Abstract
Background
Disruption of the balance between the production of ROS and their removal through enzymatic and non-enzymatic (antioxidant) processes has been proposed as a new mechanism in the pathology of polycystic ovary syndrome (PCOS). Evidence from animal models of PCOS (prenatally androgenized sheep) has suggested that treatment with insulin sensitizers, but not antiandrogens, can reduce increases in ROS.
Materials and methods
In the present study, we investigated the effects of neonatal treatment with a gonadotropin-releasing hormone (GnRH) agonist (leuprolide acetate) on prenatally androgenized sheep with testosterone propionate to determine its impact on oxidative stress molecules (ferric reducing antioxidant power [FRAP], advanced oxidation protein product [AOPP], nitric oxide [NOx], albumin) at 8, 12, and 18 months of age.
Results
Androgenized ewes (but not leuprolide-treated ewes) showed reduced total cholesterol levels associated with a decrease in the ratio of visceral to subcutaneous adiposity (adjusted to abdominal area) as determined by computed tomography. In androgenized ewes at 12 months of age, an increase in subcutaneous fat and relative decrease in the visceral fat compartment did not affect the expression of REDOX markers. At 18 months of age, however, the levels of NOx metabolites decreased in androgenized animals, but remained close to normal in ewes subjected to neonatal treatment with leuprolide acetate. Other oxidative stress parameters (FRAP, AOPP, albumin) did not vary among groups.
Conclusion
Our results demonstrate that the GnRH agonist leuprolide (as a single dose after birth) had weak effects on markers of the oxidative stress balance.
Introduction
The REDOX state reflects the balance between the production of ROS and their removal through enzymatic and non-enzymatic (antioxidant) processes. Overproduction of ROS plays a role in the development of common disorders such as diabetes mellitus, dyslipidemia, obesity, chronic obstructive pulmonary disorder, and cancer.Citation1–Citation3
Polycystic ovary syndrome (PCOS), one of the most common causes of endocrine infertility, is characterized by reproductive and metabolic disturbances as well as disruption of the REDOX state.Citation4–Citation13 Studies conducted in women with PCOS revealed increased oxidant and antioxidant markers in the blood, although the results were inconsistent because of the high heterogeneity of patients (age, body mass index, severity of hyperandrogenism) and tests used to evaluate oxidative stress.Citation13–Citation15
Animal models are widely used to understand the physiopathology of PCOS and can clarify the potential roles of disruptors in oxidative stress. Classical studies using prenatally androgenized (PA) sheep have replicated several abnormalities described in women with PCOS. Puttabyatappa et al used sheep in their study to explore the impact of prenatal and postnatal interventions with flutamide (antiandrogen) and rosiglitazone (insulin sensitizer), respectively, which demonstrated a partial response to flutamide by reducing protein-bound oxidized tyrosine moieties, 3-nitrotyrosine (NY), an oxidative stress marker, in the plasma.Citation16 The levels of antioxidant molecules such as high-molecular adiponectin and the mRNA expression were evaluated in the tissues only and were not affected by flutamide or rosiglitazone.Citation16
In rodents, pharmacological intervention has gained attention for protecting or reversing the reproductive phenotype of androgenized rodents. Recent studies demonstrated that administration of a gonadotropin-releasing hormone (GnRH) antagonist (cetrorelix acetate) during gestation (E16–E18.5) to rats treated with anti-Mullerian hormone or GNRH agonist depot (leuprolide acetate) at day 5 after birth in rats treated postnatally with testosterone propionate impaired the development of PCOS features (mainly reproductive) in the adult age.Citation17,Citation18
Although leuprolide acetate administration was not shown to alter the REDOX state in PCOS rats,Citation18 its effects have not been tested in androgenized sheep, which is considered an animal model of PCOS more similar to humans. Therefore, the aim of the present study was to examine the effects of neonatal treatment with a GnRH agonist on PA sheep with testosterone propionate, looking to determine its impact on biochemical and oxidative stress molecules.
