Abstract
Purpose
The aim of the study was to characterize the severity of the systemic inflammatory response induced by lipopolysaccharide (LPS) in animals with different resistance levels to hypoxia.
Materials and methods
Two to three months old male Wistar rats (220–240 g) were divided according to hypoxia tolerance in a hypobaric chamber. After a month, they were injected intraperitoneally with Escherichia coli LPS at a dose of 1.5 mg/kg. After 3, 6 and 24 hours of LPS injection, we studied the levels of IL-1β, C-reactive protein (CRP) and TGF-β in the serum, the expression of Hif-1α and Nf-kb in the liver, morphological disorders in the lung and ex vivo production of IL-10 by splenic cells activated by ConA.
Results
In the early periods after the injection of LPS, increase in Nf-kb expression in the liver was observed only in the rats susceptible to hypoxia. After 6 hours of LPS injection, the number of neutrophils in the interalveolar septa of the lungs of rats susceptible to hypoxia was higher than in tolerant rats. This points to the development of more pronounced LPS-induced inflammation in the rats susceptible to hypoxia and is accompanied by increased expression of Hif-1α in the liver after 6 hours of LPS administration, serum IL-1β level after 3 hours and CRP level after 24 hours. The production of the anti-inflammatory cytokine IL-10 by the spleen was significantly decreased after 6 hours of LPS injection only in the animals tolerant to hypoxia. After 24 hours of LPS injection, a significant decrease in serum TGF-β level occurred in the rats tolerant to hypoxia in comparison with the control group, which improved the survival rates of the animals.
Conclusion
We have demonstrated the differences in the severity of the LPS-induced inflammatory response in male Wistar rats with different resistance levels to hypoxia. Rats susceptible to hypoxia are characterized by a more pronounced inflammatory response induced by LPS.
Introduction
It is known that the severity of infectious and inflammatory diseases, including sepsis, mortality and survival rates depend on many patient-specific factors, in particular, ethnicity, age and sex.Citation1–Citation3 However, it remains unclear why the severity of the course, the outcome of the disease and the likelihood of secondary infections in a homogeneous population of people of the same age and sex differ.Citation3 Perhaps, one of the factors determining these differences may be the individual characteristics of resistance to the lack of oxygen, since it is known that hypoxia develops during inflammation.
Hypoxia is an important physiological stimulus for organisms. During adaptation to low oxygen content in cells and tissues, genes that participate in the metabolism of glucose and iron, angiogenesis and cell proliferation are activated. In eukaryotic cells, the key component responsible for the regulation of the molecular response to hypoxia is the transcription factor hypoxia-inducible factor 1 (HIF-1).Citation4 HIF-1 is a heterodimeric complex consisting of two subunits, HIF-1α and HIF-1β. HIF-1β is constitutively present in the cells, but HIF-1α is targeted for proteasomal degradation by a set of oxygen-sensing prolyl-hydroxylases (PHDs) during normoxic conditions. Due to the fact that oxygen is required as a cofactor for PHD-mediated hydroxylation of HIF-1α, hypoxia causes a functional inhibition of PHDs, leading to stabilization of HIF-1α. Under hypoxic conditions, HIF-1α accumulates in the cytoplasm, translocates into the nucleus, forming a complex with HIF-1β, and binds the hypoxia-response elements on the promoters of hypoxia-responsive genes, inducing their expression.Citation5,Citation6
Inflammation in various tissues develops local hypoxia due to microcirculatory disturbances, as well as an increase in oxygen demand of the immune cells during their infiltration into the inflammatory focus.Citation7 Earlier, the relationship between the molecular pathways of hypoxia and inflammation was revealed. NF-κB influences the expression of HIF-1α in both hypoxia and in response to inflammatory stimuli. The proximal part of the promoter of the HIF-1a gene contains an NF-κB–binding site.Citation8–Citation10 In 2006, Frede et al showed that lipopolysaccharide (LPS) can induce toll-like receptor 4 (TLR-4)– and NF-κB–dependent increase in mRNA and HIF-1α protein levels.Citation11 It has also been demonstrated that HIF-1α is stabilized by active forms of oxygen and the proinflammatory cytokine IL-1β.Citation12,Citation13 HIF-1α, in turn, can activate NF-κB: it is known that inhibitors promoting the ubiquitin-dependent destruction of HIF-1α also control the activity of the kinase complex I kappa B kinase (IKK), responsible for the regulation of NF-κB.Citation14,Citation15
