex, nucleus accumbens, and pons. In another functional magnetic resonance-imaging study, EA activated neurons in the cingulate cortex and modulated the activation pattern of limbic-system networks.Citation65
Acupuncture mechanism on anti-inflammation: potential role of vagus nerve activity
Based on the preceding discussion, it is likely that EA generates neuronal signals at the acupuncture point, sends these to the spinal cord and brain, and may trigger autonomic regulation of inflammatory responses in target organs. How would EA-induced sympathetic and parasympathetic nerve activities modulate pathological responsiveness in internal organs? Here, we discuss the role of vagus nerve activity as a principal parasympathetic nerve and then sympathetic nerve activity.
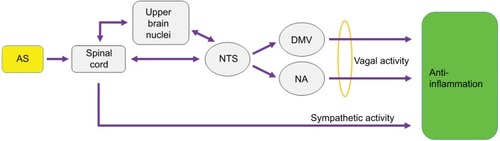
As discussed in the previous section, vagus nerve stimulation (VNS) activates nicotinic acetylcholine receptors in target cells to attenuate inflammatory responses. Here, one intriguing issue is whether the vagus nerve activity induced by EAS is functionally comparable to the VNS. It should be noted that since the VNS acts on both afferent and efferent parts of the nerve, afferent signals transmitted to the brain could indirectly affect inflammatory responses. Likewise, EA signals, if any, would be transmitted directly to the vagal efferent nerves or indirectly by way of brain pathways, and thus the vagal activity caused by EAS and VNS may share some common neural code in acting on a target organ (). Previous studies have shown that EA given at several different acupoints activates neurons in the NTS, a brain-stem location receiving somatic nerve activity and afferent vagal inputs, as demonstrated by c-Fos immunostaining and electrophysiological recordings.Citation57,Citation66,Citation67 EAS-induced neuronal activity in the NTS would send out signals to vagal efferent nerves or ascending neural paths to upper-brain neurons, and combined efferent activity may mediate anti-inflammatory effects (). In this respect, one previous study demonstrating the involvement of brain muscarinic receptor-mediated networks in anti-inflammatory regulation of EA in LPS-induced endotoxemiaCitation53 supports the notion that EAS-induced efferent vagal activity may carry the descending brain activity as acupuncture-specific neuronal signals.
Figure 2 Possible neural pathways that transmit cutaneous ASs ultimately to internal visceral organs and induce anti-inflammatory responses in target organs.
Abbreviations: AS, acupuncture signal; NTS, nucleus tractus solitarius; DMV, dorsal motor nucleus of vagus nerve; NA, nucleus ambiguus.
Torres-Rosas et al demonstrated that sciatic nerve activation by EA at the zusanli acupoint inhibited the production of major inflammatory cytokines in an animal model of polymicrobial peritonitis, and further showed that vagal activity transmitted to the adrenal gland increased dopamine production and downregulated cytokine production.Citation68 However, in another study using LPS-induced systemic inflammation animals, EA at the zusanli acupoint activated the vagal pathway connected to the spleen and attenuated the production of TNFα in the spleen.Citation39 In an animal-sepsis model with a lethal dose of LPS, vagotomy abrogated the effects of EAS (at the hegu acupoint) on anti-inflammation and animal survival,Citation53 suggesting the activation of vagal efferent by EAS. However, EAS (at the zusanli acupoint) activated NTS neurons and improved the pathologic parameters of postoperative ileus, but did not regulate the production of inflammatory cytokines.Citation66 In ischemia–reperfusion animals, vagotomy or administration of nicotinic receptor antagonist reversed EA inhibition on the release of HMGB1 and myocardial protection.Citation69 Finally, in a TNBS-induced colitis model, when given together with VNS, EAS effectively decreased the production of inflammatory cytokines and pathogenesis in the colon, but EAS alone did not increase vagal activity above VNS,Citation13 implying some augmentation effects of EAS on VNS-mediated anti-inflammation. It seems evident that vagal activity is a principal modulating factor for regulation of inflammation by EA; however, further studies are essential to verify vagal modulation of EA in different disease models using various EAS manipulations.
