Abstract
Inflammatory bowel disease (IBD) is a chronic multifactorial disease that affects the gastrointestinal tract and results from an aberrant immune response toward luminal antigens in genetically susceptible people. Most of the current therapies for IBD focus on the management of the inflammation by using corticosteroids, immune modulators, and more recently, monoclonal antibodies (biological therapy). Although these therapies provide benefit in most cases, there are still a significant number of patients who do not respond or become refractory over time, suggesting the need for alternative therapeutic options. In the last decade, it has been recognized that “dysbiosis,” an imbalanced gut microbiota, is a key element in IBD suggesting microbiome-based therapies as an attractive approach. Recently, fecal microbiota transplant (FMT) has been successfully used for the treatment of Clostridium difficile infection, and it is now under investigation for the treatment of IBD. Clinical trials data are still poor but strongly support a future introduction of FMT in therapy to manage IBD microbiome. More studies are needed to assess the optimal route of administration and the frequency of FMT, the best matched donor for each patient as well as the risks associated with FMT in IBD.
Introduction
Pathophysiology of inflammatory bowel disease (IBD) and the use of fecal microbiota transplant (FMT)
Inflammatory bowel disease is a common gastrointestinal (GI) disorder affecting approximately 10%–15% of the worldwide population,Citation1 characterized by chronic abdominal pain and altered bowel habits. Although the pathophysiology of IBD remains still largely unknown, growing evidence suggests that factors such as food habits, antibiotics, and gender, as well as psychosocial events,Citation2 may be a trigger in genetically susceptible individuals.Citation3,Citation4 In the recent years, an important role of the gut microbiota in IBD has been recognized. In the healthy status, the gut microbiota actively interacts with the human host to modulate several physiological functions such as gut development,Citation5 immune responses,Citation6,Citation7 resistance to pathogens,Citation8 and brain development and functions.Citation9,Citation10 Changes in bacteria composition and number, known as “dysbiosis,” have repeatedly been observed in IBD patients and are now recognized as a key element in gut inflammatory processes.Citation11–Citation13 Dysbiosis of the gut microbiota is characterized by a significant reduction of obligate anaerobes such as members of the phyla Bacteroidetes and Firmicutes and a sharp increase in facultative anaerobes such as the phyla Actinobacteria and Proteobacteria, such as Escherichia coli. A decrease in obligate anaerobes results in the release of anti-inflammatory compounds, which causes increased inflammation.Citation14,Citation15 Therefore, the dysbiosis correction has been considered an attractive therapeutic approach. FMT can reduce bowel permeability by increasing the production of short-chain fatty acids, especially butyrate, which helps in maintaining the integrity of the epithelial barrier and thus decreases the severity of the disease. FMT can also restore immune dysbiosis by regulating the inflammatory markers by inhibiting the activity of T cells, leukocyte adhesion, and the production of inflammatory factors.Citation16 Although microbiome-based therapies, such as probiotics and antibiotics, failed to be effective in IBD, recently, FMT is gaining a new life as a therapeutic option for the patients with a disturbed gut ecosystem.
FMT is a procedure in which fecal or stool matter is collected from a healthy donor and placed into a patients’ GI tract to correct the dysbiosis and to restore healthy conditions.Citation17 FMT has already been successfully used for the treatment of recurrent Clostridium difficile infection (rCDI) resistant to conventional antibiotic therapies,Citation18,Citation19 with an efficacy >90%.Citation20–Citation23 For this reason, it is now being considered as an experimental therapy in IBD and labeled by the Food and Drug Administration as Investigational New Drug in 2016.Citation24
Preparation and routes of administration of the new microbiota
There has been an increasing interest in the use of FMT for chronic GI infections and IBDs.Citation25 FMT requires few important steps prior to transplant that are: 1) preparation of the fecal material and 2) selection of the route of administration.