Materials and methods
Animals
All procedures were approved by the Institutional Committee for Ethics in Animal Experiments at the Federal University of Santa Maria, RS, Brazil (CEUA-UFSM number 0009/2013). The procedures conformed to the guidelines of the Brazilian National Council of Control of Animal Experimentation, which follows the “Principles of Laboratory Animal Care” established by the National Institutes of Health (Bethesda, MD, USA). For this study, multiparous females aged 3–5 years from Corriedale female sheep (a cross of the Merino and Lincoln breeds) were obtained from a local farmer (30° 20’ south latitude). The animals’ cycles were synchronized and then subjected to artificial insemination using a mix of fresh semen collected from adult rams, as previously described.Citation19 Pregnancy was confirmed at 30 days by intra-vaginal/rectal ultrasonography. The animals were raised in an extensive farming system, with free access to water supplemented with mineral salts, pellet feed (1.5% of live weight), and alfalfa hay. The androgenization protocol included administration of testosterone propionate (Androgenol™; Hertape Calier, Juatuba, Brazil) 100 mg intramuscularly (i.m.) twice per week to pregnant ewes during days 30–90 of gestation.Citation20 No treatment was necessary for controls, as supported by the literature. A group of androgenized female sheep was administered i.m. injection of a GnRH agonist (leuprolide acetate depot 7.5 mg) before 48 hours of life. All lambs were weaned at 3–4 months and had access to hay ad libitum (plus mineral salts). A group of ewes was euthanized with sodium thiopental at 12 months, including four PA ewes and five controls (C), while the remaining animals were euthanized at 19 months of age. Androgenized ewes exhibited higher levels of serum testosterone at 12 months and showed insulin resistance as defined by intravenous glucose tolerance test and higher disruption of cycles (66%; data not shown).
Laboratory measurements
An assay kit (Labtest Diagnostics®, Lagoa Santa, Brazil) was used to enzymatically evaluate the levels of total blood cholesterol, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C) triglyceride, uric acid, and glucose in the serum. Albumin, which is a scavenger antioxidant molecule, has an intra-assay coefficient of variation of 1.1%. Blood was collected by jugular venipuncture into tubes with and without EDTA (Vacutainer®; BD Biosciences, Franklin Lakes, NJ, USA) and the blood was centrifuged (2,500 ×g for 15 minutes) and stored at −80°C until use.
Oxidative stress markers: ferric-reducing ability of plasma (ferric reducing antioxidant power [FRAP]), advanced oxidation protein product (AOPP) levels in serum, nitric oxide (NOx)
A colorimetric protocol was employed using a Cobas Mira® automated analyzer (Roche Diagnostics, Basel, Switzerland) to estimate the NOx levels and the AOPP in the serum; as well, the FRAP assay was conducted, as previously described.Citation21–Citation23 Samples were processed simultaneously. The intra-assay coefficient of variation was 1%–2% for FRAP, 4% for AOPP, and 8.3% for NOx.Citation21–Citation23
Computed tomography and estimation of abdominal and subcutaneous fat
Computed tomography (CT) 64 channel (acquisition W:1,800 L:400 bone window; GE Healthcare, Little Chalfont, UK) was performed for ewes at 12 months of age within the first hour after euthanasia. According to the attenuation coefficients obtained by CT, images (region of interest) were classified as fat, muscle, or other tissues. Image processing of slices of 1 mm was performed using Analyze 12.0® software (AnalyzeDirect, Overland Park, KS, USA).Citation24 The adipose tissue quantification module from Analyze 12.0® was used to determine the segmental (3D) amount of fat, subcutaneous (SC) or visceral, in each animal. For this study, control sheep (n=5) and PA (n=4) sheep were employed.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.0 software (GraphPad, Inc., San Diego, CA, USA). Two groups were compared using Student’s t-test (if a normal distribution was expected) or Mann–Whitney U test (if an asymmetrical distribution of data was expected). Significance was assumed at P<0.05. Two-way ANOVA was used to analyze two independent variables.
