Abstract
Recurrent tonsillitis is described as when an individual suffers from several attacks of tonsillitis per year. Chronic and recurrent tonsillitis both cause repeated occurrences of inflamed tonsils which have a significant impact on a patient’s quality of life. Numerous children suffer from recurrent tonsillitis and sore throats, and these illnesses become part of their life. Antimicrobials can provide temporary relief, but in many cases, tonsillitis recurs. The cause of such recurrent infections have been identified as microorganisms which often create biofilms and a repository of infection in the wet and warm folds of the tonsils. This review discusses different treatment modalities, their advantages and disadvantages, and new treatment options focusing on biofilms. All treatment options should be selected based on evidence and individual need.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Tonsillitis
Tonsillitis is an inflammation of the pharyngeal tonsils. The inflammation may affect other areas of the back of the throat, including the adenoids and the lingual tonsils. Acute tonsillitis is an infection of the tonsils triggered by one of the several types of bacteria or viruses, and peritonsillar abscesses can also occur. Chronic tonsillitis is a tenacious infection of the tonsils which may result in tonsil stones. Recurrent tonsillitis ensues when an individual suffers from several incidents of tonsillitis per year. Both chronic and recurrent tonsillitis involve repeated occurrences of inflamed tonsils which can impact severely on a patient’s quality of life.Citation1,Citation2 Children very often suffer from tonsillitis, although it is seldom observed below the age of 2 years. Tonsillitis due to Streptococcus bacteria classically happens in children aged between 5 and 15 years, while viral tonsillitis is more prevalent in younger children.Citation3 Multiple studies report that the average prevalence of carrier status of school children for group A Streptococcus is 15.9%.Citation4,Citation5
Epidemiology of tonsillitis
Numerous children so often suffer from recurrent tonsillitis and sore throats that these illnesses become part of their life. For example, one study indicates that ~30% of peritonsillar abscesses require a tonsillectomy,Citation6 and another indicates that recurrent tonsillitis is reported in 11.7% and 12.1% of Norwegian and Turkish children, respectively.Citation7 Many of these patients are prescribed antimicrobials which typically provide temporary relief, but then the tonsillitis recurs.Citation8 Scientists working at Washington University School of Medicine identified that recurrent infections are exacerbated by the creation of biofilms by microorganisms in the wet and warm folds of the tonsils which act as a repository of infection.Citation9 A study utilizing an innovative imagining technique in single sections of human mucosal tissue reports the presence of biofilms in 70.8% of chronic tonsillitis patients.Citation10 Another study revealed that biofilms were recognized on the surface epithelium of tonsils and adenoids in many of the patients who were waiting for adenotonsillectomy due to chronic tonsillitis and adenoiditis.Citation11 Such biofilms are also observed in other otorhinolaryngology-related infections such as chronic rhinosinusitis and chronic otitis media with effusion.Citation12,Citation13
A brief overview of biofilms
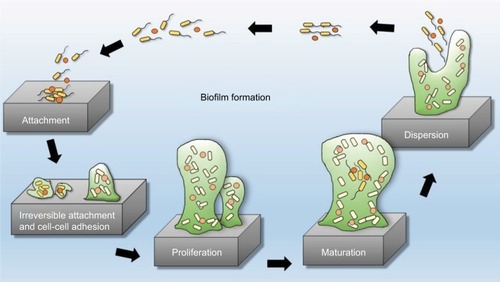
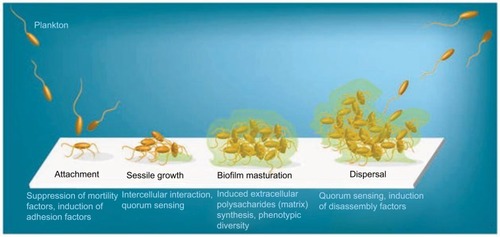
Biofilms are systematized communities of microorganisms embedded in a hydrated matrix of extracellular polymeric substances (EPSs) causing diverse persistent infections, including dental plaques, cystic fibrosis, urinary tract infections, osteomyelitis, and ear infections.Citation9,Citation14,Citation15 Biofilm formation is a prehistoric prokaryotic strategy of a microorganism to exist and grow in antagonistic settings through building innovative communities involving several processes.Citation16–Citation19 The Dutch scientist (commonly known as the Father of Microbiology) Antonie van Leeuwenhoek used his primitive but effective microscope to observe biofilms as early as 1674 and described aggregates of animalcules scraped from human tooth surfaces.Citation20,Citation21 The English phrase “survival of the fittest” arose from Darwinian evolutionary theory and describes one of the mechanisms of natural selection.Citation22,Citation23 Bacterial biofilm formation is a form of “survival of the fittest” under adverse conditions including chemical or antimicrobial treatment.Citation24,Citation25 The formation of biofilms by bacteria has three potential advantages: 1) “protection from harmful conditions in the host”, 2) “sequestration to a nutrient-rich area”, and 3) “utilization of cooperative benefits”.Citation26 Microbial biofilms were identified as a major cause of many human infections, and present in more than 65%–80% of all human bacterial infections.Citation14,Citation27–Citation30 They pose “a serious problem for public health because of the increased resistance of biofilm-associated organisms to antimicrobial agents and the potential for these organisms to cause infections in patients with indwelling medical devices”.Citation31 Biofilm formation is generally considered to arise in four core stages: 1) attachment of bacteria to a surface, 2) microcolony formation, 3) biofilm maturation, and 4) detachment (also called dispersal) of bacteria which may then colonize new areas.Citation32 Other research studies reports that the process of biofilm formation involves five stages:Citation33–Citation35 1) Microbial cells attach to surfaces reversibly.Citation36 2) Microbial cells then attach to surfaces irreversibly.Citation37 3) Cells get adsorbed on surfaces and grow into microcolonies; their physical dimensions are tens or hundreds of microns in diameter.Citation38 4) The microbial fraternity grows into a three-dimensional configuration and settles down as a biofilm as cells replicate and the EPSs accumulate.Citation39 5) Bacterial cells detach from biofilm and disperse into the bulk fluid, where they act as free-swimming bacteria and form new biofilms.Citation16,Citation17 This process of biofilm formation is depicted in and .