Preparation of FMT
Prior to FMT, the stool is collected by the donor using a sterile collection kit provided in advance. The stool can be used either immediately or frozen for later use. According to many protocols, at least 30–50 g of freshly produced stool is sufficient for a successful FMT.Citation26,Citation27 However, the stool’s weight is not an accurate measure of the microbiota quantity as it may vary among donors. Once collected, the stool is diluted in a volume of a sterile saline from three to five times larger than the starting fecal material (ie, 30 g of stool is mixed with 150 mL of saline). In addition, other solvents have been used such as water, yogurt, and milk.Citation28,Citation29 However, whether saline is preferable to preserve the microbesCitation30 better is still unknown because of the lack of comparative studies. Following homogenization, the preparation is filtered (using, eg, gauze, coffee filter, and strainer), and particles <2 mm in width are allowed to prevent any clog of the infusion tube during colonoscopy.Citation31 Cui et al reported about automatic purification of microbiota from stool using microfiltration and centrifugation.Citation32 The solution is then directly infused in the GI tract or further centrifuged to obtain a pellet composed by bacteria to be placed in gelatin capsules for oral administration. To do this, the pellet is then suspended to obtain a working concentration of 0.5 g/mL and rapidly frozen in 5- or 10 mL volume syringes. Size 1 acid-resistant hypromellose capsules are filled with 0.4 mL of fecal material and placed in size 0 acid-resistant hypromellose capsule and then nested within size 00 gelatin capsule.Citation33 Freshly prepared stool must be delivered preferably within 6 hours after emission; otherwise, the preparation must be discarded. The use of fresh material may have some logistical limitations, such as the cost of the technique, the donors’ availability, and the time associated with the screening. Recently, FMT approach using frozen materials has been developed and filled those gaps.Citation34 Indeed, the frozen material offers a larger pool of stool samples to choose from allowing the availability of stool on demand as well as the selection of the best match between donors and recipients. Similar to fresh samples, the preparation of frozen stool consists of dilution and homogenization of the sample. Before freezing, glycerol to a 10% final concentration should be addedCitation29 to protect microbial cells from the damage induced by freezing.Citation35 Although the optimization of the storage temperature is still under debate, it is preferable at −80°C, because some enzymes are still active at −20°C.Citation36
Route of administration
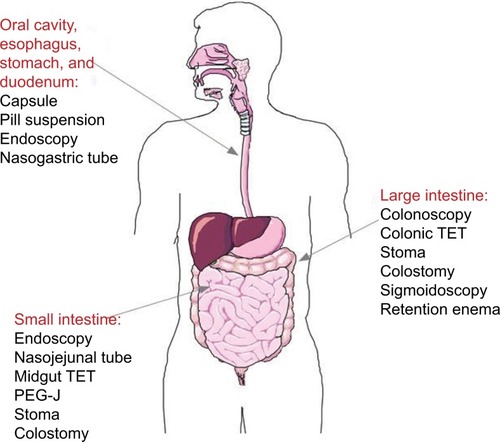
Different ways to deliver the new microbiota have been reported via the lower GI tract or upper GI tract.
By the lower digestive tract, FMT is given by colonoscopy or rectal enema. After appropriate anesthesia, an amount of 200–500 mL of donor stool is infused to the colon (terminal ileum, cecum, or sigmoid) via the endoscope channel.Citation21,Citation28,Citation37 FMT performed via colonoscopy carries the risk associated with colonoscopies, such as adverse sedative reaction and bowel perforation. Patients who are not suitable for colonoscopy may receive FMT via enema or the upper GI tract. When applied through the upper GI tract, the total volume of donor stool suspension is 10 times lower (25–50 mL), and it is delivered through nasogastric, nasojejunal, or gastrostomy tube, keeping the patient at 45° upright position for 4 hours after infusion to prevent aspiration.Citation38 However, this is the method that patients prefer less. Recently, gelatin-coated or frozen capsules are under investigation to increase the avail ability of FMT therapy in terms of accessibility and patient compliance.Citation33,Citation39 Upon filling and capping, 8–12 capsules are administered to patients.Citation39 FMT is reported to be effective by all of the routes, and the preferred method may vary with the clinical situation.Citation17 Less invasive methods, such as retention enema and nasointestinal infusion, may be safer in patients who are frail or severely ill at the time of FMT. Transendoscopic enteral tubing for FMT through the colon has been reported by Peng et al.Citation40 Other methods of delivery through small intestine stoma or percutaneous endoscopic gastrojejunostomy have also been reported.Citation40,Citation41
The choice of the delivery route will depend on the type of microbiota to transplant (gastric juice can activate certain bacteria species and damage others), the overall health status of the patient, and the disease to be treated (CDI vs colitis). In addition, the number of administration is crucial, because a single dose may be adequate for CDI, but not for IBD. Furthermore, despite the beneficial effect of the transplanted microbiota, it is known that the host shapes the microbiota,Citation42,Citation43 suggesting that host genes and diet can limit FMT effectiveness. summarizes various routes of administration of FMT.