Results
Weight, visceral fat, and biochemical features of control (C), PA, and prenatally androgenized and GnRH-treated (LA) sheep
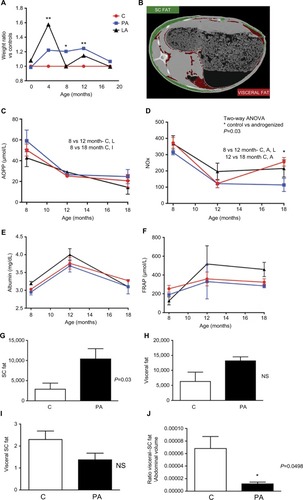
shows the biochemical profile of control sheep (C) against PA or prenatally androgenized sheep treated neonatally with leuprolide acetate depot (LA) at 12 and 18 months of age. At 12 months, total cholesterol levels were reduced in PA ewes with a mean ± SD of 54.86±9.2 mg/dL compared to C (68.5±9.9 mg/dL, P<0.001); LA sheep (68.5±4.9 mg/dL) were between C and PA (). No significant differences were observed in the levels of HDL-C (), LDL-C (), or triglycerides () among the three groups at 12 months. Basal glucose was very similar in the three groups, with values of 70.1±12.7, 72.8±8.11, and 70.±11.3 mg/dL in the C, PA, and LA groups, respectively (). The triglyceride-glucose (TyG) index, a marker of insulin resistance and metabolic syndrome,Citation25–Citation28 was not changed between the control (C) and androgenized groups (PA or LA). As shown in , there were no significant differences in TYG index values in the C group (1,362±392), PA group (1,288±244.3), and LA group (1,336±1,206.5). Remarkably, uric acid levels were decreased in PA ewes whose levels at 12 months were 0.51±0.07 mg/dL, while in C ewes this value was 0.83±0.33 mg/dL and in LA ewes it was 0.6±0.28 mg/dL (Student’s t-test P=0.04 PA vs C; ). PA sheep showed higher weights than controls at 12 months of age (). These animals exhibited an increased mass of SC depot (P=0.03; ) estimated by CT 3D analysis of an abdominal segment (), and a similar visceral fat depot () and trend to a lower SC to visceral ratio () were observed. Curiously, the ratio of SC to visceral ratio adjusted to the abdominal volume (PA animals showed higher weights) revealed a reduced amount of visceral fat in young PA ewes ().
Figure 1 Biochemical profile of C, PA, and LA groups at 12 and 18 months of age.
Figure 2 Weight, oxidative stress markers, and fat depot analysis (CT).
Notes: (A) Ratio of weight of C (red), PA sheep (blue), and LA sheep (black). (B) CT image of an abdominal section; images in red and green identify (with the support of the software analyze 12.0) subcutaneous and visceral fat depots, respectively. Measures of AOPP (C), NOx (D), albumin (E), and FRAP (F) staring from 8th to 18th month of age in c sheep (red), PA sheep (blue), and LA sheep (black). Values of (G) subcutaneous fat mass and (H) visceral fat, both obtained by the use of analyze 12.0 software. (I) Ratio of visceral to subcutaneous fat and (J) ratio of visceral to subcutaneous fat adjusted to the abdominal area. Results (C–J) show mean ± SEM. *Indicates statistical significance of weight in kilograms with P<0.05 (ANOVA). **P<001.
Abbreviations: AOPP, advanced oxidation products of proteins; C, control; CT, computed tomography; FRAP, ferric-reducing antioxidant power; LA, prenatally androgenized sheep treated with leuprolide acetate; NOx, nitric oxide; NS, nonsignificant; PA, prenatally androgenized; SC, subcutaneous; SEM, standard error of the mean.
Notes: Animals at 12 months: (A) total cholesterol, (B) HDL cholesterol, (E) LDL cholesterol, (F) triglycerides, (I) glucose, (J) TYG index, (M) uric acid. Animals at 18 months: (C) total cholesterol, (D) HDL cholesterol, (G) LDL cholesterol, (H) triglycerides, (K) glucose, (L) TYG index, (N) uric acid. Results show mean + SEM. Number of ewes evaluated at 12 months: C, n=7; Pa, n=6; LA, n=3. Number of ewes evaluated at 18 months: C, n=4; Pa, n=4; LA, n=3. Superscript letters a and b indicate that they are statistically different (ANOVA test).
