Abstract
Solid tumor development requires angiogenesis and is correlated to the expression of inflammatory markers through cellular metabolic and energetic adaptation. While high glycolysis rates enable the cancer cell compartment to generate adenosine triphosphate (ATP), very little is known about the impact of low intracellular ATP concentrations within the vascular endothelial cell compartment, which is responsible for tumor angiogenesis. Here, we investigated the effect of 2-deoxy-D-glucose (2-DG), a glucose analog that inhibits glycolysis through intracellular ATP depletion, on human brain microvascular endothelial cell (HBMEC) angiogenic properties. While preformed capillaries remained unaffected, we found that in vitro tubulogenesis was dose-dependently decreased by 2-DG and that this correlated with reduced intracellular ATP levels. Procarcinogenic signaling was induced with phorbol 12-myristate 13-acetate (PMA) and found to trigger the proinflammatory marker cyclooxygenase-2 (COX-2) and endoplasmic reticulum (ER) stress marker GRP78 expression, whose inductions were potentiated when PMA was combined with 2-DG treatment. Inversely, PMA-induced matrix-metalloproteinase-9 (MMP-9) gene expression and protein secretion were abrogated in the presence of 2-DG, and this can be partially explained by reduced nuclear factor-κB signaling. Collectively, we provide evidence for an intracellular ATP requirement in order for tubulogenesis to occur, and we link increases in ER stress to inflammation. A better understanding of the metabolic adaptations of the vascular endothelial cells that mediate tumor vascularization will help the development of new drugs and therapies.
Introduction
Tumor-associated angiogenesis, a fundamental process in tumor growth and metastasis, consists of recruiting endothelial cells (EC) toward an angiogenic stimulus.Citation1 The cells subsequently proliferate and differentiate to form endothelial tubes and capillary-like structures in order to deliver nutrients and oxygen to the tumor and to remove the products of its metabolism. In recent years several pathways have, in addition to stimulation of tumor angiogenesis, been suggested to contribute to the cell metabolic adaptations required for carcinogenesis, which include decreased tumoral apoptosis, increased invasion and metastasis, immune suppression, and tumor-associated inflammation.Citation2,Citation3
An interesting link between overexpression of the pro-inflammatory marker cyclooxygenase-2 (COX-2) and tumor angiogenesis was recently described as one such metabolic adaptative phenotype.Citation4–Citation6 This is supported by the fact that in normal cerebral cortex COX-2 is only present in neurons but absent from vascular EC.Citation7,Citation8 It is however still unknown whether the EC-associated COX-2 correlates with high malignancy. Furthermore, little is known about the molecular events that dictate metabolic adaptation of EC in response to procarcinogenic stimuli. It is tempting to suggest that specific inhibition of metabolic pathways may offer a novel therapeutic approach that would simultaneously inhibit tumor-induced angiogenesis and inflammatory phenotypes.Citation9,Citation10
While human brain microvascular endothelial cells (HBMEC) play an essential role as structural and functional components of the blood–brain barrier (BBB), its disruption by the brain tumor-secreted matrix metalloproteinase-9 (MMP-9) is believed to favor tumor invasion.Citation11,Citation12 Recent studies delineated a unique brain endothelial phenotype in which MMP-9 secretion by HBMEC was increased upon treatment with the tumor-promoting agent phorbol 12-myristate 13-acetate (PMA).Citation13,Citation14 Inhibition of MMP-9 secretion was demonstrated to reduce both in vitro invasion and angiogenesis in human microvascular EC.Citation15 In fact, adenoviral-mediated MMP-9 downregulation inhibited human dermal microvascular EC migration in cell wounding and spheroid migration assays, and reduced capillary-like tube formation, demonstrating the key role of MMP-9 in EC network organization.Citation15 Therefore, among all MMP, the MMP-9 secreted from brain EC may be of importance in brain tumor-associated neovascularization.
Among the numerous signaling pathway candidates, nuclear factor-κB (NF-κB) can regulate the expression of COX-2 through endoplasmic reticulum (ER) stress and, in part, through induction of the ER chaperone GRP78/BiP, which is expressed at high levels in a variety of tumors and which confers drug resistance to both proliferating and dormant cancer cells.Citation16 Importantly, it was recently demonstrated that partial reduction of GRP78 substantially reduced tumor microvessel density.Citation17 In the current study, we treated HBMEC with 2-deoxy-D-glucose (2-DG) which, once phosphorylated by hexokinase to 2-deoxyglucose-6-phosphate, cannot be further metabolized and leads to a blockade of glycolysis and to depletion of intracellular adenosine triphosphate (ATP). In vitro tubulogenesis, expression of ER stress marker GRP78, PMA-induced MMP-9 secretion, and COX-2 were also assessed in order to provide a metabolic and adaptative link between endothelial inflammation and angiogenesis.