Figure 1 FMT: routes of administration.
Note: Images were created with SmartDraw software (https://www.smartdraw.com).
Abbreviations: FMT, fecal microbiota transplant; PeG-J, percutaneous endoscopic gastrojejunostomy; TeT, transendoscopic enteral tubing.
Donor selection, screening, and stool composition to impact the efficacy of FMT
Donor selection is a big challenge for FMT in IBD. Because the major concern of the FMT is the possibility to spread viral or bacterial infections through the stools, FMT requires a healthy donor chosen among patient-selected (relative or friend) or anonymous person with no risk factors for transmissible diseases or any issues that may alter the microbiota composition, such as drugs or antibiotics. Thus, potential donors undergo a selection process that involves a preliminary interview followed by at least two rounds of blood and stool tests.Citation44
In the first step, all potential donors fill out a questionnaire about their medical history and life habits to identify risk factors that can be eventually undetectable through the blood or stool test. The key issues addressed are about “infectious diseases,” such as exposure to human immunodeficiency virus (HIV), hepatitis B virus or hepatitis C virus, syphilis, human T-lymphotropic virus I and II, malaria, trypanosomiasis, and tuberculosis; use of illegal drugs; risky sexual behavior; previous reception of organ transplant or blood products; recent (<6 months) body tattoo, piercing, and acupuncture; recent (<6 months) travel to tropical countries, countries at a high risk of transmissible diseases, or traveler’s diarrhea; recent (<6 months) immunization with live attenuated virus; individual working with animals (increases the risk of zoonoses); “GI, metabolic, and neurological disorders,” such as history of irritable bowel syndrome, IBD, chronic constipation, and other chronic GI disorders; history or high risk factor for GI cancer or polyposis; history of neurological/neurodegenerative disorders; history of psychiatric conditions; overweight and obesity (body mass index >25); and “drugs that can impair gut microbiota,” such as recent exposure to antibiotics, immunotherapy, and chemotherapy as well as treatment with proton pump inhibitors.Citation44 Donors who report significant exposure to one or more risk factors on their questionnaires are deemed to be unsuitable donors. In the second step, selected donors are scheduled for an in-person health assessment by the medical staff and are further checked for any recent onset of harmful events, by submitting a new form in which they briefly describe any changes to their health, diet, and bowel habits; travel to any tropical area; new sexual partners; and recent ingestion of substances that can be harmful for the recipient or that can affect the donor gut microbiota (antibiotics) since the previous screening. The above recommendations are supported by excellent safety data collected from several randomized clinical trials.Citation18,Citation21,Citation45,Citation46 In addition, stool specimens and blood are collected and analyzed for infectious diseases potentially transmittable to the recipient. Some tests are mandatory such as HIV; hepatitis A, B, and C; Salmonella, Shigella, and Campylobacter; ova and parasites; and Enterobacteriaceae, Enterococcus, Helicobacter pylori, and C. difficile,Citation31 while others are optional according to geographical areas, clinical conditions of the recipients, and medical history of the donors.Citation44 The questionnaire and test results are reviewed to determine the donors’ suitability. Donors who test positive for potential transmissible pathogens are deemed and confidentially informed and referred for appropriate treatment. Standard donors must repeat the questionnaire and resubmit blood and stool specimens for testing every 3–6 months to confirm their suitability as donors.Citation31 Of note, each donor has a different microbiota composition that makes difficult to obtain a homogenous FMT treatment, suggesting that an intrinsic characteristic of the stool donor may play a crucial role on the outcome of the FMT. In 2015, a study by Moayyedi et al observed that patients who received stool from the same donor (donor B) achieved remission more often than others,Citation17,Citation46 suggesting that the efficacy of the FMT is related to the donor stool “richness.” Indeed, in the same year, a study by Grinspan and KellyCitation47 reported that the microbiota of donor B was enriched in members of the Lachnospiraceae family and Ruminococcus genus accounting for the greater efficacy of the FMT treatment. Same observations were described by Sokol in 2016.Citation48 Moreover, another study revealed the importance of anti-inflammatory bacteria such as Faecalibacterium prausnitzii, whose levels are low in patients with Crohn’s disease (CD).Citation49,Citation50 