Abbreviations: C, control; HDL, high-density lipoprotein; la, prenatally androgenized sheep treated with leuprolide acetate; LDL, low-density lipoprotein; Pa, prenatally androgenized; SEM, standard error of the mean.

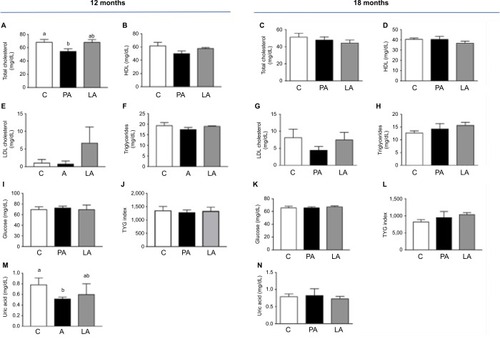
At 18 months of age (and similar weight), as shown in , the differences in biochemical variables among control (C) and the two androgenized groups (PA and LA) disappeared. Total cholesterol (), HDL (), and LDL () were similar among the three groups. Basal glucose () and basal triglyceride () levels were similar among groups: glucose levels were 66±6.7 mg/dL in C sheep, 66±3.7 mg/dL in PA sheep, and 67.7±2.5 mg/dL in LA sheep. Triglyceride levels in the serum were 12.7±2.5, 14.33±3.5, and 15.67±2.08 mg/dL in C, PA, and LA sheep, respectively; these values were not significantly different (P=0.08; ). TYG did not change among groups. However, there was an increasing trend in TYG in LA ewes (1,038±98.8 mg/dL) vs C ewes (828±128.4 mg/dL; Student’s t-test P=0.06); the values for PA sheep were intermediate (953.7±291.1 mg/dL; ). Uric acid levels did not vary among these three groups (). Insulin resistance estimated by the intravenous glucose tolerance test was increased in PA and LA sheep at 16 months of age, as previously reported.Citation29
Oxidative stress markers and antioxidant capacity (albumin) in serum and plasma
The levels of oxidants (AOPP and FRAP), NOx, and molecules with antioxidant function (albumin) from month 8 to 18 of life are shown in . The serum levels of AOPP (related to oxidation of proteins) decreased over time and did not differ between the three groups (). At month 18, the AOPP values were 20.6±6.1, 24.7±6.8, and 14.37±6.3 µmol/L in the C, PA, and LA groups, respectively. Evaluation of FRAP (associated with the reducing ability of plasma) showed similar results among groups. Starting at month 8, the levels of FRAP in the C, PA, and LA groups were 253.8±36, 190.7±4.1, and 125±43 µmol/L, respectively. At 12 months of age, FRAP levels in the C, PA, and LA groups were 358.3±73, 329.8±184, and 517±194 µmol/L, respectively. C, PA, and LA ewes at 18 months showed no differences in FRAP levels, with values of 323.4±35.8, 284±20, and 459±76.07 µmol/L in the C, PA, and LA groups, respectively (). NOx levels showed a slight decrease from month 8 to 18 of age in the sheep. The initial values of NOx at month 8 were 372±33.4, 313.7±14.5, and 368.8±50 in the C, PA, and LA animals, respectively (). These values decreased at month 12 in the three groups (123±25.2, 121.7±25.2, and 196±52 in the C, PA, and LA groups, respectively). Remarkably, the NOx values at month 18 were significantly decreased in PA ewes compared to those in C ewes. The NOx levels were 255.9±26.4 and 113.6±40 in the C and PA groups, respectively (two-way ANOVA; P=0.048). Albumin, an antioxidant scavenger molecule, was also evaluated (). Albumin levels were increased in the three groups at 12 months. At month 18, the albumin levels were 3.27±0.04, 3.1±0.2, and 3.1±0.2 mg/dL in the C, PA, and LA groups, respectively ().
Discussion
The results of this study revealed a limited impact of GnRH agonist (leuprolide acetate) on modifying metabolic and oxidative stress parameters in PA sheep.