Materials and methods
Materials
2-Deoxy-D-glucose, sodium dodecylsulfate (SDS) and bovine serum albumin (BSA) were purchased from Sigma- Aldrich Canada (Oakville, Canada). Electrophoresis reagents were purchased from Bio-Rad (Mississauga, Canada). The enhanced chemiluminescence (ECL) reagents were from Perkin Elmer (Waltham, MA). Micro bicinchoninic acid protein assay reagents were from Pierce (Rockford, IL). The polyclonal antibodies against IκB and phospho-IκB were purchased from Cell Signaling (Danvers, MA). The monoclonal antibodies against GRP78 and GAPDH were from Advanced Immunochemical Inc (Long Beach, CA). Horseradish peroxidase-conjugated donkey anti-rabbit and anti-mouse IgG secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). All other reagents were from Sigma-Aldrich Canada.
Cell culture
HBMEC were characterized and generously provided by Dr. Kwang Sik Kim of the Johns Hopkins University School of Medicine (Baltimore, MD). These cells were positive for factor VIII-Rag, carbonic anhydrase IV, and Ulex Europeus Agglutinin I; they took up fluorescently labeled, acetylated low-density lipoprotein and expressed gamma glutamyl trans-peptidase, demonstrating their brain EC-specific phenotype.Citation18 HBMEC were immortalized by transfection with simian virus 40 large T antigen and maintained their morphological and functional characteristics for at least 30 passages.Citation19 HBMEC were maintained in RPMI 1640 (Gibco, Burlington, Canada) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (iFBS) (HyClone Laboratories, Logan, UT), 10% (v/v) NuSerum (BD Bioscience, Mountain View, CA), modified Eagle’s medium nonessential amino acids (1%) and vitamins (1%) (Gibco), sodium pyruvate (1 mM) and EC growth supplement (30 μg/mL). Culture flasks were coated with 0.2% type-I collagen to support the growth of HBMEC monolayers. Cells were cultured at 37°C under a humidified atmosphere containing 5% CO2. All experiments were performed using passages 3 to 28.
Endothelial cell morphogenesis assay
Tubulogenesis was assessed using Matrigel aliquots of 50 μL, plated into individual wells of 96-well tissue culture plates (Costar, Amherst, MA) and allowed to polymerize at 37°C for 30 min. After brief trypsinization, HBMEC were washed and resuspended at a concentration of 106 cells/mL in serum-free medium. Twenty-five microliters of cell suspension (25,000 cells/well) and 75 μL of medium with serum were added into each culture well. Cells were allowed to form capillary-like tubes at 37°C in 5% CO2/95% air for 20 h in the presence or absence of different 2-DG concentrations. The formation of capillary-like structures was examined microscopically and images (100×) were recorded using a Retiga 1300 camera (QImaging, Surrey, Canada) and an Eclipse TE2000-U microscope (Nikon, Melville, NY). The extent to which capillary-like structures formed in the gel was quantified by analysis of digitized images to determine the thread length of the capillary-like network, using a commercially available image analysis program (Northern Eclipse; Empix, Mississauga, Canada) as described and validated previously.Citation20,Citation21 For each experiment, four randomly chosen areas were quantified by counting the number of tubes formed. Tubulogenesis data are expressed as a mean value derived from at least three independent experiments.
Gelatin zymography
Gelatin zymography was used to assess the extent of proMMP-2 and proMMP-9 activity as described previously.Citation22 Briefly, an aliquot (20 μL) of the culture medium was subjected to SDS-PAGE in a gel containing 0.1 mg/mL gelatin. The gels were then incubated in 2.5% Triton X-100 and rinsed in nanopure distilled H2O. Gels were further incubated at 37°C for 20 h in 20 mM NaCl, 5 mM CaCl2, 0.02% Brij-35, 50 mM Tris-HCl buffer, pH 7.6, then stained with 0.1% Coomassie Brilliant blue R-250 and destained in 10% acetic acid, 30% methanol in H2O. Gelatinolytic activity was detected as unstained bands on a blue background.
Immunoblotting procedures
Proteins or nuclear extracts were isolated from control and treated cells using the NE-PER Nuclear and cytoplasmic extraction kit (Pierce) and were separated by SDS–polyacrylamide gel electrophoresis (PAGE). After electrophoresis, proteins were electrotransfered to polyvinylidene difluoride membranes which were then blocked for 1 h at room temperature with 5% nonfat dry milk in Tris-buffered saline (150 mM NaCl, 20 mM Tris–HCl, pH 7.5) containing 0.3% Tween-20 (TBST). Membranes were further washed in TBST and incubated with the primary antibodies (1/1000 dilution) in TBST containing 3% bovine serum albumin, followed by a 1 h incubation with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (1/2,500 dilution) in TBST containing 5% nonfat dry milk. Immunoreactive material was visualized by enhanced chemiluminescence (Amersham Biosciences, Baie d’Urfée, Canada).
Analysis of cell death by flow cytometry
Cell death was assessed by flow cytometry as described previously.Citation23 Adherent and floating cells were harvested by trypsin digestion and gathered to produce a single cell suspension. The cells were pelleted by centrifugation and washed with phosphate-buffered saline (PBS). Then, 105 cells were pelleted, resuspended in 200 μL of buffer solution, and stained with annexin-V-fluorescein isothiocyanate and propidium iodide (PI) according to the manufacturer’s protocol (BD Biosciences, Mississauga, Canada). The cells were diluted by adding 300 μL of buffer solution and processed for data acquisition and analysis on a Becton Dickinson FACS Calibur flow cytometer using CellQuest Pro software.