Therefore, the richness of the donor is an important parameter to take in consideration for a successful FMT. Another aspect to take into account is the donor–recipient microbiota compatibility. For instance, a donor strain belonging to a species already present in the recipient microbiota is more likely to establish in his/her gut,Citation51 maximizing the FMT effect. Donor selection is a big challenge considering all the different aspects that can affect the outcome of FMT in IBD. Donor screening and consequently FMT activities can be facilitated by the development of stool banks. To do this, the Microbiome Health Research Institute (OpenBiome, Cambridge, MA, USA), a nonprofit organization dedicated to expanding safe access to FMT, has built an international public stool bank that allows to screen and process the donor stool in a standardized manner, at the same time offering a platform for investigating other microbiome-associated diseases.Citation52
FMT in IBD
The role of FMT for rCDI has been largely documented and demonstrated to have a cure rate of 90% in >500 reported cases to date.Citation22,Citation23 Considering the fact that microbiota composition is also profoundly modified in IBD, FMT is considered a promising approach for the treatment of IBD. Preliminary clinical reports of FMT in patients with ulcerative colitis (UC) and CD showed clinical remission that was maintained over long-term follow-up in many casesCitation53,Citation54 and in few other cases also reported endoscopic and histologic remission.Citation55 A recent review and meta-analysis of nine studies that included 122 patients (79 with UC, 39 CD, and four with unclassified IBD) who received FMT found a remission rate of 36.2%.Citation56 However, the remission was higher in younger patients (7–20 years old) and in patients with CD (64.1% and 60.5%, respectively) compared with UC patients where only 22% achieved remission. Other small studies conducted in both adult and pediatric patients showed controversial results. Angelberger et al reported the results of a small study conducted in five patients with moderate to severe UC who received FMT via a nasojejunal tube.Citation57 None of them showed remission after 12 weeks except for one patient who showed an improvement in the Mayo Score. In a different study involving four pediatric UC patients, Suskind et al did not observe any clinical improvement after a single administration of FMT via nasogastric tube.Citation58 On the other hand, Kunde et al showed a high rate of success when they performed FMT via enema in nine pediatric UC patients once a day for 5 consecutive days.Citation59 Indeed, seven patients showed remission after 1 week and six of them up to 1 month. Moreover, in a different study involving 15 adult patients with steroid-dependent UC who received FMT via upper colonoscopy, Cui et al reported clinical improvement in eight patients (57%) with four of them maintaining long-term remission.Citation32
Suskind et al were the first to report that FMT might be a therapeutic option in CD.Citation60 Vaughn et al studied about increased intestinal microbial diversity following FMT in CD patients. In this study, eleven of 19 patients (58%) showed improved clinical response.Citation61 In another study by He et al, FMT efficacy and safety were evaluated in CD patients with inflammatory mass. A total of 25 patients were enrolled, and all the patients received initial FMT followed by FMT every 3 months; 17 of the 25 (68%) patients achieved a clinical response, and 13 of the 25 (52%) patients achieved clinical remission at 3 months. Sustained clinical remission was found in 12 (48.0%), eight (32.0%), and five (22.7%) patients at 6, 12, and 18 months, respectively.Citation62 A study of 30 patients by Cui et al on FMT through midgut for refractory CD showed that the clinical improvement and remission rates based on clinical activity at the first month were 86.7% (26 of 30) and 76.7% (23 of 30).Citation63
Unfortunately, these studies lack uniformity in treatment protocols and route of administration and did not include control (placebo) groups; therefore, it is complicated to make a solid conclusion about the safety and efficacy of FMT in IBD. Recently, two randomized placebo control trials evaluating the efficacy of FMT in IBD were published. In a study by Moayyedi et al, 75 patients with UC were randomized to receive FMT or placebo via enema once a week for 6 weeks.Citation46 The results showed that the treated group achieved higher remission compared with the placebo (24% vs 9%). The second trial enrolled 50 patients with mild to severe UC and randomized to receive stool from a healthy donor or autologous fecal microbiota via nasoduodenal delivery at weeks 0 and 3.Citation45 There was no statistically significant difference in clinical or endoscopic remission between the two groups.