As shown above, neonatal treatment with androgens led to reduced levels of NOx at 18 months of age, which was prevented by administration of LA. NOx is produced by endothelial cells and acts as a second messenger to mediate the inhibition of platelet aggregation and anti-inflammatory and vasodilatation functions. We evaluated the major stable metabolites of NOx, particularly nitrites and nitrates (NOx). NOx has various other functions in reproduction, cardiac function, and cellular signaling.
Although LA improved NOx levels, the treatment did not modify the levels of other oxidative stress markers (FRAP and AOPP) or albumin, glucose, total cholesterol (and its fractions), uric acid, or TYG index. Our results partially agree with those obtained for sheep postnatally treated for 8 weeks with flutamide (an antiandrogen), which did not modify the levels of protein-bound oxidized tyrosine moiety NY, an oxidative stress marker in the plasma.Citation16 Our results agree with those of a study in which androgenized rats were treated with LA depot on day 5 of life; although reproductive function was improved, androgens (testosterone or androstenedione) or oxidant and antioxidant molecules in the serum and plasma were not modified.Citation18 In the previous study, however, disruption of oxidative stress markers was associated with a worse metabolic profile (TYG) in female Wistar rats androgenized after birth, which was not observed in our study using sheep. However, as recently described, PA and LA sheep showed significantly elevated insulin levels in an intravenous glucose tolerance test, suggesting insulin resistance in both androgenized groups.Citation29
Androgen levels or the presence of hyperandrogenemia also reflects changes in the oxidative stress status, as gender is thought to influence disruptions in the oxidative/antioxida-tive balance. However, treatment with LA did not change the androgens levels (data not shown).Citation29
Interestingly, despite higher weights at 12 months of age, PA sheep exhibited a proportionally lower ratio of visceral to SC fat (estimated by 3D CT) and lower total cholesterol. These features were similar to those described by Veiga-Lopez et al who reported an increased total amount of visceral fat and reduced ratio of visceral to SC fat in sheep treated with high levels of bisphenol A during gestation.Citation24
We evaluated three REDOX markers in an animal model (sheep), which replicate several abnormalities of PCOS and which were followed from month 8 to 18 of life. One limitation of this study was the small number of animals employed (increased chance of beta error). Additionally, we did not evaluate the levels of adipokines (adiponectin, leptin, irisin), which would help to clarify the effects of these molecules on overall inflammation.
In summary, our findings indicate that administration of LA had minimal effects on the REDOX balance and metabolic parameters in PA sheep.
Conclusion
Our results confirm the limited impact of reproductive drugs such as leuprolide acetate (a GnRH agonist) on markers of the oxidative stress balance.
Ethics approval
All procedures were approved by the Institutional Committee for Ethics in Animal Experiments at the Federal University of Santa Maria, RS, Brazil (CEUA-UFSM number 0009/2013).
Acknowledgments
We would like to thank Dr Michael Philcock, Mayo Clinic (Analyze 12.0 software license) and Prof João Francisco Coelho de Oliveira (in memoriam) for his great contribution to the design of the study. This work was supported by CAPES Foundation and the National Council for Scientific and Technological Development (CNPq) Brazil, grant 445019/2014-0 (http://www.cnpq.br/).
Disclosure
The authors report no conflicts of interest in this work.