Anti-inflammatory regulation of EA via sympathetic nerve pathway
Possible involvement of sympathetic nerve activity in neuroimmune regulation has been implicated by adrenergic neuronal activity inducing iNKT cells, whose inhibition attenuated immunosuppression and bacterial infection ().Citation33 Can a concept of anti-inflammatory regulation via the suppression of sympathetic activity be applied to acupuncture-mediated anti-inflammation? Previous reports have suggested that in a physiological state, EA may either increase or decrease sympathetic nerve activity in several organ systems. For instance, EAS improves rectal motility by inhibiting sympathetic nerve activity, but suppresses gastric motility by increasing sympathetic activity.Citation70,Citation71 EA increases cellular uptake of glucose by increasing sympathetic nerve activity.Citation72 It has further been reported that EA was involved in cardiovascular baroreceptor reflex by modulating hypothalamic inputs to rostroventrolateral medulla, an area relaying signals to preganglionic sympathetic neurons in the spinal cord.Citation73 In relation to inflammatory responses under a pathological state, EA attenuates inflammatory reaction by activating postganglionic sympathetic nerve activity in carrageenan- and zymosan-induced animal models.Citation74,Citation75 However, in LPS-induced endotoxemia animals, EA inhibits peripheral sympathetic activity and increases vagal activity to regulate systemic inflammatory responses.Citation53 This suggests that (possibly reflecting differential experimental systems) either increased or decreased sympathetic nerve activities are involved in mediating anti-inflammatory effects of EA.
Martelli et al claimed that instead of vagus nerve activity, sympathetic activity, which is evoked by electrical stimulation of splanchnic nerve connected to celiac ganglia, might be responsible for regulating anti-inflammatory response in the spleen.Citation31,Citation32 Therefore, it is tempting to explore the possibility that anti-inflammation by EAS is modulated by splanchnic nerve activity transmitted to celiac ganglia. Immune cells, such as iNKT cells, and a subpopulation of CD4+ T cells are known to receive adrenergic inputs from sympathetic nerve terminals and mediate immunosuppression and inflammation ().Citation23,Citation33 Therefore, one experimental approach to explore the mechanistic basis of the regulation of sympathetic nerve activity by EA would be to investigate whether EAS activates preganglionic neurons in the spinal cord and subsequent celiac ganglia neurons, and analyze the activation of immune cells in pathologic target organs, such as spleen and liver, in association with sympathetic nerve connectivity.
Regulation of ischemic brain injury by EA
EA has been widely used for the treatment of stroke and cerebral ischemia.Citation76 To understand the biological basis for its efficacy, rodent models of cerebral ischemia–reperfusion and middle cerebral artery occlusion are used widely. Histological and behavioral examinations demonstrated that EA manipulation protected neural tissue from injury and improved motor impairment and cognitive function.Citation77–Citation81 Studies at cellular and molecular levels showed that EAS regulated the expression of apoptosis-related genes, such as BCL2 and BAX,Citation77,Citation82 inhibited the production of HMGB1 production,Citation83,Citation84 known to induce inflammation by activating TLR4 and RAGE,Citation85 and downregulated mRNA and protein levels of MMP2, Aqp4, and Aqp9.Citation86,Citation87 EAS also activates several signaling molecules and related pathways, including stimulation of STAT3Citation88 and PI3K,Citation89 and upregulates levels of glutamate receptor GluR2 for neuroprotection.Citation90 Finally, EA affects the activation of microglial cells and astrocytes and the production of astroglial lactate transporter (MCT1), implying that EA may exert its protective function by acting on glial cells and neurons in the central nervous system.Citation78,Citation80,Citation81,Citation91
Despite numerous reports on pathologic responses by EA, mechanistic studies underlying acupuncture efficacy on cerebral ischemia have been limited. Chavez et al describedCitation76 on a mechanistic basis a list of possible beneficial effectors of acupuncture (both EA and MA) in brain areas after ischemic stroke. In an experimental stroke model, EAS has been shown to attenuate cerebral ischemia by activating α7-nicotinic acetylcholine receptors in penumbra and also vagal motor neurons.Citation92 However, it is unclear how EA-modulated vagal activity is linked to cholinergic nerve activity in ischemic cerebral tissue and leads to pathological responsiveness in target tissue. Further studies demonstrating the protective effects of EAS on brain and spinal tissue from ischemic damage are critical to gain insights into the mechanistic basis of anti-inflammatory mechanisms of EA.