Altogether, the results of FMT in IBD may look disappointing, especially if compared with the impressive results obtained in patients with rCDI (about 90% remission). However, these data highlight the fact that IBD is not a pure microbiota-driven disease such as CDI, but it is far more complex, suggesting that results obtained in CDI trials cannot be directly transposed to IBD. Furthermore, the studies of FMT in IBD have involved only a few patients so far and are quite inconsistent in many aspects, such as the age (pediatric vs adult patients), the control groups, dose and preparation of donor feces, delivery method, and frequency of FMT. Therefore, more randomized controlled placebo studies are needed to clarify and optimize the role of FMT in IBD.
Adverse effects of FMT
Potential side effects of FMT can be categorized into short-term and long-term. While short-term events can also be distinguished between side effects related to the delivery method or to FMT itself, very little information exists regarding long-term events considering the lack of lengthy prospective trials to assess its safety.
Short-term side effects
Regardless of the delivery method, the common side effects following FMT include a mild fever and mild GI symptoms (abdominal discomfort, flatulence, diarrhea, constipation, and vomiting) that usually resolve within few weeks.Citation18,Citation57,Citation64 The route of administration seems to affect the side effects profile. For example, high fever and rise of the C-reactive protein have been described via the nasojejunal routeCitation65 and rarely have been reported for aspiration pneumonia,Citation66 while perforation, bleeding, and effects related to anesthesia have been described for the colonoscopy.Citation23 Transmission of enteric pathogens through fecal donor material appears to be rare considering the screening procedure that donors undergo. However, two cases of intestinal infection (norovirus) at 2 and 12 days post-FMT were reported.Citation67 Mortality has been reported in the literature.Citation17,Citation68 One case described an aspiration event related to the delivery method of FMT, while in the second case, the patient died 13 days after FMT secondary to progressive pneumonia, for which the patient was treated with antibiotics before and after FMT. However, the latter can be unrelated to FMT, but rather associated with patient’s comorbidity.
Long-term side effects
The major concern about the safety of FMT in IBD is the assessment of long-term side effects. However, due to the lack of long prospective studies, there is not much information collected about long-term side effects, and many are speculative. A great theoretical risk may be the induction of chronic diseases based on the alteration of gut microbiota, which includes obesity, diabetes, atherosclerosis, and colon cancer. Significant gain of weight was reported in a woman who received FMT from an overweight donor. The recipient was also overweight at the time of FMT; thus, the data must be interpreted with caution.Citation69 However, studies in rodents have corroborated the abovementioned hypothesis. Some groups have reported transferring colitis from different knockout models to wild-type mice.Citation70,Citation71 The transplantation of human microbiota from obese subjects to rodents leads to obesity in rodents,Citation72 and FMT from lean donors increases insulin sensitivity in obese individuals with metabolic syndrome and thus legitimates the concern about the long-term risks of FMT. Finally, long-term follow-up combined with the analysis of screened donors and recipient specimens will be crucial to assess the safety and future adverse events.