Analysis of autophagy by fluorescent microscopy and flow cytometry
HBMEC were seeded onto 12-well plates and cultured for 18 h to reach 80% confluence. Cells were then exposed to different concentrations of 2-DG in the presence or absence of PMA (1 μM) in serum-free medium at 37°C in 5% CO2/95% air. Acridine orange was added to the culture medium at a final concentration of 0.5 μg/mL and incubated for 15 min in the dark at 37°C. The cells were then washed two times with medium lacking phenol red. Cells were immediately analyzed by flow cytometry or images taken with a fluorescent microscope using 510–560 nm for excitation and 590 nm for emission.
Intracellular ATP levels assay
Cells were treated and 10X–100X-diluted lysates kept on ice as described by the manufacturer (Sigma). One hundred microliters of ATP mix was added to the lysates and incubated at room temperature for 3 min. Somatic cell ATP-releasing reagent (1X) was prepared and added to the cell lysates. From this solution 100 μL was added to the ATP mix prior to analysis by spectrofluorimetry.
Statistical analysis
Statistical analyses were performed with Student’s t-test when one group was compared with the control group. To compare two or more groups with the control group, one-way analysis of variance (ANOVA) with Dunnett’s post hoc test was used. All statistical analyses were performed using Prism software (GraphPad, La Jolla, CA). Differences with P < 0.05 were considered significant.
Results
2-Deoxy-D-glucose depletes intracellular ATP and inhibits in vitro capillary-like structure formation in HBMEC
We first tested the effects of 2-DG against the angiogenic properties of HBMEC. Cells were seeded on top of Matrigel and left to adhere as described in the Methods section. Upon capillary-like structure formation, we then added 10–100 mM 2-DG or Mannose. No effect was found on the integrity of the preformed structures (). In contrast, when various concentrations of 2-DG were added at the very early time points (ie, 30 min after cell seeding on top of Matrigel), cell structure formation was significantly decreased with a half maximal inhibitory concentration (IC50) of 4.1 mM (). Cell survival was assessed with annexin- V-fluorescein isothiocyanate (apoptosis) and propidium iodide (necrosis) and was not significantly affected by 2-DG (). Mannose did not affect the capacity of the cells to form structures (not shown). We also validated 2-DG’s ability to deplete intracellular ATP levels in HBMEC. While vehicle or 2-DG treatment did not affect total protein content levels (), we found that intracellular ATP levels decreased by ~20% in vehicle-treated cells during the 24 h incubation prior to which tube formation was assessed (, left panel). When cells were treated with 100 mM 2-DG, >60% of intracellular ATP was depleted within the first hour of treatment, and >90% depletion was achieved at 4 h of treatment (, right panel). ATP-dependent inhibition of tubulogenesis was investigated next to find out whether any extracellular matrix (ECM) degrading events were involved in this 2-DG effect.
Figure 1 2-DG (mM) depletes intracellular ATP and inhibits in vitro capillary-like structure formation in HBMEC. In order to assess the impact of ATP requirement for in vitro tubulogenesis, HBMEC were seeded on top of Matrigel as described in the Methods section and left to form structures. A) Capillaries were then treated with 2-DG (mM) or Mannose (mM). B) The impact of 2-DG was also monitored during the formation of the structures, where cells were treated with various concentrations of 2-DG 30 min after seeding of the cells on top of Matrigel, and structure formation monitored after 18 h. Representative phase contrast pictures were taken. C) The extent of three-dimensional capillary-like structure formation (tubulogenesis) and of cell survival was assessed as described in the Methods section. D) Intracellular ATP as well as total protein content were assessed as described in the Methods section in vehicle- or 2-DG-treated cells. The length of the tube network was quantitated using Northern Eclipse software.
Notes: Values are means of two independent experiments (*P < 0.01 versus control alone); bars, ± SD.
Abbreviations: HBMEC human brain microvasular endothelial cell; ATP, adenosine triphosphate; 2-DG, 2 deopy-D-glucose.
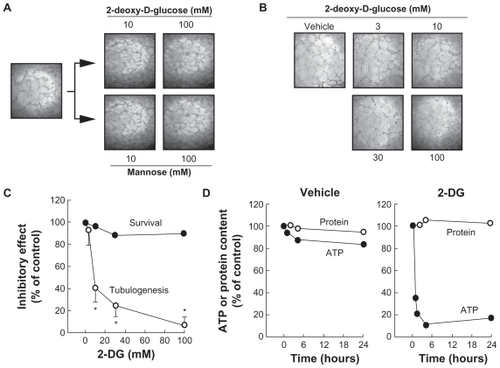
2-Deoxy-D-glucose inhibits PMA-induced MMP-9 secretion in HBMEC
Among the secreted enzymes involved in ECM degradation, matrix metalloproteinases (MMP) are well documented as being involved in cell migration and tubulogenesis.Citation13,Citation24 More specifically, MMP-2 and MMP-9 are secreted by numerous cell types and their presence is often representative of angiogenesis.Citation25,Citation26 HBMEC were serum-starved, treated for 18 h with 2-DG, and the conditioned media were harvested to measure the levels of MMP-2 and of MMP-9 in both control and in PMA-treated cells by gelatin zymography. While MMP-2 extracellular levels were unaffected by 2-DG or by PMA (), MMP-9 levels were undetectable in basal conditions but were significantly increased in PMA-treated cells (, lower panel); the simultaneous presence of 2-DG significantly attenuated the MMP-9 levels seen in the presence of PMA with an IC50 of ~18.1 mM (). Collectively, these results suggest that changes in intracellular ATP levels do not inhibit MMP vesicular trafficking since MMP-2 secretion was unaffected, but do affect signal transducing events such as those triggered by PMA and which control MMP-9 secretion.