Figure 1 Four different stages of biofilm development.
Figure 2 Five stages of biofilm development.
Note: Reproduced by permission from Perfectus Biomed Limited http://perfectusbiomed.com/cbe-meeting-anti-biofilm-technologies/.Citation151
Note: Islam MS, Richards JP, Ojha AK. Targeting drug tolerance in mycobacteria: a perspective from mycobacterial biofilms. Expert Rev Anti Infect Ther. 2012;10(9):1055–1066, Taylor & Francis Ltd, http://www.tandfonline.com reprinted by permission of the publisher.Citation150

Distinct features of biofilm bacteria
Bacteria found inside biofilms have distinct features different from those of free-swimming (planktonic) bacteria of the same classes and possess a very high level of resistance to commonly used antimicrobial remedies, biocides and antiseptics, and the host immune response.Citation40–Citation42 Older, mature, and impenetrable biofilms are consistently more resistant to antimicrobials than younger, less dense biofilms.Citation42 Bacterial cells residing in the outermost parts of the biofilm are more vulnerable to the host’s defenses and antimicrobials, although these microorganisms possess numerous defensive mechanisms. The biofilm is formed of various microbial communities that create a complex three-dimensional physical barrier which hinders the diffusional penetration of antimicrobials.Citation17,Citation43,Citation44 The metabolic activity of the bacteria residing in the exterior layer of biofilm alters the local pH to be more acidic and creates anoxic zones that help to degrade antimicrobials.Citation45–Citation48 The biofilm also creates nutrient-depleted areas which act on microbes to put them into a stationary or dormant phase, which may also contribute toward antibiotic resistance.Citation49,Citation50 The extracellular matrix of the biofilm secretes polymers that bind and deactivate antimicrobials, forming an antibiotic “sink”.Citation51 These properties of biofilms (inadequate diffusion of nutrients, restricted antimicrobial transmission, and the alteration of the environment to produce a more hostile environment) combine to produce a widespread resistance and tolerance to antimicrobials.Citation16,Citation43–Citation56 In addition, microbes entrenched in a biofilm can exist even in the presence of high concentrations of bactericidal antimicrobials, although they are abundantly sensitive to those antimicrobials in culture plates under planktonic conditions.Citation57 This complex phenomenon is known as the “recalcitrance of biofilm bacteria toward antibiotics”,Citation58 and microorganisms found in biofilms can be up to 500–1,000 times more tolerant to antibacterial compounds than their planktonic counterparts.Citation59–Citation62 Additionally, many studies report that as soon as a biofilm is rooted and fixed, microbes develop resistance to several categories of physicochemical aggression, including ultraviolet light, heavy metals, low pH, changes in hydration or salinity, and phagocytosis.Citation63–Citation67
Recurrent tonsillitis and tonsillectomy
Chronic tonsillitis affecting both children and adults is a serious health problem,Citation68,Citation69 and while the definition of severe recurrent tonsillitis varies, severity is described as five or more episodes of true tonsillitis a year, symptoms for at least a year, and episodes that are disabling and prevent normal functioning.Citation70,Citation71 In one study, the lifetime prevalence of recurrent tonsillitis is described as 11.7% (95% CI, 11.0%–12.3%) with a significant preponderance of females.Citation7 Recurrent tonsillitis is typically treated by either surgery or, when the patient does not meet tonsillectomy benchmarks or there are surgical or medical contraindications, by medical antimicrobial intervention.Citation72,Citation73
While tonsillectomy (surgical removal of the tonsils, with or without adenoidectomy) as a treatment modality has been practiced for over 100 years for children, much controversy exists around its value. As, for example, in 1951, the British Medical Journal reported that “it is better to delay a decision than to hurry it, and above all to avoid operating on tonsils which have been recently inflamed”.Citation74 One study showed that 0.6 episode of any type of a sore throat was reported in the first year after surgery compared to medical intervention,Citation75 and another reported that surgery could lead to life-threatening complications. A Swedish cohort study reports that among post-tonsillectomy patients 20 years later, there was a higher incidence of “chronic, immune-mediated diseases … in the operated group”, with a statistically significant relationship between post-tonsillectomy and chronic disease, with a relative risk (RR) at 9.41 and a CI from 1 (1.13 < RR < 78.14).Citation76 However, another research study focusing on adults found that tonsillectomy promotes and improves long-term health and quality of life, thus saving health resources.Citation77
The decision to operate should therefore be taken with care based on an individual patient’s needs and history, plus current research evidence.Citation74,Citation76,Citation78,Citation79 In making such decisions, secondary care doctors and family medicine practitioners need to collaborate because the decision whether a tonsillectomy is necessary is quite difficult and both the general practitioner (GP) and the otolaryngologist must contribute equally.Citation74 The GP knows about the patient’s frequency, duration, and severity of tonsillitis, whereas the ear, nose and throat specialist will evaluate symptoms relating to nasal and Eustachian impediment, and will assess whether symptoms are due to tonsillitis or chronic sinusitis.Citation74