Contraindications to FMT
FMT is often perceived as “natural” remedy by many patients and physicians, However, considering the fact that the transfer of complex microbiota can modify the host phenotype with unknown long-term effects, it is preferable to exclude certain categories of patients in which the delivery of FMT may worsen their condition, or it may even be fatal. For example, patients with severe bowel disease cannot undergo colonoscopy, while those with severe immunosuppression and decompensated liver cirrhosis are excluded considering the potential risk of enteric microbe transmission from donor’s stool. However, recently, Kelly et al published a retrospective study of immunocompromised patients who received FMT to treat rCDI in which they show that there were no infectious complications following FMT in these potentially “at-risk” patients.Citation23
Discussion
IBD is a chronic intestinal disorder, causes of which are not fully elucidated yet. Despite the availability of different therapeutic options, treatment dissatisfaction is still high, implying a reduced life quality of the patients and increased social, sanitary, and economic burden worldwide. Therefore, the identification of new players involved in the physiopathology of IBD will improve and expand the therapeutic armamentarium.Citation73
As early as the 1900s, physicians recognized that bacteria might promote development and maintenance of symptoms in colitis.Citation74 In the last decade, thanks to advanced molecular techniques, profound differences in the composition and functions of the microbiota have been demonstrated in patients with IBD compared with healthy subjects,Citation75–Citation77 recognizing a role of the gut microbiome in the etiology and pathogenesis of IBD. The high therapeutic efficacy of FMT in the treatment of rCDI is impressive, and although the microbial basis of IBD is far more complex and variable than that of rCDI, microbiome-based therapies are an important area of investigation for these chronic diseases. FMT is the first way to directly alter the intestinal microbiome; therefore, it is considered a promising therapy in IBD. However, standardized controlled studies are necessary to define which patient is eligible, frequency and optimal timing in which FMT should be proposed (at the early or late point of the disease course), and the optimal donor for each patient. Robust safety data of FMT are still missing, and long-term clinical follow-up will be crucial to address this issue. In addition, animal and human data showed that donor microbiota could induce certain chronic disease that is gut-driven.Citation69–Citation72 Thus, more information is necessary on the modification of host microbiota composition and future research on metabolic pathways and microbial genes in the GI tract, and their effects on protein expression will shed light on the role of the gut microbiota in these chronic conditions. Moreover, it will be interesting to identify active components of the gut microbiota that could be isolated and used as therapeutic agents in IBD. The rationale for that comes from the studies of Sokol et al, which identified the protective role of the F. prausnitzii in the intestinal inflammatory process.Citation49 Recently, the anti-inflammatory molecule produced by this commensal bacterium has been identified,Citation78,Citation79 suggesting that, in the near future, “artificial” microbiota can be generated and used to counterbalance the dysbiosis in a more standardized and controlled way compared with FMT. Finally, it is well accepted that IBD pathogenesis is related to an aberrant cross talk between the host immune system and gut microbiota;Citation3 however, thus far, the only approach envisaged was to inhibit the overactivated immune system. With our current understanding of the microbiological basis for IBD, it should be taken into account both players, and probably it should target them simultaneously to achieve optimal results.
Author contributions
TS and PR contributed to conception and design. TS, PR, AO, and VG contributed to analysis and interpretation. PR, TS, AO, and VG drafted the article. TS, PR, AO, and VG critically revised the article and approved the final version of the article. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- LovellRMFordACGlobal prevalence of and risk factors for irritable bowel syndrome: a meta-analysisClin Gastroenterol Hepatol2012107712.e4721.e422426087
- BarbaraGFeinle-BissetCGhoshalUCThe intestinal microenvironment and functional gastrointestinal disordersGastroenterology2016150613051318
- KhorBGardetAXavierRJGenetics and pathogenesis of inflammatory bowel diseaseNature2011474735130731721677747
- GazouliMWoutersMMKapur-PojskićLLessons learned – resolving the enigma of genetic factors in IBSNat Rev Gastroenterol Hepatol2016132778726726033
- Murgas TorrazzaRNeuJThe developing intestinal microbiome and its relationship to health and disease in the neonateJ Perinatol201131Suppl 1S29S3421448201