Perspectives: neural coding of acupuncture signals
All sensory information in humans and animals is received by specific receptors, and the nerve signals generated are transmitted to and perceived by the brain. Modality, location, intensity, and duration are basic features of sensory stimulation at the periphery, and act as basic elements to transduce unique signals to sensory neurons in the brain.Citation93 Here, the initial stimulation requires the process of neural coding so that the sensory signals can be interpreted through brain circuitry.Citation94
Would cutaneous acupuncture stimulation generate its own specific neural responses? Neuroimaging studies on acupuncture indicate the possible existence of neural correlates of acupuncture.Citation95 Receptive fields of sensory neurons, important to determine perception sensitivity to a stimulus, are affected by receptor density at the stimulation area. We have recently found that α6/β1 integrin receptors are highly expressed at the zusanli acupoint after acupuncture stimulation.Citation35 Since integrin activity plays an important role in mediating intercellular signaling,Citation96 it will be interesting to explore the distribution and activation of specific types of integrin receptors responding after stimulation at other acupoints. Moreover, it will be of great importance to investigate whether EA manipulation at the acupoint can generate a unique sensory modality, eg, deqi is interpreted as a special sensation distinct from typical somatosensation, which is shared between acupuncturist and patient. EAS induces afferent signals, and may directly affect visceral autonomic nerve activity. Alternatively, it is transmitted to the cerebrum and generates brain acupuncture signals (). We speculate that acupuncture stimulation may trigger the responsiveness of sensory receptors and generate neural activity in its own specific way, which may be encoded in the cerebral cortex and autonomic neuronal center and exert its effects on regulating inflammation. Future investigations to explore whether acupuncture-specific vagal activity exists and acts on cells in target organs will be of great importance to gain insights into the mechanistic basis of acupuncture.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C0096).
Disclosure
The authors report no conflicts of interests in this work.
References
- ZhuWYeYLiuYMechanisms of acupuncture therapy for cerebral ischemia: an evidence-based review of clinical and animal studies on cerebral ischemiaJ Neuroimmune Pharmacol201712457559228527041
- ShiPSunLLLeeYSTuYElectroacupuncture regulates the stress-injury-repair chain of events after cerebral ischemia/reperfusion injuryNeural Regen Res201712692593028761425
- ParkJYKimYKKimSYAcupuncture modulates brain neural activity in patients: a systematic review and meta-analysisOrient Pharm Exp Med2017172111126
- World Health OrganizationWHO International Standard Terminologies on Traditional Medicine in the Western Pacific RegionGenevaWHO2007
- StuxGPomeranzBAcupuncture: Textbook and AtlasHeidelbergSpringer2012
- MaWLiZLuZProtective effects of acupuncture in cardiopulmonary bypass-induced lung injury in ratsInflammation20174041275128428493083
- KongJGollubRHuangTAcupuncture de qi, from qualitative history to quantitative measurementJ Altern Complement Med200713101059107018166116
- KimDHHuangdi’s Internal ClassicSeoulEuiseongdang2002
- WhitePBishopFHardyHSouthampton needle sensation questionnaire: development and validation of a measure to gauge acupuncture needle sensationJ Altern Complement Med200814437337918576920
- ParkHParkJLeeHLeeHDoes deqi (needle sensation) exist?Am J Chin Med2002301455012067096
- KongJFufaDTGerberAJPsychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal painJ Pain200561556415629419
- MacPhersonHAsgharAAcupuncture needle sensations associated with de qi: a classification based on experts’ ratingsJ Altern Complement Med200612763363716970533
- JinHGuoJLiuJAnti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitisAm J Physiol Gastrointest Liver Physiol20173133G192G20228546285