Treatments aimed at disrupting biofilms
Microbial biofilm formation is responsible for the development of acute-to-chronic infection in several diseases including cystic fibrosis, periodontitis, infective endocarditis, persistent otitis media, chronic rhinosinusitis, chronic tonsillitis, prostatitis, chronic osteomyelitis, atopic dermatitis, onychomycosis, dental caries, infectious kidney stones, and chronic wounds.Citation80–Citation83 Biofilms can also form on any surface, living or nonliving, even on clinical devices like pacemakers, implants, and catheters, and are very difficult to eradicate, which accentuates clinical consequences; for example, pseudomonal infections can affect any part of the human body. Furthermore, the microorganisms’ adaptive capability and genetic changes within the biofilm lead to resistance to all known antimicrobial medicines. Pseudomonal infections in particular become really difficult to be treated and can threaten human life.Citation83,Citation84 It is thought that 99% of the biosphere’s bacteria live in and that microbial communities gain an advantage living in this state.Citation85 Consequently, microbial biofilms are thought to significantly affect human health by increasing morbidity, mortality, and health care cost. Biofilms not only add to hospital-acquired infections (HAIs) by increasing their chronicity and persistence but also colonize in other areas of the environment instigating corrosion, fouling of water pipes, and food and pharmaceutical decomposition.Citation14,Citation86–Citation88 Another study reported that microbial biofilms can stick onto and infect all medical devices such as orthopedic prostheses and intravascular catheters and promote up to 60% of HAIs.Citation89
Microorganisms in biofilms are distinctively more resistant to antimicrobial agents and environmental insults and are therefore very difficult to eradicate.Citation42,Citation90–Citation94 Biofilms in general (and chronic tonsillitis specifically) can therefore lead to substantial economic costs for countries and individuals and health concerns and are an evolving public health problem in both high- and low-resource settings.Citation77,Citation95–Citation100 For this reason, multiple research studies have attempted to resolve the issues of both biofilms and recurrent tonsillitis.Citation59,Citation61,Citation101–Citation108
The explosion of antibiotic resistance throughout the world of many microbial strains has put pressure on the research and medical communities to find an alternative strategy for the management of biofilm-mediated diseases.Citation61 “Perhaps new antibiotics are not the only way to combat bio-film infections if we could make ineffective older antibiotics active again.”Citation59 In one study, a 2-amino-imidazole molecule was developed which was capable of disrupting biofilms through making microorganisms which were previously antibiotic-resistant more vulnerable to older antimicrobials.Citation59,Citation62 Immunotherapy (using cyclic dinucleotides) has been effective in the management of different cancers, and this molecule has also been utilized as a therapeutic strategy for biofilm-related infections. Immunoprophylaxis and immunotherapy might therefore provide new tools to combat Staphylococcus epidermis biofilm formation.Citation109,Citation110 Recently, multiple studies revealed that a 3,5-cyclic diguanylic acid (c-di-GMP)-binding protein was found in biofilm communities.Citation111,Citation112 BdcA (a protein that enhances biofilm dispersal) confiscates c-di-GMP and minimizes its local concentration and is partly responsible for the reduction and downregulation of EPSs of biofilms and for the upregulation of swimming, swarming, and planktonic microbes.Citation111,Citation112 This phenomenon has been observed in Pseudomonas sp. and Rhizobium meliloti biofilm communities.Citation111,Citation112 Multiple groups of scientists recently reported that CdrA (an adhesin compound) which is produced by biofilms in response to high levels of c-di-GMP binds with Psl and stabilizes biofilm structure.Citation38,Citation106,Citation113 Multiple research studies have identified at least three extracellular polysaccharides (Alginate, Pel, and Psl) that are important factors for structure maintenance and antibiotic resistance of biofilm.Citation114–Citation123 Another study revealed that exogenous addition of D-amino acidsCitation109 disrupted preformed biofilms by disturbing adhesive fiber interactions and was also effective in preventing biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa.Citation124–Citation126 Another research study reported that biofilm-disassembly molecule is norspermidine which has a similar dispersal mechanism to D-amino acids by targeting the exopolysaccharides.Citation125 The biofilm-inhibiting properties of norspermidine were detected in S. aureus and Escherichia coli pellicle biofilm.Citation125 Current research therefore needs to focus on the development of nor-spermidine, BdcA, D-amino acids, and other polyamines as a novel antibiofilm approach, and medical communities should no longer depend exclusively on antimicrobials (which are increasingly ineffective with many pathogenic microorganisms because of resistance) and surgery to treat infectious diseases.Citation104,Citation111,Citation112,Citation124,Citation125