- RoundJLLeeSMLiJThe Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiotaScience2011332603297497721512004
- IvanovIIAtarashiKManelNInduction of intestinal Th17 cells by segmented filamentous bacteriaCell2009139348549819836068
- CandelaMPernaFCarnevaliPInteraction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 productionInt J Food Microbiol2008125328629218524406
- MatsumotoMKibeROogaTCerebral low-molecular metabolites influenced by intestinal microbiota: a pilot studyFront Syst Neurosci20137923630473
- CryanJFDinanTGMind-altering microorganisms: the impact of the gut microbiota on brain and behaviourNat Rev Neurosci2012131070171222968153
- GeversDKugathasanSDensonLAThe treatment-naive microbiome in new-onset Crohn’s diseaseCell Host Microbe201415338239224629344
- MorganXCTickleTLSokolHDysfunction of the intestinal microbiome in inflammatory bowel disease and treatmentGenome Biol2012139R7923013615
- SokolHSeksikPThe intestinal microbiota in inflammatory bowel diseases: time to connect with the hostCurr Opin Gastroenterol201026432733120445446
- HensonMAPhalakPMicrobiota dysbiosis in inflammatory bowel diseases: in silico investigation of the oxygen hypothesisBMC Syst Biol201711114529282051
- KimDZengMYNúñezGThe interplay between host immune cells and gut microbiota in chronic inflammatory diseasesExp Mol Med2017495e33928546562
- ShenZHZhuCXQuanYSRelationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantationWorld J Gastroenterol201824151429358877
- KellyCRKahnSKashyapPUpdate on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlookGastroenterology2015149122323725982290
- van NoodEVriezeANieuwdorpMDuodenal infusion of donor feces for recurrent Clostridium difficileN Engl J Med2013368540741523323867
- LefflerDALamontJTClostridium difficile infectionN Engl J Med2015372161539154825875259
- AustinMMellowMTierneyWMFecal microbiota transplantation in the treatment of Clostridium difficile infectionsAm J Med2014127647948324582877
- CammarotaGMasucciLIaniroGRandomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infectionAliment Pharmacol Ther201541983584325728808
- KassamZLeeCHYuanYHuntRHFecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysisAm J Gastroenterol2013108450050823511459
- KellyCRIhunnahCFischerMFecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patientsAm J Gastroenterol201410971065107124890442
- MooreTRodriguezABakkenJSFecal microbiota transplantation: a practical update for the infectious disease specialistClin Infect Dis201458454154524368622
- ZhangFCuiBHeXMicrobiota transplantation: concept, methodology and strategy for its modernizationProtein Cell20189546247329691757
- GoughEShaikhHMangesARSystematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infectionClin Infect Dis20115310994100222002980
- CostelloSPTuckerECLa BrooyJSchoemanMNAndrewsJMEstablishing a fecal microbiota transplant service for the treatment of Clostridium difficile infectionClin Infect Dis201662790891426628567
- MattilaEUusitalo-SeppäläRWuorelaMFecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infectionGastroenterology2012142349049622155369
- SatokariRMattilaEKainulainenVArkkilaPESimple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection – an observational cohort studyAliment Pharmacol Ther2015411465325355279
- LiaoCHShollenbergerLMSurvivability and long-term preservation of bacteria in water and in phosphate-buffered salineLett Appl Microbiol2003371455012803555
- TauxeWMDhereTWardARacsaLDVarkeyJBKraftCSFecal microbiota transplant protocol for clostridium difficile infectionLab Med2015461e19e2325805532
- CuiBLiPXuLStep-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitisJ Transl Med20151329826363929
- YoungsterIRussellGHPindarCZiv-BaranTSaukJHohmannELOral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infectionJAMA2014312171772177825322359
- HamiltonMJWeingardenARSadowskyMJKhorutsAStandardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infectionAm J Gastroenterol2012107576176722290405
- FullerBJCryoprotectants: the essential antifreezes to protect life in the frozen stateCryo Letters200425637538815660165
- BahlMIBergströmALichtTRFreezing fecal samples prior to DNA extraction affects the Firmicutes to Bacteroidetes ratio determined by downstream quantitative PCR analysisFEMS Microbiol Lett2012329219319722325006
- KellyCRKhorutsAStaleyCEffect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trialAnn Intern Med2016165960961627547925
- AasJGessertCEBakkenJSRecurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tubeClin Infect Dis200336558058512594638
- HirschBESaraiyaNPoethKSchwartzRMEpsteinMEHonigGEffectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infectionBMC Infect Dis20151519125885020
- PengZXiangJHeZColonic transendoscopic enteral tubing: a novel way of transplanting fecal microbiotaEndosc Int Open201646E610E61327556065
- NiXFanSZhangYCoordinated hospital-home fecal microbiota transplantation via percutaneous endoscopic cecostomy for recurrent steroid-dependent ulcerative colitisGut Liver201610697598027282271