Figure 2 2-DG inhibits PMA-induced MMP-9 secretion in HBMEC. HBMEC were serum-starved in the presence of various concentrations of 2-DG in combination with vehicle or 1 μM PMA for 18 h. A) Conditioned media were then harvested and gelatin zymography was performed in order to detect proMMP-9 and proMMP-2 hydrolytic activity as described in the Methods section. B) Scanning densitometry was used to quantify the extent of either basal proMMP-2 gelatin hydrolysis (open circles), or proMMP-9 (closed circles) in PMA-treated cells.
Note: Data shown are representative of two independent experiments.
Abbreviations: 2-DG, 2-dexy-D-glucose; HBMEC, mitovasuila endothelial cells; MMP, matrix metalloproteinase; PMA, phorbol 12-myristate 13-acetate; GA DPH, glyceraldetyde 3- phisiphate dehydrogenase; PAGE, pohyacrylanide electrophoresis.
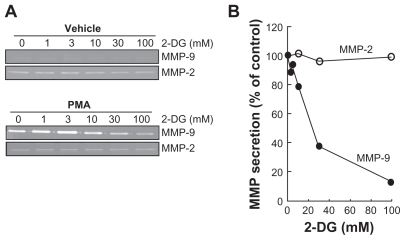
2-Deoxy-D-glucose inhibits PMA-induced IκB phosphorylation and nuclear translocation of the p65 subunit of NF-κB
Among MMP-9 expression regulators, the NF-κB signaling pathway has been suggested.Citation27 We therefore first assessed whether this signaling was activated upon PMA treatment and whether it was reflected in IκB degradation. PMA-mediated phosphorylation of IκB was assessed. HBMEC were treated for 45 min with 1 μM PMA following preincubation with either vehicle or with 100 mM 2-DG. Preincubation with vehicle followed by PMA treatment rapidly led to IκB phosphorylation and to a concomitant decrease in IκB (, upper panels). When cells were preincubated with 2-DG (, lower panels), PMA-mediated IκB phosphorylation was significantly reduced () as quantified by scanning densitometry. Nuclear translocation of the p65 and p50 subunits of NF-κB is among the direct consequences of IκB phosphorylation. When nuclear extracts were isolated, we found that lower p65 nuclear translocation () occurred when cells were treated both with PMA and 2-DG as compared to PMA alone (). This is in agreement with the reduced phosphorylation of IκB observed in .
Figure 3 2-DG inhibits PMA-induced IκB phosphorylation that leads to IκB degradation. A) HBMEC were serum-starved for 30 min in the presence of either vehicle or 100 mM 2-DG. Cells were then incubated for the indicated time with 1 μM PMA. Lysates were isolated, electrophoresed via SDS-PAGE and immunodetection of phosphorylated IκB (P-IκB), IκB, and of GA PDH proteins was performed as described in the Methods section. B) Quantification was performed by scanning densitometry of the autoradiograms. Data were expressed as x-fold induction over basal untreated cells of the P-IκB/IκB ratios in vehicle pre-treated cells (open circles) and 2-DG pre-treated cells (closed circles).
Abbreviations: 2-DG, 2-dexy-D-glucose; HBMEC, mitovasuila endothelial cells; MMP, matrix metalloproteinase; PMA, phorbol 12-myristate 13-acetate; GA DPH, glyceraldetyde 3- phisiphate dehydrogenase; PAGE, pohyacrylanide electrophoresis.
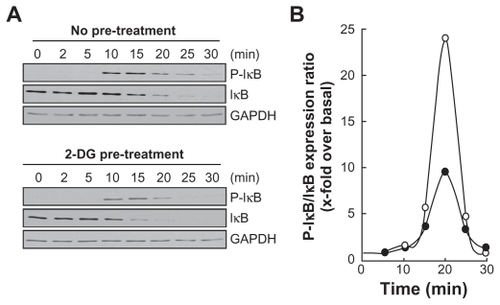
Figure 4 2-DG inhibits PMA-induced nuclear translocation of the p65 subunit of NF-κB. A) HBMEC were serum-starved for 30 min in the presence of either vehicle or 100 mM 2-DG. Cells were then incubated for the indicated time with 1 μM PMA. Nuclear extracts were isolated, electrophoresed via SDS-PAGE and immunodetection of the p65 subunit of NF-κB protein was performed as described in the Methods section. B) Quantification was performed by scanning densitometry of the autoradiograms. Data were expressed as x-fold induction over basal untreated cells of the vehicle pretreated cells (open circles) and 2-DG pre-treated cells (closed circles).