Other studies have identified additional ways of disrupting biofilms. Bioactive enzymes such as dispersin or Proteinase K studied in orthopedic implants made bacteria more susceptible to antibiotics and finally eradicated the biofilm by affecting polymers or proteins of the biofilm structure.Citation127 Several cytotoxic agents have also been found to successfully eliminate biofilms from implant surfaces, with citric acid being reported to be the most successful in eradicating biofilms on titanium surfaces.Citation128 Multiple research studies have identified that electrical current can successfully detach S. aureus and S. epidermis biofilms from stainless steel implants.Citation129–Citation131 Another study observed that biofilms of S. epidermis on stainless steel fasteners were successfully eradicated through pulsed electromagnetic fields in combination with gentamicin.Citation132 A new cluster of research studies have used laser-generated shockwaves to effectively break up biofilms.Citation133 The technique was performed using a Q-switched, ND:YAG rhythmically laser functioning at a “rep rate of 10 Hz with 1500 mJ pulses centered at 1064 nm. The laser pulses were used to create shockwave pulses in Al coated polycarbonate substrates and a resulting peak stress of greater than 50 MPa” was able to reduce 55% living microorganisms.Citation134 The laser technique offers another way of disrupting biofilms and is useful in the management of infected wounds, where standard treatment modalities such as topical antimicrobials or the removal of dead, damaged, or infected tissue are unsuccessful or injurious. One study found that just 4–10 seconds of the laser therapy was able to disperse 97.9% of P. aeruginosa from biofilms on nitinol stents to single-celled planktonic microorganisms that can be more easily treated with antibiotics.Citation135 Another found that laser-generated shockwaves therapy quickly disrupts the biofilms in infected wounds to eliminate the microorganisms and intensify the effectiveness of topical antimicrobials in the residual biofilm. Such interventions will promote patients’ quality of life by reducing healing times and morbidity, and save health care costs.Citation136
N-acetyl-cysteine (NAC) is an antioxidant mediator which reduces the variety of microbial bacteria on biofilm emergence and evolution,Citation137 inhibits the manufacturing of the extracellular polysaccharide matrix,Citation138 and promotes the disruption of mature biofilms.Citation133 NAC has been found to reduce Streptococcus pneumoniae and Haemophilus influenzae adhesion to human oropharyngeal epithelial cells in laboratory experiments.Citation138 Chronic infections raise prostaglandin levels, and NAC effectively reduces these levels and helps to disrupt the biofilms.Citation139–Citation142 Correspondingly, aspirin-like non-steroidal anti-inflammatory drugs (NSAIDs) decrease biofilm production and completely block fungal infections.Citation143 NAC interacts with the sulfhydryl group of enzymes involved in EPS production or excretion, which reduces the activity of these molecules or inhibits cysteine utilization.Citation144 NAC, therefore, decreases in vitro biofilm formation,Citation145 and other research on salicylates shows a similar negative effect on the production of biofilm.Citation146 A study which applied both found that therapeutic doses of acetylsalicylic acid (ASA) and NAC diminish tonsillar mucosal biofilm formation in chronic or recurrent tonsillitis.Citation102 Another Iraqi study found a strong correlation between the biofilm of Streptococcus pyogenes and recurrent tonsillitis and that three types of vinegar eradicated streptococcal biofilm remarkably: date (100%), apple (95.5%), and grape (90.9%).Citation105 A later study also demonstrated the potential of vinegar in eradicating tonsillar biofilm.Citation101 In a laboratory experiment, while washing and cleaning with a soft brush did not remove the chronic tonsillitis biofilm layer on the tonsil surface, using a harder brush removed more biofilm.Citation103 Researchers believe that the physical removal of biofilm (by brushing or using ultrasound-activated bubbles) from the tonsil surface in vivo will lead to greater effectiveness of topical antimicrobials and decrease the need for systemic antimicrobials.Citation103
Conclusion
Recurrent or chronic tonsillitis is currently a global public health issue which can severely impair an individual’s quality of life.Citation77,Citation147 Microbial biofilms are a major cause of repeated tonsillitis in both pediatric and adult cohorts, and more research is needed to develop new treatment strategies.Citation107,Citation148,Citation149 Treatment modalities should however be based on careful selection and individual consideration of the potential impact of biofilms on cases of recurrent tonsillitis.Citation74 Rather than developing or using more potent antimicrobials, doctors should ensure they are up-to-date with research and the treatment of biofilms, including the application of topical agents, the physical removal of biofilms, and other innovative treatments.
Acknowledgments
The authors are grateful to Dr Zakirul Islam, Associate Professor and Head of The Department, Pharmacology and Therapeutics, Eastern Medical College, Comilla, Bangladesh for his cooperation in converting the video abstract from a PowerPoint file to video format.