- DavidLAMauriceCFCarmodyRNDiet rapidly and reproducibly alters the human gut microbiomeNature2014505748455956324336217
- RawlsJFMahowaldMALeyREGordonJIReciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selectionCell2006127242343317055441
- CammarotaGIaniroGTilgHEuropean consensus conference on faecal microbiota transplantation in clinical practiceGut201766456958028087657
- RossenNGFuentesSvan der SpekMJFindings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitisGastroenterology20151491110.e4118.e425836986
- MoayyediPSuretteMGKimPTFecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trialGastroenterology20151491102.e6109.e625857665
- GrinspanAMKellyCRFecal microbiota transplantation for ulcerative colitis: not just yetGastroenterology20151491151826021232
- SokolHToward rational donor selection in faecal microbiota transplantation for IBDJ Crohns Colitis201610437537626746170
- SokolHPigneurBWatterlotLFaecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patientsProc Natl Acad Sci U S A200810543167311673618936492
- SokolHSeksikPFuretJPLow counts of Faecalibacterium prausnitzii in colitis microbiotaInflamm Bowel Dis20091581183118919235886
- LiSSZhuABenesVDurable coexistence of donor and recipient strains after fecal microbiota transplantationScience2016352628558658927126044
- KazerouniABurgessJBurnsLJWeinLMOptimal screening and donor management in a public stool bankMicrobiome201537526675010
- BennetJDBrinkmanMTreatment of ulcerative colitis by implantation of normal colonic floraLancet198918630164
- BorodyTJGeorgeLAndrewsPBowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome?Med J Aust198915010604
- BorodyTJWarrenEFLeisSSuraceRAshmanOTreatment of ulcerative colitis using fecal bacteriotherapyJ Clin Gastroenterol2003371424712811208
- ColmanRJRubinDTFecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysisJ Crohns Colitis20148121569158125223604
- AngelbergerSReinischWMakristathisATemporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantationAm J Gastroenterol2013108101620163024060759
- SuskindDLSinghNNielsonHWahbehGFecal microbial transplant via nasogastric tube for active pediatric ulcerative colitisJ Pediatr Gastroenterol Nutr2015601272925162366
- KundeSPhamABonczykSSafety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitisJ Pediatr Gastroenterol Nutr201356659760123542823
- SuskindDLBrittnacherMJWahbehGFecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s diseaseInflamm Bowel Dis201521355656325647155
- VaughnBPVatanenTAllegrettiJRIncreased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s diseaseInflamm Bowel Dis20162292182219027542133
- HeZLiPZhuJMultiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn’s disease complicated with inflammatory massSci Rep201771475328684845
- CuiBFengQWangHFecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial resultsJ Gastroenterol Hepatol2015301515825168749
- KumpPKGröchenigHPLacknerSAlteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitisInflamm Bowel Dis201319102155216523899544
- VermeireSJoossensMVerbekeKDonor species richness determines faecal microbiota transplantation success in inflammatory bowel diseaseJ Crohns Colitis201610438739426519463
- BaxterMAhmadTColvilleASheridanRFatal aspiration pneumonia as a complication of fecal microbiota transplantClin Infect Dis201561113613725805303
- SchwartzMGluckMKoonSNorovirus gastroenteritis after fecal microbiota transplantation for treatment of Clostridium difficile infection despite asymptomatic donors and lack of sick contactsAm J Gastroenterol201310881367
- ShaSLiangJChenMSystematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and childrenAliment Pharmacol Ther201439101003103224641570
- AlangNKellyCRWeight gain after fecal microbiota transplantationOpen Forum Infect Dis201521ofv00426034755
- GarrettWSLordGMPunitSCommunicable ulcerative colitis induced by T-bet deficiency in the innate immune systemCell20071311334517923086
- ElinavEStrowigTKauALNLRP6 inflammasome regulates colonic microbial ecology and risk for colitisCell2011145574575721565393
- RidauraVKFaithJJReyFEGut microbiota from twins discordant for obesity modulate metabolism in miceScience20133416150124121424009397
- RawlaPSunkaraTRajJPRole of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectivesJ Inflamm Res20181121522629844695
- WallisFCThe surgery of colitisBr Med J190912505101320764215
- NagalingamNALynchSVRole of the microbiota in inflammatory bowel diseasesInflamm Bowel Dis201218596898421936031
- ManichanhCBorruelNCasellasFGuarnerFThe gut microbiota in IBDNat Rev Gastroenterol Hepatol201291059960822907164
- FrankDNAmandALStFeldmanRABoedekerECHarpazNPaceNRMolecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseasesProc Natl Acad Sci U S A200710434137801378517699621
- MiquelSLeclercMMartinRIdentification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitziiMBio201562 pii.e00300-15
- QuévrainEMaubertMAMichonCIdentification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s diseaseGut201665341542526045134