Abbreviations: HBMEC, mitovasuila endothelial cells; PAGE, pohyacrylanide electrophoresis. PMA, phorbol 12-myristate 13-acetate; 2-DG-glucose.
2-Deoxy-D-glucose potentiates PMA-induced cyclooxygenase-2 and GRP78 expression
As PMA is also a well documented inducer of cyclooxygenase- 2 (COX-2),Citation28 we determined whether 2-DG also inhibited a possible NF-κB signaling pathway that leads to COX-2 induction. Cells were first treated with PMA or a combination of PMA/2-DG or PMA/Man (mannose served as an osmotic control). PMA induced COX-2 expression, which was exacerbated in PMA/2-DG-treated cells, which had increased GRP78 expression further (, left panel). These effects were not observed in PMA/Man-treated cells (, right panel). To investigate the dose-dependent effect, cells were incubated with vehicle or PMA in the presence of various 2-DG concentrations for 24 h. No COX-2 expression was observed in the absence of PMA, while a slight basal increase in the ER stress marker GRP78 was noticed with increasing 2-DG treatment (, left panel). As expected, COX-2 expression was significantly induced by PMA (, right panel), and this induction was dose-dependently potentiated by 2-DG (). This 2-DG-mediated potentiation is in contrast to the results seen with MMP-9 and with the reduced NF-κB signaling described above ( and ). Interestingly, we also found that the 2-DG-mediated expression of the ER stress marker GRP78 was potentiated upon PMA treatment (). Altogether, our data suggest ATP depletion affects PMA-mediated signaling independent of NF-κB and results in both decreased MMP-9 levels and in increased COX-2 expression. The latter is possibly a consequence of ER stress induction.
Figure 5 2-DG potentiates PMA-induced cyclooxygenase-2 and GRP78 expression. A) HBMEC were serum starved for 18 h in the presence or absence of 1 μM PMA and in combination with either 30 mM 2-DG or 30 mM Man. Lysates were isolated, electrophoresed via SDS-PAGE, and immunodetection of COX-2, GRP78, and GA PDH was performed as described in the Methods section. B) HBMEC were treated as in (A) with various doses of 2-DG. Lysates were isolated, electrophoresed via SDS-PAGE, and immunodetection of COX-2, GRP78, and GA PDH was performed as described in the Methods section. C) Scanning densitometry of COX-2 expression was only performed in PMA-treated cells since no COX-2 was detectable in vehicle-treated cells. D) Scanning densitometry of GRP78 expression was performed in vehicle- and in PMA-treated cells.
Abbreviations: HBMEC, mitovasuila endothelial cells; PMA, phorbol 12-myristate 13-acetate; 2-DG-glucose; PAGE, pohyacrylanide electrophoresis; GA DPH, glyceraldetyde, 3-phisiphate; man, mannose.
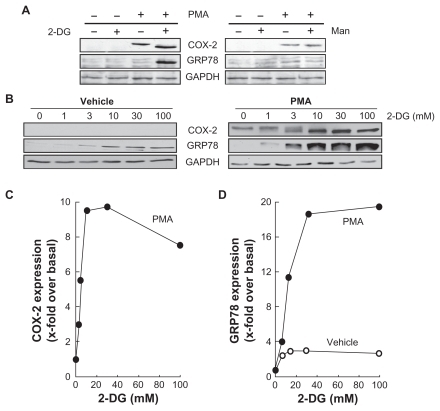
Increased endoplasmic reticulum stress, rather than MMP-mediated EC M hydrolysis, correlates with cyclooxygenase-2 expression in HBMEC
As a consequence of the results above, we suspected a correlative relationship that would direct the molecular events observed following intracellular ATP depletion within HBMEC. Accordingly, we plotted GRP78 vs COX-2 as well as MMP-9 vs COX-2 and confirmed a positive correlation between ER stress and COX-2 expression (). As for MMP-9 and its possible involvement in the ECM degradation required for tubulogenesis to occur, we instead found an inverse correlation when plotted against COX-2 expression (). Collectively, this suggests that intracellular ATP needs are crucial for cell survival signaling through ER stress-mediated events that would lead to a proinflammatory phenotype.
Figure 6 Increased endoplasmic reticulum stress, rather than MMP-mediated EC M hydrolysis, correlates with cyclooxygenase-2 expression in HBMEC. The effects of PMA treatments in the presence of increasing 2-DG concentrations were plotted in order to assess any correlation between A) ER stress (GRP78 expression) and inflammation (COX-2 expression), and B) between EC M hydrolysis (MMP-9 expression) and inflammation.
Abbreviations: EC M,extracellular matrix; MMP, matrix metallo prsteinase; HBMEC, mitovasuila endothelial cells; PMA, phorbol 12-myristate 13-acetate; 2-DG-glucose; ER, endoplasmic reticulam.