- ZhangRLaoLRenKBermanBMMechanisms of acupuncture-electroacupuncture on persistent painAnesthesiology20131202482503
- LinJGChenWLAcupuncture analgesia: a review of its mechanisms of actionsAm J Chin Med200836463564518711761
- TakeuchiOAkiraSPattern recognition receptors and inflammationCell2010140680582020303872
- BorovikovaLVIvanovaSZhangMVagus nerve stimulation attenuates the systemic inflammatory response to endotoxinNature2000405678545846210839541
- WangHYuMOchaniMNicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammationNature2003421692138438812508119
- de JongeWJvan der ZandenEPBijlsmaMFStimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathwayNat Immunol20056884485116025117
- WangHLiaoHOchaniMCholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsisNat Med200410111216122115502843
- TraceyKJThe inflammatory reflexNature2002420691785385912490958
- Rosas-BallinaMOchaniMParrishWRSplenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemiaProc Natl Acad Sci U S A200810531110081101318669662
- Rosas-BallinaMOlofssonPSOchaniMAcetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuitScience201133460529810121921156
- ChiuIMvon HehnCAWoolfCJNeurogenic inflammation and the peripheral nervous system in host defense and immunopathologyNat Neurosci20121581063106722837035
- GhiaJEBlennerhassettPCollinsSMImpaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depressionJ Clin Invest200811862209221818451995
- BockxIVerdrenghKVander ElstIHigh-frequency vagus nerve stimulation improves portal hypertension in cirrhotic ratsGut201261460461222187073
- PereiraMRLeitePEThe Involvement of parasympathetic and sympathetic nerve in the inflammatory reflexJ Cell Physiol201623191862186926754950
- KooALiangIMicrovascular filling pattern in rat liver sinusoids during vagal stimulationJ Physiol19792951191199521923
- KooALiangIParasympathetic cholinergic vasodilator mechanism in the terminal liver microcirculation in ratsExp Physiol1979643149159
- BellonoNWBayrerJRLeitchDBEnterochromaffin cells are gut chemosensors that couple to sensory neural pathwaysCell20171701185198.e1628648659
- MartelliDMcKinleyMJMcAllenRMThe cholinergic anti-inflammatory pathway: a critical reviewAuton Neurosci2014182656924411268
- MartelliDYaoSTMcKinleyMJMcAllenRMReflex control of inflammation by sympathetic nerves, not the vagusJ Physiol201459271677168624421357
- WongCHJenneCNLeeWYLegerCKubesPFunctional innervation of hepatic iNKT cells is immunosuppressive following strokeScience2011334605210110521921158
- LangevinHMChurchillDLCipollaMJMechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupunctureFASEB J200115122275228211641255
- ShinHCParkJYLimHDNamgungUInduction of α6 and β1 integrins by acupuncture stimulation in ratsBiochem Biophys Res Commun2017491362963528760342
- LuxGHagelJBäckerPAcupuncture inhibits vagal gastric acid secretion stimulated by sham feeding in healthy subjectsGut1994358102610297926899
- LiuZLiuYXuHEffect of electroacupuncture on urinary leakage among women with stress urinary incontinence: a randomized clinical trialJAMA2017317242493250128655016
- WuXKStener-VictorinEKuangHYEffect of acupuncture and clomiphene in Chinese women with polycystic ovary syndrome: a randomized clinical trialJAMA2017317242502251428655015
- LimHDKimMHLeeCYNamgungUAnti-inflammatory effects of acupuncture stimulation via the vagus nervePloS One2016113e015188226991319
- da SilvaMDBobinskiFSatoKLKolkerSJSlukaKASantosARIL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle painMol Neurobiol2015511193124961568
- SonYSParkHJKwonOBJungSCShinHCLimSAntipyretic effects of acupuncture on the lipopolysaccharide-induced fever and expression of interleukin-6 and interleukin-1β mRNAs in the hypothalamus of ratsNeurosci Lett20023191454811814650
- CeccherelliFGagliardiGMatterazzoGVisentinRGironGThe role of manual acupuncture and morphine administration on the modulation of capsaicin-induced edema in rat paw: a blind controlled studyAcupunct Electrother Res19962117148791904