The result of HIF-1 activation depends on the context in which it was activated (hypoxia or inflammation). If HIF-1 is elevated by hypoxia, the transcription of various targeted genes is enhanced, which makes it possible to adapt to the lack of oxygen. Being initiated by the TLR-4– and NF-κB– dependent pathways, the genes of proinflammatory cytokines become active.Citation16 According to the literature, HIF can play both anti-inflammatory and proinflammatory roles under conditions of inflammation.Citation17 In mice, HIF-1 was described as a protective factor in the acute colitis model: deficiency of HIF-1α in animals with colitis resulted in high mortality, and in surviving mice, to more severe clinical manifestations.Citation18 In contrast, in systemic infections such as sepsis, the increase in HIF-1α levels results in greater mortality and elevated levels of proinflammatory cytokines (IL-1β, TNF-α) in the blood serum and decrease in levels of anti-inflammatory cytokine IL-10, which promotes activation of the immune response.Citation19 The activation of HIF-1 as a potential prognostic marker of sepsis is being discussed.Citation20
Human population, as well as other species of animals, is heterogeneous in hypoxia tolerance.Citation21,Citation22 The genetic polymorphism of the HIF1α gene affects the severity and outcome of infectious and inflammatory diseases. For example, it is established that the polymorphism of the gene HIF1α (1772T allele), which determines its high level of expression, is a risk factor for the development of abdominal aortic aneurysm and oral lichen planus.Citation23,Citation24 The other polymorphic HIF-1α rs12434439 GG genotype plays a protective role for rheumatoid arthritis development,Citation25 but gene polymorphism HIF-1α rs11549467 is associated with the risk of COPD.Citation26
It is known that laboratory animals are divided by their resistance to hypoxia.Citation27–Citation30 Animals that are tolerant and susceptible to hypoxia differ by many parameters (such as antioxidant activity, mitochondrial enzyme complex I activity, etc),Citation31 including the content of HIF-1α. It has been demonstrated that in rats susceptible to hypoxia, under normal conditions, the level of HIF-1α in the neocortex is 1.7 times higher than in tolerant rats.Citation27 Earlier, we demonstrated that at different periods after acute hypoxic exposure, rats tolerant and susceptible to hypoxia are characterized by the variability in expression of Nf-kb and TGF-β cytokine content, modulating inflammatory responses.Citation32 This may cause the distinctive features of the development of inflammation. The obtained data indicate that animals with different tolerance levels to hypoxia have various adaptive capabilities and predisposition to the development of inflammatory diseases: in the susceptible animals, the oxidative stress marker 8-isoprostane increases after hypoxic exposure, which is associated with the damage to cellular macromolecules and increase in the level of TGF-β.Citation32
In experimental researches, one of the widespread models of inflammation is LPS-induced systemic inflammatory response syndrome (SIRS). LPS is a cell wall component of gram-negative bacteria, which is recognized by TLR-4 on the surface of immunocompetent cells.Citation33,Citation34 LPS is a common inflammatory stimulus in clinical and laboratory studies, and its effects on NF-κB and inflammatory mediators have been well characterized.Citation35,Citation36 Injection of high doses of LPS leads to the development of severe inflammatory response, which manifests itself via hypercoagulation with the development of disseminated intravascular coagulation (DIC) syndrome, acute respiratory distress syndrome, vacuolar cell degeneration, dystrophic changes and necrosis in the liver and endotoxemia.Citation37–Citation39
Hypoxia, resulting from the microcirculatory disorders, caused by DIC plays a key role in the progression of SIRS and sepsis.Citation37 The severity of hypoxic damage to tissues and organs not only depends on the microcirculatory disorders, but is also mostly determined by individual resistance to hypoxia, which is not taken into consideration in clinical and experimental studies.
Therefore, the aim of the study was to determine the differences in the severity of the systemic inflammatory response caused by the administration of LPS in male Wistar rats with different resistance levels to hypoxia.
Materials and methods
Experimental animals
Male Wistar rats (n=60), 2–3 months old and weighing 220–240 g, were purchased from the animal breeding facility branch “Stolbovaya” of the Federal State Budget Institution of Science, the “Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency”, Russia. Six rats per cage (18.5×60×38 cm) were housed in a temperature-regulated room under 12:12 hour light–dark cycle and relative humidity between 40% and 50% and with unlimited access to water and food (“Char”, JSC “Range-Agro”, Turakovo, Russia). We obtained permission from the Bioethics Committee of the Science Research Institute of Human Morphology (Protocol No. 16, November 11, 2015) for conducting the study. All manipulations with animals were carried out according to the European convention for the protection of vertebrate animals used for experimental and other scientific purposes (ets no. 123), Strasbourg, 2006, and all efforts were made to minimize the suffering and distress of animals.