Disclosure
The authors report no conflict of interest in this work.
References
- American Academy of OtolaryngologyTonsillitis2018 Available from: http://www.entnet.org/content/tonsillitisAccessed January 6, 2018
- HayesKChronic and recurrent tonsillitis: what to know2017 Available from: https://www.verywell.com/chronic-and-recurrent-tonsillitis-1191984Accessed January 6, 2018
- ShahUKTonsillitis and peritonsillar abscess. Drugs & Diseases. Otolaryngology and Facial Plastic SurgeryMedscape Available from: https://emedicine.medscape.com/article/871977-overview#a6Accessed January 6, 2018
- PichicheroMECaseyJRDefining and dealing with carriers of group A StreptococciContemp Pediatr20032014653
- WaldERCommentary: antibiotic treatment of pharyngitisPediatr Rev200122825525611483850
- HerzonFSHarrisPMosher Award thesis. Peritonsillar abscess: incidence, current management practices, and a proposal for treatment guidelinesLaryngoscope19951058 Pt 3 Suppl 74117
- KvestadEKvaernerKJRoysambETambsKHarrisJRMagnusPHeritability of recurrent tonsillitisArch Otolaryngol Head Neck Surg2005131538338715897415
- WardDBacterial biofilms may be source of recurrent tonsillitis Medicine & HealthWashington University in St. Louis2018 Available from: https://source.wustl.edu/2003/09/bacterial-biofilms-maybe-source-of-recurrent-tonsillitis/Accessed January 6, 2018
- CholeRAFaddisBTAnatomical evidence of microbial biofilms in tonsillar tissues: a possible mechanism to explain chronicityArch Otolaryngol Head Neck Surg2003129663463612810467
- KaniaRELamersGEVonkMJDemonstration of bacterial cells and glycocalyx in biofilms on human tonsilsArch Otolaryngol Head Neck Surg2007133211512117309977
- Al-MazrouKAAl-KhattafASAdherent biofilms in adenotonsillar diseases in childrenArch Otolaryngol Head Neck Surg20081341202318209130
- SaylamGTatarECTatarIÖzdekAKorkmazHAssociation of adenoid surface biofilm formation and chronic otitis media with effusionArch Otolaryngol Head Neck Surg2010136655055520566904
- SandersonARLeidJGHunsakerDBacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitisLaryngoscope200611671121112616826045
- CostertonJWStewartPSGreenbergEPBacterial biofilms: a common cause of persistent infectionsScience199928454181318132210334980
- DonlanRMCostertonJWBiofilms: survival mechanisms of clinically relevant microorganismsClin Microbiol Rev200215216719311932229
- Hall-StoodleyLCostertonJWStoodleyPBacterial biofilms: from the environment to infectious diseaseNat Rev Microbiol2004229510815040259
- Hall-StoodleyLStoodleyPBiofilm formation and dispersal and the transmission of human pathogensTrends Microbiol200513171015639625
- Purevdorj-GageBCostertonWJStoodleyPPhenotypic differentiation and seeding dispersal in nonmucoid and mucoid Pseudomonas aeruginosa biofilmsMicrobiology2005151Pt 51569157615870466
- Mai-ProchnowALucas-ElioPEganSHydrogen peroxide linked to lysine oxidase activity facilitates biofilm differentiation and dispersal in several gram-negative bacteriaJ Bacteriol2008190155493550118502869
- BorhanWMDababoMAThompsonLDSaleemMPashleyNAcute necrotizing herpetic tonsillitis: a report of two casesHead Neck Pathol20159111912224338612
- SlavkinHCBiofilms, microbial ecology, and Antoni van LeeuwenhoekJ Am Dent Assoc199712844924959103803
- FasoloAThe Theory of Evolution and Its ImpactMilanSpringer2012
- NeumannJJThe Role of Metaphor in the Darwin Debates: Natural Theology, Natural Selection, and Christian Production of Counter-Metaphor [master’s thesis]College Station, TXTexas A&M University2012
- TilahunAHaddisSTeshaleAHadushTReview on biofilm and microbial adhesionInt J Microbiol Res2016736373
- BrownMRWGilbertPMicrobiological Quality Assurance: A Guide Towards Relevance and Reproducibility of InoculaBoca Raton, NYCRC Press1995
- JeffersonKKWhat drives bacteria to produce a biofilm?FEMS Microbiol Lett2004236216317315251193
- ChambersJRSauerKThe MerR-like regulator BrlR impairs Pseudomonas aeruginosa biofilm tolerance to colistin by repressing PhoPQJ Bacteriol2013195204678468823935054
- JooHSOttoMMolecular basis of in-vivo biofilm formation by bacterial pathogensChem Biol201219121503151323261595
- LebeauxDChauhanARenduelesOBeloinCFrom in vitro to in vivo models of bacterial biofilm-related infectionsPathogens20132228835625437038
- CostertonJWIntroduction to biofilmInt J Antimicrob Agents1999113–421722110394973
- DonlanRMBiofilm formation: a clinically relevant microbiological processClin Infect Dis20013381387139211565080
- LandiniPAntonianiDBurgessJGNijlandRMolecular mechanisms of compounds affecting bacterial biofilm formation and dispersalAppl Microbiol Biotechnol201086381382320165945
- RennerLDWeibelDBPhysicochemical regulation of biofilm formationMRS Bull201136534735522125358
- BanerjeePSinghMSharmaVBiofilm formation: a comprehensive reviewInt J Pharm Res Health Sci201532556560
- SauerKCamperAKEhrlichGDCostertonJWDaviesDGPseudomonas aeruginosa displays multiple phenotypes during development as a biofilmJ Bacteriol200218441140115411807075
- ThomasWENilssonLMForeroMSokurenkoEVVogelVShear-dependent “stick-and-roll” adhesion of type 1 fimbriated Escherichia coliMol Microbiol20045351545155715387828
- FlemmingHCWingenderJThe biofilm matrixNat Rev Microbiol20108962363320676145
- BorleeBRGoldmanADMurakamiKSamudralaRWozniakDJParsekMRPseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrixMol Microbiol201075482784220088866