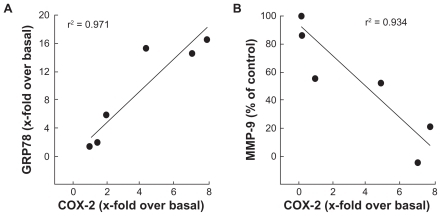
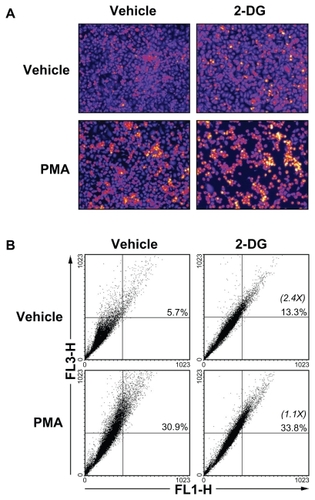
2-Deoxy-D-glucose-induced autophagy is abrogated in PMA-treated HBMEC
Given that 2-DG treatment of the cells did not result in any cytotoxic effect (), we tested the possible implication of autophagy, a crucial component of the cellular stress adaptation response that maintains cell homeostasis and that may act as a survival mechanism that can rescue cells from apoptotic/necrotic death. HBMEC were treated in the presence of 100 mM 2-DG in combination or not with PMA. The acidic autophagic vesicles were visualized by supravital staining with a pH-sensitive dye acridine orange and fluorescent microscopy used to for visualization (). We observed that 2-DG, but more importantly PMA, triggered autophagic vacuoles formation which effect was slightly increased in the presence of combined 2-DG/PMA treatment. When flow cytometry was used to quantify the extent of autophagy, we found that 2-DG induced by 2.4-times autophagy (). PMA treatment alone triggered extensive autophagy, but when combined to 2-DG no increase (1.1-times over basal) in autophagy was observed.
Figure 7 2-DG induced autophagy is abrogated in PMA-treated HBMEC. Autophagy was assessed as described in the Methods section in vehicle, 100 mM 2-DG-, 1 μM PMA-, and 100 mM 2-DG/1 μM PMA-treated HBMEC. A) Representative microphotographs of the formation of acidic vesicular organelles were taken upon acridine orange staining. B) The extent of autophagy was further confirmed by flow-cytometry of acridine orange stained cells and the percent of autophagy indicated for each condition. In between parenthesis, the extent of autophagy induction is indicated.
Abbreviations: HBMEC, mitovasuila endothelial cells; PMA, phorbol 12-myristate 13-acetate; 2-DG-glucose.
Discussion
The adaptive mechanisms responsible for EC survival under pro-carcinogenic and low energy conditions remain poorly documented. Although increasing interest has been manifested towards cancer therapies that target cell metabolism, very few studies have specifically assessed the impact of targeting the EC metabolic compartment. In fact, EC are believed to be metabolically robust and to adapt to pro-carcinogenic paracrine stimulation and conditions such as encountered within the hypoxic tumor microenvironment.Citation29,Citation30 In our study, we induced in vitro procarcinogenic stimulation of brain microvascular endothelium using PMA in combination with ATP-depleting culture conditions in order to assess EC angiogenic and inflammatory responses. While preformed endothelial structures remained unaffected by 2-DG-mediated ATP depletion, we show that minimal supply of energy is required by HBMEC for tubulogenesis processes to occur and that the acquisition of a proinflammatory phenotype better correlates with induction of ER stress than with increased ECM degradation capacities. This is among the first demonstration linking molecular players such as GRP78 (ER stress) and COX-2 (inflammation) expression to the adaptative survival mechanisms of vascular EC’s response to procarcinogenic and ATP-depleting conditions. More importantly, this also in part demonstrates the crucial requirement for ATP in the early time points of tubulogenesis processes, while preformed vessels may adopt metabolic adaptative mechanisms enabling them to resist energy depletion. Alternate strategies, aside from using energy depletion agents, must therefore be envisioned in order to target preformed tumor-associated vessels, while optimization of antiangiogenic therapies may be complemented with ATP-depleting strategies.
EC are the first cells to be exposed to decreases in PO2 within a hypoxic tumor-associated environment. Accordingly, tight regulation of ATP and GTP turnover exists, which enables EC to maintain their high-energy phosphates during hypoxia.Citation31 In EC, ATP is also predominantly generated by glycolysis and O2 consumption is comparatively low.Citation29 Although accelerated glycolysis is one of the biochemical characteristics of cancer cells metabolism, one can safely extrapolate that this metabolic shift alteration, in part consequent to the hypoxic tumor microenvironment, must also occur within the tumor-associated vascular endothelium. Accordingly, it was demonstrated that low energy demand and high glycolytic activity may explain why the coronary endothelium is less severely injured than cardiomyocytes in ischemic and anoxic hearts.Citation29 Targeting glycolysis in the tumor-associated vascular compartment may become an attractive antiangiogenic approach. As such, besides affecting cell death and stress signaling pathways, 2-DG was recently shown to activate autophagy via ER stress rather than ATP depletion in cancer cell lines.Citation32 Autophagy is a crucial component of the cellular stress adaptation response that maintains mammalian homeostasis, a process believed to protect against neurodegenerative and inflammatory conditions, aging, and cancer. Similar to tumor cells defense mechanisms, one can therefore hypothesize that the ER stress consequent to 2-DG treatment in tumor-associated EC may contribute to autophagy and promote cell survival (this study). While protective effects of autophagy were observed in the cancer cell compartment in both laser-induced cell deathCitation33 and in resveratrol-induced cytotoxicity in glioma cells,Citation34 inhibition of autophagy was on the other hand found to potentiate antiangiogenic effects of sulforaphane in an EC model.Citation35 Our study provides evidence supporting the latter assumption whereas 2-DG-mediated inhibition of autophagy was only observed in carcinogen-treated HBMEC (). Deployment of therapeutic strategies to block autophagy for cancer and/or antiangiogenic therapy may therefore be envisioned.