- WangKZhangRXiangXDifferences in neural-immune gene expression response in rat spinal dorsal horn correlates with variations in electroacupuncture analgesiaPLoS One201278e4233122879942
- LuKWHsuCKHsiehCLYangJLinYWProbing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36-ST37 acupoints on CFA-induced inflammatory painSci Rep201662212326906464
- LiaoHYHsiehCLHuangCPLinYWElectroacupuncture attenuates CFA-induced inflammatory pain by suppressing Nav1.8 through S100B, TRPV1, opioid, and adenosine pathways in miceSci Rep201774253128211895
- LiALaoLWangYElectroacupuncture activates corticotrophin-releasing hormone-containing neurons in the paraventricular nucleus of the hypothalamus to alleviate edema in a rat model of inflammationBMC Complement Altern Med200882018474100
- BaekYHChoiDYYangHIParkDSAnalgesic effect of electroacu-puncture on inflammatory pain in the rat model of collagen-induced arthritis: mediation by cholinergic and serotonergic receptorsBrain Res200510571–218118516139820
- LiJLiJChenRCaiGTargeting NF-κB and TNF-α activation by electroacupuncture to suppress collagen-induced rheumatoid arthritis in model ratsAltern Ther Health Med20152142634
- TianLHuangYXTianMGaoWChangQDownregulation of electroacupuncture at ST36 on TNF-αin rats with ulcerative colitisWorld J Gastroenterol2003951028103312717850
- GondimDVCostaJLRochaSSBritoGARibeiroRAValeMLAntinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular jointCan J Physiol Pharmacol201290439540522443108
- LiuFFangJShaoXLiangYWuYJinYElectroacupuncture exerts an anti-inflammatory effect in a rat tissue chamber model of inflammation via suppression of NF-κB activationAcupunct Med201432434034524820260
- ZhangDSongXJLiSYEvaluation of liver function and electroacupuncture efficacy of animals with alcoholic liver injury by the novel imaging methodsSci Rep201663011927443832
- SongJGLiHHCaoYFElectroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous systemAnesthesiology2012116240641422222470
- GoldmanNChenMFujitaTAdenosine A1 receptors mediate local anti-nociceptive effects of acupunctureNat Neurosci201013788388820512135
- TuWZChengRDChengBAnalgesic effect of electroacu-puncture on chronic neuropathic pain mediated by P2X3 receptors in rat dorsal root ganglion neuronsNeurochem Int201260437938622269805
- JiRRZhangZWZhouYZhangQHanJSInduction of c-Fos expression in the rostral medulla of rats following electroacupuncture stimulationInt J Neurosci1993723–41831918138374
- LiuJHLiJYanJExpression of c-Fos in the nucleus of the solitary tract following electroacupuncture at facial acupoints and gastric distension in ratsNeurosci Lett2004366221521915276250
- KimSKKimJWooHSElectroacupuncture induces Fos expression in the nucleus tractus solitarius via cholecystokinin A receptor signaling in ratsNeurol Res201032Suppl 111611920034459
- TakahashiTAcupuncture for functional gastrointestinal disordersJ Gastroenterol200641540841716799881
- YinJChenJDGastrointestinal motility disorders and acupunctureAuton Neurosci20101571313720363196
- OuyangHYinJWangZPasrichaPJChenJDElectroacupuncture accelerates gastric emptying in association with changes in vagal activityAm J Physiol Gastrointest Liver Physiol20022822G390G39611804862
- SakaiSHoriEUmenoKKitabayashiNOnoTNishijoHSpecific acupuncture sensation correlates with EEGs and autonomic changes in human subjectsAuton Neurosci2007133215816917321222
- LonghurstJCTjen-A-LooiSAcupuncture regulation of blood pressure: two decades of researchInt Rev Neurobiol201311125727124215927
- ZhangWTJinZCuiGHRelations between brain network activation and analgesic effect induced by low vs. high frequency electrical acupoint stimulation in different subjects: a functional magnetic resonance imaging studyBrain Res2003982216817812915252
- NapadowVMakrisNLiuJKettnerNWKwongKKHuiKKEffects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRIHum Brain Mapp200524319320515499576
- FangJFFangJQShaoXMElectroacupuncture treatment partly promotes the recovery time of postoperative ileus by activating the vagus nerve but not regulating local inflammationSci Rep201773980128051128