- AlpkvistEPicioreanuCvan LoosdrechtMCHeydenAThree-dimensional biofilm model with individual cells and continuum EPS matrixBiotechnol Bioeng200694596197916615160
- HentzerMGivskovMPharmacological inhibition of quorum sensing for the treatment of chronic bacterial infectionsJ Clin Invest200311291300130714597754
- CholeRAFaddisBTEvidence for microbial biofilms in cholesteatomasArch Otolaryngol Head Neck Surg2002128101129113312365883
- StewartPSAntimicrobial tolerance in biofilmsMicrobiol Spectr20153310
- McConougheySJHowlinRGrangerJFBiofilms in periprosthetic orthopedic infectionsFuture Microbiol201498987100725302955
- StoodleyPSauerKDaviesDGCostertonJWBiofilms as complex differentiated communitiesAnnu Rev Microbiol20025618720912142477
- HuangCTYuFPMcFetersGAStewartPSNonuniform spatial patterns of respiratory activity within biofilms during disinfectionAppl Environ Microbiol1995616225222567793945
- de BeerDStoodleyPRelation between the structure of an aerobic biofilm and mass transport phenomenaWater Sci Technol19953281118
- de BeerDStoodleyPLewandowskiZMeasurement of local diffusion coefficients in biofilms by microinjection and confocal microscopyBiotechnol Bioeng199753215115818633959
- StoodleyPWefelJGiesekeADeBeerDvon OhleCBiofilm plaque and hydrodynamic effects on mass transfer, fluoride delivery, and cariesJ Am Dent Assoc200813991182119018762628
- WaltersMC3rdRoeFBugnicourtAFranklinMJStewartPSContributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycinAntimicrob Agents Chemother200347131733212499208
- FuxCAWilsonSStoodleyPDetachment characteristics and oxacillin resistance of Staphylococcus aureus biofilm emboli in an in vitro catheter infection modelJ Bacteriol2004186144486449115231780
- HøibyNRecent advances in the treatment of Pseudomonas aeruginosa infections in cystic fibrosisBMC Med201193221463524
- AnwarHStrapJLCostertonJWEstablishment of aging biofilms: a possible mechanism of bacterial resistance to antimicrobial therapyAntimicrob Agents Chemother1992367134713511510427
- BorrielloGWernerERoeFKimAMEhrlichGDStewartPSOxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilmsAntimicrob Agents Chemother20044872659266415215123
- BrownMRAllisonDGGilbertPResistance of bacterial biofilms to antibiotics: a growth-rate related effect?J Antimicrob Chemother19882267777833072331
- ShahDZhangZKhodurskyAKaldaluNKurgKLewisKPersisters: a distinct physiological state of E. coliBMC Microbiol20061253
- StewartPSCostertonJWAntibiotic resistance of bacteria in biofilmsLancet2001358927613513811463434
- AnderlJNFranklinMJStewartPSRole of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacinAntimicrob Agents Chemother20004471818182410858336
- LebeauxDGhigoJMBeloinCBiofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibioticsMicrobiol Mol Biol Rev201478351054325184564
- PoteraCAntibiotic resistance: biofilm dispersing agent rejuvenates older antibioticsEnviron Health Perspect20101187A288
- SedlacekMJWalkerCAntibiotic resistance in an in vitro subgingival biofilm modelOral Microbiol Immunol200722533333917803631
- WorthingtonRJRichardsJJMelanderCSmall molecule control of bacterial biofilmsOrg Biomol Chem201210377457747422733439
- RogersSAHuigensRW3rdCavanaghJMelanderCSynergistic effects between conventional antibiotics and 2-aminoimidazole-derived antibiofilm agentsAntimicrob Agents Chemother20105452112211820211901
- EspelandEMWetzelRGComplexation, stabilization, and UV photolysis of extracellular and surface-bound glucosidase and alkaline phosphatase: implications for biofilm microbiotaMicrob Ecol200142457258512024240
- Le Magrex-DebarELemoineJGelleMPJacquelinLFChoisyCEvaluation of biohazards in dehydrated biofilms on foodstuff packagingInt J Food Microbiol2000551–323924310791750
- LeidJGShirtliffMECostertonJWStoodleyPHuman leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilmsInfect Immun200270116339634512379713
- McNeillKHamiltonIRAcid tolerance response of biofilm cells of Streptococcus mutansFEMS Microbiol Lett20032211253012694906
- TeitzelGMParsekMRHeavy metal resistance of biofilm and planktonic Pseudomonas aeruginosaAppl Environ Microbiol20036942313232012676715
- WagnerSJungHNauFSchmittHRelevance of infectious diseases in a pediatric practiceKlin Padiatr1993205114178445847
- PoteraCForging a link between biofilms and diseaseScience199928354091837183910206887
- Management of sore throat and indications for tonsillectomyNational Clinical Guideline No. 34EdinburghScottish Intercollegiate Guidelines Network, Royal College of Physicians Available from: http://www.sdl.academic.chula.ac.th/Sore%20Throat/Sign.pdfAccessed January 11, 2018
- McKerrowWSRecurrent tonsillitisAm Fam Physician20026691735173612449272
- El HennawiDEDGeneidAZaherSAhmedMRManagement of recurrent tonsillitis in childrenAm J Otolaryngol201738437137428385329
- GeorgalasCCTolleyNSNarulaATonsillitisBMJ Clin Evid200920090503
- GaleAHRefresher course for general practitioners: pros and cons of tonsillectomyBr Med J19511469813313514812127
- BurtonMJGlasziouPPChongLYVenekampRPTonsillectomy or adenotonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitisCochrane Database Syst Rev20141911CD001802
- JohanssonEHultcrantzETonsillectomy—clinical consequences twenty years after surgery?Int J Pediatr Otorhinolaryngol200367998198812907054
- SenskaGAtayHPütterCDostPLong-term results from tonsillectomy in adultsDtsch Arztebl Int20151125084985526763379
- StuckBAGötteKWindfuhrJPGenzwürkerHSchrotenHTenenbaumTTonsillectomy in childrenDtsch Arztebl Int20081054985286119561812