Our study documents the effects of limited intracellular ATP levels not only on the EC angiogenic properties but also on their capacity to express inflammatory markers. We showed that ATP depletion upon procarcinogenic stimulation with PMA exacerbates COX-2 expression in HBMEC, an effect correlated to ER stress-mediated mechanism. It has been established that COX-2 is usually undetectable in normal tissues, but can be induced through several stimuli, including mitogens, growth factors, hormones, and cytokines. Citation36 In recent years, many molecular pathways have been proposed to explain how increased COX-2, and the resulting prostaglandin over-production, may contribute to carcinogenesis.Citation37 COX-2 mediates this role through the specific production of PGE2 that acts to inhibit apoptosis, promote cell proliferation, stimulate angiogenesis, and decrease immunity.Citation38 To date, only few reports document an association between COX-2 expression and survival in EC consequent to tissue injury. In fact, it is believed that sepsis, consequent to the release of the endotoxin lipopolysaccharide (LPS) by Gram-negative bacteria, is regulated through induction of COX-2 expression. One can therefore envision a close parallel between proinflammatory molecular mechanisms involved in endotoxin-induced brain tissue injury and inflammation associated to brain tumor development. Interestingly, LPS-induced COX-2 expression was recently reported in bEnd.3 mouse brain endothelial cells.Citation39 This study actually provides strong support to our study as the contribution of NF-κB (p65) activation was reported to also regulate COX-2.Citation39 Noteworthy, LPS-induced adenosine was demonstrated to promote angiogenesis by the upregulation of VEGF expression in macrophages.Citation40 Whether such a conceptual model impacts on the link between proinflammatory COX-2 and increased tumor angiogenesis remains to be further investigated.
On the other hand, moderate activity of the ER stress response system exerts an antiapoptotic function and supports tumor cell survival and chemoresistance, whereas more severe aggravation may exceed the protective capacity of this system and turn on its proapoptotic module.Citation38 In our study we demonstrate in vitro, through the combination of two pharmacologic approaches, that increased COX-2 expression only occurs within those EC which possess low intracellular ATP levels and which are cultured under procarcinogenic conditions. 2-DG-mediated ATP depletion combined with PMA-induced ER stress synergize and inhibit autophagy potential of PMA-treated cells, an effect which ultimately impacts on EC angiogenic properties (ie, low tubulogenesis and diminished MMP-9 secretion). Similar cytotoxic outcome was recently documented in human breast cancer cells where the HIV protease inhibitor nelfinavir (Viracept; Agouron Pharmaceuticals Inc., La Jolla, CA) and the COX-2 inhibitor celecoxib (Celebrex; Pfizer, New York, NY) were combined and produced aggravated ER stress, which caused pronounced toxicity.Citation38
In summary, the present study has demonstrated that minimal ATP is required for EC-mediated tubulogenesis to occur. Moreover, we provide evidence that combined ATP depletion and procarcinogenic signaling trigger exacerbated ER stress that leads to the acquisition of a proinflammatory phenotype as reflected by increased COX-2 induction. Altogether, the combined output of those mechanisms may further impact on the cell survival adaptative response and trigger antiangiogenic effects within the tumor-associated vascular endothelium.
Acknowledgments
BA holds a Canada Research Chair in Molecular Oncology from the Canadian Institutes of Health Research. ET is a Fonds de Recherche sur la Nature et les Technologies (FQRNT) awardee. This study was funded by a grant from the Natural Sciences and Engineering Research Council of Canada to BA.
Disclosure
The authors report no conflicts of interest in this work.