- HuZChenBTongJHuangDLuSThe change of C-Fos expression in ovariectomized rats following electroacupuncture treatment: an immunohistochemistry studyAcupunct Electrother Res19931821171247902641
- Torres-RosasRYehiaGPeñaGDopamine mediates vagal modulation of the immune system by electroacupunctureNat Med201420329129524562381
- ZhangJYongYLiXVagal modulation of high mobility group box-1 protein mediates electroacupuncture-induced cardioprotection in ischemia-reperfusion injurySci Rep201551550326499847
- ChenJSongGQYinJKoothanTChenJDElectroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogsAm J Physiol Gastrointest Liver Physiol20082953G614G62018653722
- YuZZhangNLuCXElectroacupuncture at ST25 inhibits jejunal motility: role of sympathetic pathways and TRPV1World J Gastroenterol20162251834184326855542
- BenrickAKokosarMHuMAutonomic nervous system activation mediates the increase in whole-body glucose uptake in response to electroacupunctureFASEB J20173183288329728404742
- Tjen-A-LooiSCGuoZLFuLWLonghurstJCParaventricular nucleus modulates excitatory cardiovascular reflexes during elec-troacupunctureSci Rep201662591027181844
- KimHWKangSYYoonSYLow-frequency electroacupuncture suppresses zymosan-induced peripheral inflammation via activation of sympathetic post-ganglionic neuronsBrain Res20071148697517367766
- KimHWUhDKYoonSYLow-frequency electroacupuncture suppresses carrageenan-induced paw inflammation in mice via sympathetic post-ganglionic neurons, while high-frequency EA suppression is mediated by the sympathoadrenal medullary axisBrain Res Bull200875569870518355649
- ChavezLMHuangSSMacDonaldILinJGLeeYCChenYHMechanisms of acupuncture therapy in ischemic stroke rehabilitation: a literature review of basic studiesInt J Mol Sci20171811E227029143805
- LiuFJiangYJZhaoHJYaoLQChenLDElectroacupuncture ameliorates cognitive impairment and regulates the expression of apoptosis-related genes Bcl-2 and Bax in rats with cerebral ischaemia-reperfusion injuryAcupunct Med201533647848426376847
- LiuWWangXYangSElectroacupuncture improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic strokeLife Sci201615131332226979777
- YuanSZhangXBoYLiWZhangHJiangQThe effects of electroacupuncture treatment on the postoperative cognitive function in aged rats with acute myocardial ischemia-reperfusionBrain Res20141593192925446007
- TaoJZhengYLiuWElectro-acupuncture at LI11 and ST36 acupoints exerts neuroprotective effects via reactive astrocyte proliferation after ischemia and reperfusion injury in ratsBrain Res Bull2016120142426524137
- ChenYZhouJLiJElectroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia–reperfusion via inhibition of microglial activation and attenuation of oxidative stress in ratsBrain Res20121432364522129788
- ChuangCHHsuYCWangCCHuCKuoJRCerebral blood flow and apoptosis-associated factor with electroacupuncture in a traumatic brain injury rat modelAcupunct Med201331439540324055977
- ZhuXLChenXWangWElectroacupuncture pretreatment attenuates spinal cord ischemia-reperfusion injury via inhibition of high-mobility group box 1 production in a LXA4 receptor-dependent mannerBrain Res2017165911312028089662
- WangQWangFLiXElectroacupuncture pretreatment attenuates cerebral ischemic injury through α7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in ratsJ Neuroinflammation201292422277256
- SchmidtAMYanSDYanSFSternDMThe multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responsesJ Clin Invest2001108794995511581294
- XuHZhangYSunHChenSWangFEffects of acupuncture at GV20 and ST36 on the expression of matrix metalloproteinase 2, aquaporin 4, and aquaporin 9 in rats subjected to cerebral ischemia/reperfusion injuryPLoS One201495e9748824828425
- LvEDengJYuYNrf2-ARE signals mediated the anti-oxidative action of electroacupuncture in an MPTP mouse model of Parkinson’s diseaseFree Radic Res201549111296130726118717
- ZhouHZhangZWeiHActivation of STAT3 is involved in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptors in ratsBrain Res2013152915416423880371