- WindfuhrJPIndications for tonsillectomy stratified by the level of evidenceGMS Curr Top Otorhinolaryngol Head Neck Surg201615 Doc09
- AparnaMSYadavSBiofilms: microbes and diseaseBraz J Infect Dis200812652653019287843
- SotoSMImportance of biofilms in urinary tract infections: new therapeutic approachesAdv Biol20142014543974
- ZhaoGUsuiMLLippmanSIBiofilms and inflammation in chronic woundsAdv Wound Care (New Rochelle)20132738939924527355
- Reyes-DariasJAKrellTRiboswitches as potential targets for the development of anti-biofilm drugsCurr Top Med Chem2017171719451953
- SharmaGRaoSBansalADangSGuptaSGabraniRPseudomonas aeruginosa biofilm: potential therapeutic targetsBiologicals20144211724309094
- DonlanRMNew approaches for the characterization of prosthetic joint biofilmsClin Orthop Relat Res20054371219
- FigueiredoAMSFerreiraFABeltrameCOCôrtesMFThe role of biofilms in persistent infections and factors involved in ica-independent biofilm development and gene regulation in Staphylococcus aureusCrit Rev Microbiol201743560262028581360
- ZumsteinVBetschartPAlbrichWCBiofilm formation on ureteral stents – incidence, clinical impact, and preventionSwiss Med Wkly2017147w1440828165539
- VickeryKHuHJacombsANBradshawDADevaAKA review of bacterial biofilms and their role in device associated infectionHealthc Infect20131826166
- BryersJDMedical biofilmsBiotechnol Bioeng2008100111818366134
- LewisKRiddle of biofilm resistanceAntimicrob Agents Chemother2001454999100711257008
- SpoeringALLewisKBiofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobialsJ Bacteriol2001183236746675111698361
- DaveyMEO’tooleGAMicrobial biofilms: from ecology to molecular geneticsMicrobiol Mol Biol Rev200064484786711104821
- BridierABriandetRThomasVDubois-BrissonnetFResistance of bacterial biofilms to disinfectants: a reviewBiofouling20112791017103222011093
- El KhatibMTranQTNasrallahCProvidencia stuartii form biofilms and floating communities of cells that display high resistance to environmental insultsPLoS One2017123e017421328334028
- ZhouGShiQSHuangXMXieXBThe three bacterial lines of defense against antimicrobial agentsInt J Mol Sci2015169217112173326370986
- FishKEOsbornAMBoxallJCharacterizing and understanding the impact of microbial biofilms and the extracellular polymeric substance (EPS) matrix in drinking water distribution systemsEnviron Sci Water Res Technol20162614630
- SadekuzzamanMYangSMizanMFRHaSDCurrent and recent advanced strategies for combating biofilmsComp Rev Food Sci Food Safety2015144491509
- ZhaoXZhaoaFWangJZhongZBiofilm formation and control strategies of foodborne pathogens: food safety perspectivesRSC Adv201773667036683
- DuarteVMMcGrathCLShapiroNLBhattacharryaNHealth-care costs of acute and chronic tonsillar conditions in the pediatric population in the United StatesInt J Pediatr Otorhinolaryngol201579692192525912631
- StelterKTonsillitis and sore throat in childrenGMS Curr Top Otorhinolaryngol Head Neck Surg201413 Doc07
- Al-SaadiMAKAbdul-LateefLAKareemMADetection of biofilm formation and effect of vinegar on biofilm of Streptococcus pyogenes isolated from patients with tonsillitisInt J Pharm Tech Res201699236242
- BulutFMericFYorgancilarEEffects of N-acetyl-cysteine and acetylsalicylic acid on the tonsil bacterial biofilm tissues by light and electron microscopyEur Rev Med Pharmacol Sci201418233720372525535147
- CiftciZDeveliogluOArbakSOzdoganogluTGultekinEA new horizon in the treatment of biofilm-associated tonsillitisTher Adv Respir Dis201483788324741004
- ConnaughtonAChildsADylewskiSSabesanVJBiofilm disrupting technology for orthopedic implants: what’s on the horizon?Front Med2014122
- IsmaelNF“Vinegar” as anti-bacterial biofilm formed by Streptococcus pyogenes isolated from recurrent tonsillitis patients, in vitroJordan J Biol Sci201363191197
- KostakiotiMHadjifrangiskouMHultgrenSJBacterial bio-films: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic eraCold Spring Harb Perspect Med201334a01030623545571
- RömlingUBalsalobreCBiofilm infections, their resilience to therapy and innovative treatment strategiesJ Intern Med2012272654156123025745
- WuHMoserCWangHZHøibyNSongZJStrategies for combating bacterial biofilm infectionsInt J Oral Sci2015711725504208
- TopalianSLWeinerGJPardollDMCancer immunotherapy comes of ageJ Clin Oncol201129364828483622042955
- Van MellaertLShahrooeiMHofmansDEldereJVImmunoprophylaxis and immunotherapy of Staphylococcus epidermidis infections: challenges and prospectsExpert Rev Vaccines201211331933422380824
- MaQGuishanZWoodTKEscherichia coli BdcA controls biofilm dispersal in Pseudomonas aeruginosa and Rhizobium melilotiBMC Res Notes2011444722029875
- MaQYangZPuMPetiWWoodTKEngineering a novel c-di-GMP-binding protein for biofilm dispersalEnviron Microbiol201113363164221059164
- HaDGO’TooleGAc-di-GMP and its effects on biofilm formation and dispersion: a Pseudomonas aeruginosa reviewMicrobiol Spectr20153210
- FriedmanLKolterRGenes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilmsMol Microbiol200451367569014731271
- FriedmanLKolterRTwo genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrixJ Bacteriol2004186144457446515231777
- JacksonKDStarkeyMKremerSParsekMRWozniakDJIdentification of psl, a locus encoding a potential exopolysaccharide that is essential for Pseudomonas aeruginosa PAO1 biofilm formationJ Bacteriol2004186144466447515231778
- MatsukawaMGreenbergEPPutative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm developmentJ Bacteriol2004186144449445615231776