References
- HanahanDFolkmanJPatterns and emerging mechanisms of the angiogenic switch during tumorigenesisCell1996863533648756718
- RojasAFigueroaHMoralesEFueling inflammation at tumor microenvironment: the role of multiligand/RAGE axisCarcinogenesis20103133434120028726
- HayesACancer, cyclo-oxygenase and nonsteroidal anti-inflammatory drugs – can we combine all threeVet Comp Oncol2007511319754797
- CostaCSoaresRReis-FilhoJSCyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancerJ Clin Pathol20025542943412037025
- FournierLSNovikovVLucidiVMR monitoring of cyclooxygenase-2 inhibition of angiogenesis in a human breast cancer model in ratsRadiology200724310511117329684
- Müller-DeckerKFürstenbergerGThe cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesisMol Carcinog20074670571017546626
- BrederCDSaperCBExpression of inducible cyclooxygenase mRNA in the mouse brain after systemic administration of bacterial lipopolysaccharideBrain Res199671364698724976
- YamagataKAndreassonKIKaufmannWEExpression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoidsNeuron1993113713868352945
- CascanteMBorosLGComin-AnduixBMetabolic control analysis in drug discovery and diseaseNat Biotechnol20022024324911875424
- BorosLGCascanteMLeeWNMetabolic profiling of cell growth and death in cancer: applications in drug discoveryDrug Discov Today2002736437211893545
- LakkaSSGondiCSRaoJSProteases and glioma angiogenesisBrain Pathol20051532734116389945
- BonoiuAMahajanSDYeLMMP-9 gene silencing by a quantum dot-siRNA nanoplex delivery to maintain the integrity of the blood brain barrierBrain Res2009128214215519477169
- AnnabiBRojas-SutterlinSLarocheMThe diet-derived sulforaphane inhibits matrix metalloproteinase-9-activated human brain microvascular endothelial cell migration and tubulogenesisMol Nutr Food Res20085269270018435488
- RoomiMWMonterreyJCKalinovskyTDistinct patterns of matrix metalloproteinase-2 and -9 expression in normal human cell linesOncol Rep20092182182619212645
- JadhavUChigurupatiSLakkaSSInhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cellsInt J Oncol2004251407141415492832
- VerfaillieTGargADAgostinisPTargeting ER stress induced apoptosis and inflammation in cancerCancer Lett2010 10.1016/j.canlet.2010.07.016.In press
- DongDNiMLiJCritical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor developmentCancer Res20086849850518199545
- StinsMFGillesFKimKSSelective expression of adhesion molecules on human brain microvascular endothelial cellsJ Neuroimmunol19977681909184636
- GreiffenbergLGoebelWKimKSInteraction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cellsInfect Immun199866526052679784531
- McLaughlinNAnnabiBSik KimKThe response to brain tumor-derived growth factors is altered in radioresistant human brain endothelial cellsCancer Biol Ther200651539154517102591
- LamySGingrasDBéliveauRGreen tea catechins inhibit vascular endothelial growth factor receptor phosphorylationCancer Res20026238138511809684
- SinaALord-DufourSAnnabiBCell-based evidence for aminopeptidase N/CD13 inhibitor actinonin targeting of MT1-MMP-mediated proMMP-2 activationCancer Lett200927917117619264392
- CurrieJCFortierSSinaAMT1-MMP down-regulates the glucose 6-phosphate transporter expression in marrow stromal cells: a molecular link between pro-MMP-2 activation, chemotaxis, and cell survivalJ Biol Chem20072828142814917229722
- AbécassisIOlofssonBSchmidMRhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasionExp Cell Res200329136337614644158
- HandsleyMMEdwardsDRMetalloproteinases and their inhibitors in tumor angiogenesisInt J Cancer200511584986015729716
- NoëlAJostMMaquoiEMatrix metalloproteinases at cancer tumor-host interfaceSemin Cell Dev Biol200819526017625931
- DongJJimiEZeissCConstitutively active NF-kappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory diseaseGenes Dev2010241709171720713516
- TahanianELord-DufourSDasAInhibition of tubulogenesis and of carcinogen-mediated signaling in brain endothelial cells highlight the antiangiogenic properties of a mumbaistatin analogChem Biol Drug Des20107548148820486934
- MertensSNollTSpahrREnergetic response of coronary endothelial cells to hypoxiaAm J Physiol1990258H6896942316683
- ShryockJCRubioRBerneRMRelease of adenosine from pig aortic endothelial cells during hypoxia and metabolic inhibitionAm J Physiol1988254H2232293344813
- TretyakovAVFarberHWEndothelial cell tolerance to hypoxiaJ Clin Invest1995957387447860755
- XiHKurtogluMLiuH2-Deoxy-D: -glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletionCancer Chemother Pharmacol2010
- LiJQinZLiangZThe prosurvival role of autophagy in Resveratrol-induced cytotoxicity in human U251 glioma cellsBMC Cancer2009921519566920
- KrmpotAJJanjetovicKDMisirkicMSProtective effect of autophagy in laser-induced glioma cell death in vitroLasers Surg Med20104233834720432283
- NishikawaTTsunoNHOkajiYThe inhibition of autophagy potentiates anti-angiogenic effects of sulforaphane by inducing apoptosisAngiogenesis20101322723820694744
- DuboisRNAbramsonSBCroffordLCyclooxygenase in biology and diseaseFASEB J199812106310739737710
- AntonacopoulouAGTsamandasACPetsasTEGFR, HER-2 and COX-2 levels in colorectal cancerHistopathology20085369870619102009
- ChoHYThomasSGoldenEBEnhanced killing of chemo-resistant breast cancer cells via controlled aggravation of ER stressCancer Lett2009282879719345476
- ShihRHYangCMInduction of heme oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2 expression in mouse brain endothelial cellsJ Neuroinflammation201078621118574
- HaraYKurodaNInoueKUp-regulation of vascular endothelial growth factor expression by adenosine through adenosine A2 receptors in the rat tongue treated with endotoxinArch Oral Biol20095493299419712927