- SunNZouXShiJLiuXLiLZhaoLElectroacupuncture regulates NMDA receptor NR1 subunit expression via PI3-K pathway in a rat model of cerebral ischemia-reperfusionBrain Res200510641–29810716289403
- LiuZChenXGaoYInvolvement of GluR2 up-regulation in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptor in miceSci Rep20155949025830356
- LuYZhaoHWangYElectro-acupuncture up-regulates astrocytic MCT1 expression to improve neurological deficit in middle cerebral artery occlusion ratsLife Sci2015134687226037401
- ChiLDuKLiuDBoYLiWElectroacupuncture brain protection during ischemic stroke: a role for the parasympathetic nervous systemJ Cereb Blood Flow Metab201838347949128281385
- GardnerEPJohnsonKOPrinciples of Neural Science5th edNew YorkMcGraw-Hill2013
- JohnsonKONeural codingNeuron200026356356610896153
- DhondRPKettnerNNapadowVDo the neural correlates of acupuncture and placebo effects differ?Pain20071281–281217267130
- HynesROIntegrins: bidirectional, allosteric signaling machinesCell2002110667368712297042
- GengYChenDZhouJSynergistic effects of electroacupuncture and mesenchymal stem cells on intestinal ischemia/reperfusion injury in ratsInflammation20163941414142027221138
- FangBQinMLiYElectroacupuncture preconditioning and postconditioning inhibit apoptosis and neuroinflammation induced by spinal cord ischemia reperfusion injury through enhancing autophagy in ratsNeurosci Lett201764213614128188848
- HoTYLoHYChaoDCElectroacupuncture improves trini-trobenzene sulfonic acid-induced colitis, evaluated by transcriptomic studyEvid Based Complement Alternat Med2014201494219624995035
- SongXMWuXJLiJGThe effect of electroacupuncture at ST36 on severe thermal injury-induced remote acute lung injury in ratsBurns20154171449145826188895
- WangKJuZYongYChenTSongJZhouJThe effects of electroacupuncture on the apelin/APJ system in the spinal cord of rats with inflammatory painAnesth Analg201612361603161027632349
- DongZQZhuJLuDZChenQXuYLEffect of electroacupuncture in “zusanli” and “kunlun” acupoints on TLR4 signaling pathway of adjuvant arthritis ratsAm J Ther Epub2016829
- ZhangHHeSHuYZhengHAntagonism of cannabinoid receptor 1 attenuates the anti-inflammatory effects of electroacupuncture in a rodent model of migraineAcupunct Med201634646347027834685
- ChenYLeiYMoLQElectroacupuncture pretreatment with different waveforms prevents brain injury in rats subjected to cecal ligation and puncture via inhibiting microglial activation, and attenuating inflammation, oxidative stress and apoptosisBrain Res Bull201612724825927771396
- LiawJJPeplowPVEffect of electroacupuncture on inflammation in the obese Zucker fatty rat model of metabolic syndromeJ Acupunct Meridian Stud201692737927079228
- WenCKLeeTYElectroacupuncture prevents white adipose tissue inflammation through modulation of hypoxia-inducible factors-1α-dependent pathway in obese miceBMC Complement Altern Med20151545226714835
- ZhuJChenXYLiLBElectroacupuncture attenuates collagen-induced arthritis in rats through vasoactive intestinal peptide signalling-dependent re-establishment of the regulatory T cell/T-helper 17 cell balanceAcupunct Med201533430531125979865
- LisboaMRGondimDVErvolinoEEffects of electroacu-puncture on experimental periodontitis in ratsJ Periodontol201586680181125741581
- HanDLiuZWangGZhangYWuZElectroacupuncture improves cognitive deficits through increasing regional cerebral blood flow and alleviating inflammation in CCI ratsEvid Based Complement Alternat Med20172017517316828491108
- ShenMHZhangCBZhangJHLiPFElectroacupuncture attenuates cerebral ischemia and reperfusion injury in middle cerebral artery occlusion of rat via modulation of apoptosis, inflammation, oxidative stress, and excitotoxicityEvid Based Complement Alternat Med20162016943865027123035
- LiuWWangXZhengYElectroacupuncture inhibits inflammatory injury by targeting the miR-9-mediated NF-κB signaling pathway following ischemic strokeMol Med Rep20161321618162626718002
- LanLTaoJChenAElectroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathwayInt J Mol Med2013311758023165960