- MaLJacksonKDLandryRMParsekMRWozniakDJAnalysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure post attachmentJ Bacteriol2006188238213822116980452
- VasseurPVallet-GelyISosciaCGeninSFillouxAThe pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formationMicrobiology2005151Pt 398599715758243
- ColvinKMIrieYTartCSThe Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrixEnviron Microbiol20121481913192822176658
- WeiQMaLZBiofilm matrix and its regulation in Pseudomonas aeruginosaInt J Mol Sci20131410209832100524145749
- FranklinMJNivensDEWeadgeJTHowellPLBiosynthesis of the Pseudomonas aeruginosa extracellular polysaccharides, alginate, Pel, and PslFront Microbiol2011216721991261
- LimoliDHJonesCJWozniakDJBacterial extracellular polysaccharides in biofilm formation and functionMicrobiol Spectr20153310
- FujiiND-amino acids in living higher organismsOrig Life Evol Biosph200232210312712185671
- Kolodkin-GalIRomeroDCaoSClardyJKolterRLosickRD-amino acids trigger biofilm disassemblyScience2010328597862762920431016
- CavaFLamHde PedroMAWaldorMKEmerging knowledge of regulatory roles of D-amino acids in bacteriaCell Mol Life Sci201068581783121161322
- CampocciaDMontanaroLArciolaCRA review of the biomaterials technologies for infection-resistant surfacesBiomaterials201334348533855423953781
- NtroukaVISlotDELouropoulouAVan der WeijdenFThe effect of chemotherapeutic agents on contaminated titanium surfaces: a systematic reviewClin Oral Implants Res201122768169021198891
- ErcanBKummerKMTarquinioKMWebsterTJDecreased Staphylococcus aureus biofilm growth on anodized nanotubular titanium and the effect of electrical stimulationActa Biomater2011773003301221515421
- Del PozoJLRouseMSEubaGThe electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitisAntimicrob Agents Chemother200953104064406819651912
- van der BordenAJvan der MeiHCBusscherHJElectric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidisBiomaterials200526336731673515979141
- PickeringSABaystonRScammellBEElectromagnetic augmentation of antibiotic efficacy in infection of orthopedic implantsJ Bone Joint Surg Br200385458859312793569
- HansenENZmistowskiBParviziJPeriprosthetic joint infection: what is on the horizon?Int J Artif Organs2012351093595023371923
- TaylorZDNavarroAKealeyCPBacterial biofilm disruption using laser-generated shockwavesConf Proc IEEE Eng Med Biol Soc201020101028103221096997
- KizhnerVKrespiYPHall-StoodleyLStoodleyPLaser-generated shockwave for clearing medical device biofilmsPhotomed Laser Surg201129427728221182450
- FrancisNCYaoWGrundfestWSTaylorZDLaser-generated shock-waves as a treatment to reduce bacterial load and disrupt biofilmIEEE Trans Biomed Eng201764488288927323358
- SchwandtLQVan WeissenbruchRStokroosIVan Der MeiHCBusscherHJAlbersFWPrevention of biofilm formation by dairy products and N-acetylcysteine on voice prostheses in an artificial throatActa Otolaryngol2004124672673115515498
- RiiseGCQvarfordtILarssonSEliassonVAnderssonBAInhibitory effect of N-acetylcysteine on adherence of Streptococcus pneumoniae and Haemophilus influenzae to human oropharyngeal epithelial cells in vitroRespiration20006755255811070462
- RicciottiEFitzGeraldGAProstaglandins and inflammationArterioscler Thromb Vasc Biol2011315986100021508345
- Kiecolt-GlaserJKStress, food, and inflammation: psychoneuro-immunology and nutrition at the cutting edgePsychosom Med201072436536920410248
- HsiehCCHsiehSCChiuJHWuYLProtective effects of N-acetylcysteine and a prostaglandin E1 analog, alprostadil, against hepatic ischemia: reperfusion injury in ratsJ Tradit Complement Med201441647124872935
- BlasiFPageCRossoliniGMThe effect of N-acetylcysteine on biofilms: implications for the treatment of respiratory tract infectionsRespir Med201611719019727492531
- WitkinSSJeremiasJLedgerWJA localized vaginal allergic response in women with recurrent vaginitisJ Allergy Clin Immunol19888124124163422256
- AlemMADouglasLJEffects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicansAntimicrob Agents Chemother2004481414714693516
- PéRez-GiraldoCRodriguez-BenitoAMoranFJHurtadoCBlancoMTGomez-GarciaACInfluence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidisJ Antimicrob Chemother19973956436469184365
- XuXMSansores-GarciaLChenXMMatijevic-AleksicNDuMWuKKSuppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylateProc Natl Acad Sci U S A19999695292529710220459
- TorrettaSRosazzaCPaceMEIofridaEMarchisioPImpact of adenotonsillectomy on pediatric quality of life: review of the literatureItal J Pediatr201743110729178907
- AlasilSMOmarRIsmailSYusofMYDhabaanGNAbdullaMAEvidence of bacterial biofilms among infected and hypertrophied tonsils in correlation with the microbiology, histopathology, and clinical symptoms of tonsillar diseasesInt J Otolaryngol2013201340823824454384
- TorrettaaSLorenzo DragoLMarchisioPRecurrences in chronic tonsillitis substained by tonsillar biofilm-producing bacteria in children. Relationship with the grade of tonsillar hyperplasyInt J Pediatr Otorhinolaryngol201377220020423137856
- IslamMSRichardsJPOjhaAKTargeting drug tolerance in mycobacteria: a perspective from mycobacterial biofilmsExpert Rev Anti Infect Ther20121091055106623106280
- CBE Regulatory Meeting: Anti-Biofilm Technologies: Pathways to Product Development [webpage on the Internet]Perfectus Biomed2013 Available from http://perfectusbiomed.com/cbe-meeting-anti-biofilm-technologies/Accessed July 23, 2018