References
- LiuZRenZZhangJRole of ROS and nutritional antioxidants in human diseasesFront Physiol2018947729867535
- JiangLDiazPTBestTMStimpflJNHeFZuoLMolecular characterization of redox mechanisms in allergic asthmaAnn Allergy Asthma Immunol2014113213714224986036
- SinghRDeviSGollenRRole of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-lifeDiabetes Metab Res Rev201531211312624845883
- FranksSPolycystic ovary syndromeN Engl J Med1995333138538617651477
- NormanRJDewaillyDLegroRSHickeyTEPolycystic ovary syndromeLancet2007370958868569717720020
- BalenAHomburgRFranksSDefining polycystic ovary syndromeBMJ2009338a296819218318
- Diamanti-KandarakisEArgyrakopoulouGEconomouFKandarakiEKoutsilierisMDefects in insulin signaling pathways in ovarian steroidogenesis and other tissues in polycystic ovary syndrome (PCOS)J Steroid Biochem Mol Biol20081093–524224618440223
- DeepikaMLNaliniSMaruthiGAnalysis of oxidative stress status through Mn test and serum MDA levels in PCOS womenPak J Biol Sci201417457457725911850
- AzzizRCarminaEChenZPolycystic ovary syndromeNat Rev Dis Primers201621605727510637
- VictorVMRovira-LlopisSBañulsCInsulin resistance in PCOS patients enhances oxidative stress and leukocyte adhesion: role of myeloperoxidasePLoS One2016113e015196027007571
- AbruzzeseGACerrroneGEGamezJMLipid accumulation product (LAP) and visceral adiposity index (VAI) as markers of insulin resistance and metabolic associated disturbances in young Argentine women with polycystic ovary syndromeHorm Metab Res2017491232927571188
- BañulsCRovira-LlopisSMartinez de MarañonAMetabolic syndrome enhances endoplasmic reticulum, oxidative stress and leukocyte-endothelium interactions in PCOSMetabolism20177115316228521868
- Behboudi-GandevaniSAmiriMBidhendi YarandiRThe risk of metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysisClin Endocrinol (Oxf)201888216918428930378
- ZhangRLiuHBaiHOxidative stress status in Chinese women with different clinical phenotypes of polycystic ovary syndromeClin Endocrinol (Oxf)2017861889627489079
- ZhangJZhangYLiuHAntioxidant properties of high-density lipoproteins are impaired in women with polycystic ovary syndromeFertil Steril201510351346135425813288
- PuttabyatappaMAndriessenVMesquittaMZengLPennathurSPadmanabhanVDevelopmental programming: impact of gestational steroid and metabolic milieus on mediators of insulin sensitivity in prenatal testosterone-treated female sheepEndocrinology201715892783279828911168
- TataBMimouniNEHBarbotinALElevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthoodNat Med201824683484629760445
- Serrano MujicaLKBertolinKBridiAThe impact of postnatal leuprolide acetate treatment on reproductive characteristics in a rodent model of polycystic ovary syndromeMol Cell Endocrinol201744212513327988272
- MirandaVOOliveiraFCDiasJHEstrus resynchronization in ewes with unknown pregnancy statusTheriogenology201810610310729049921
- OrtegaHHReyFVelazquezMMPadmanabhanVDevelopmental programming: effect of prenatal steroid excess on intraovarian components of insulin signaling pathway and related proteins in sheepBiol Reprod20108261065107520147730
- BenzieIFStrainJJThe ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assayAnal Biochem1996239170768660627
- MedeirosMSSchumacher-SchuhACardosoAMIron and oxidative stress in Parkinson’s disease: an observational study of injury biomarkersPLoS One2016111e014612926751079
- TatschEBochiGVPereiraRSA simple and inexpensive automated technique for measurement of serum nitrite/nitrateClin Biochem201144434835021185277
- Veiga-LopezAMoellerJSreedharanRDevelopmental programming: interaction between prenatal BPA exposure and postnatal adiposity on metabolic variables in female sheepAm J Physiol Endocrinol Metab20163103E238E24726646100
- Simental-MendíaLERodríguez-MoránMGuerrero-RomeroFThe product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjectsMetab Syndr Relat Disord20086429930419067533
- González-TorresLVázquez-VelascoMOlivero-DavidRGlucomannan and glucomannan plus Spirulina added to pork significantly block dietary cholesterol effects on lipoproteinemia, arylesterase activity, and CYP7A1 expression in Zucker fa/fa ratsJ Physiol Biochem201571477378426475369
- PintoBAMeloTMFlisterKFEarly and sustained exposure to high-sucrose diet triggers hippocampal ER stress in young ratsMetab Brain Dis201631491792727154727
- QuCZhouXYangGLiLLiuHLiangZThe natural logarithm of zinc-α2-glycoprotein/HOMA-IR is a better predictor of insulin sensitivity than the product of triglycerides and glucose and the other lipid ratiosCytokine2016799610226797477
- Tonellotto dos SantosJEscarião da NóbregaJSerrano MujicaLKPrenatal androgenization of ewes as a model of hirsutism in polycystic ovary syndromeEndocrinology2018159124056406430376052