Determination of resistance to hypobaric hypoxia
Hypoxic tolerance was determined by measuring the time taken for the onset of gasping. According to the literature, this parameter characterizes the organism’s resistance to hypoxia.Citation27–Citation30 Adult male Wistar rats were exposed, one at a time, to simulated hypobaric hypoxia, equivalent to the altitude of 11,500 m, as described previouslyCitation27–Citation30,Citation32,Citation40–Citation42 in an animal decompression chamber coupled to a mercury barometer (equivalent to 180 mmHg). All the decompressions and recompressions were achieved gradually at the rate of 600 m (≈40 mmHg)/min to prevent any tissue injury to the organism as a result of a sudden fall or rise in ambient pressure. The airflow in the chamber was 2 L/min, while the relative humidity was maintained at 40%–50%. The time taken for appearance of the first sign of gasping, a characteristic hyperventilatory response, was recorded using an electronic stopwatch. Based on their gasping time, animals were categorized into three groups: normal (80–240 seconds, n=12), tolerant (>240 seconds, n=25) and susceptible (<80 seconds, n=23). Normal rats were not used in the experiments. After the experiment, all rats were found to be alive and to have resumed normal activity without any evident sign of pathology.
Modeling of SIRS
One month after the determination of resistance to hypoxia,Citation30,Citation42 rats in the experimental groups were injected intraperitoneally with LPS from Escherichia coli O26:B6 (Sigma-Aldrich, St Louis, MO, USA) at a dose of 1.5 mg/kg, leading to pathological changes in target organs.Citation43 The animals were euthanized with an overdose of carbon dioxide gas using a gradual fill (30% chamber volume per minute) technique after 3, 6 and 24 hours of LPS injection (five or eight animals for each term). The timing choice was determined by the fact that Nf-kb expression increased after 1-2 and 6 hours, and pronounced pathological changes in the target organs developed on the first day after the administration of LPS.Citation44,Citation45 In control groups, the rats tolerant (n=5) and susceptible (n=5) to hypoxia received an intraperitoneal injection of physiological saline.
Mortality of animals from SIRS
Within a day after the injection of LPS, some of the animals died. Mortality rates of rats in response to the injection of LPS were 3 out of 18 (17%) in the susceptible to hypoxia group and 2 out of 20 (10%) in the tolerant group. The mortality, caused by development of endotoxin shock, appeared within 6 hours after the injection of LPS. The choice of dose of LPS was considered and approved by the Bioethics Committee of the Science Research Institute of Human Morphology (Protocol No. 16, November 11, 2015).
Real-time PCR (quantitative PCR)
Expression of Hif-1a and Nf-kb in the liver was analyzed by real-time quantitative reverse transcription PCR. Slices of tissue from the liver, of about 30 mm3 volume, were submerged in IntactRNA Reagent (Evrogen JSC, Moscow, Russia) immediately after harvesting, incubated overnight at 4°C and stored at −80° until use. Total RNA was isolated from the tissue samples using RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Estimated purified RNA concentration in the eluate was 0.1 g/L; quality of the material was controlled by electrophoresis.
Reverse transcription of the total RNA to randomly primed single-stranded cDNA was done using MMLV RT Kit (Evrogen JSC); the synthesis was carried out at 39°C for 1 hour. After heat inactivation of the enzyme, the reaction mixture was diluted with 2 volumes of sterile RNase-free water for further use and storage; final dilution of the mixture in PCR constituted 1:250. PCRs were set in duplicates on the basis of qPCRmix-HS SYBR (Evrogen JSC) with oli-gonucleotide primers (custom-made by SYNTOL, Moscow, Russia) in 0.2–0.4 µM final concentrations. Primers for PCR were selected using the online Primer-BLAST program in accordance with the generally accepted requirements. Structures of the oligonucleotides with symbols and descriptions of the corresponding targets are given in . Amplification with detection and digital analysis of fluorescence in real time was carried out on DT-96 Real-Time PCR Cycler (DNA-Technology JSC, Moscow, Russia) in a standard mode at 95°C for 5 minutes followed by the PCR cycle, consisted of: 95°C for 15 seconds, 62°C for 10 seconds + reading and 72°C for 20 seconds, this was repeated for 45 times. Characteristic values (Cp) were automatically generated by nonlinear regression analysis, and the relative expression values were calculated by the approach originally introduced by PfafflCitation46 using b2m (see ) as the reference target.
Table 1 PCR primer structures and targets definition
Sample collection
Venous blood from jugular veinsCitation47 was centrifuged for 20 minutes at 200×g. The obtained serum was frozen at −70°C and stored for no more than 2 months. The lungs were fixed in Carnoy’s solution (60 mL ethanol, 30 mL chloroform and 10 mL glacial acetic acid) for 2 hours and were embedded in paraffin according to routine procedures. Histological sections of 4–5 µm thickness were produced and stained with H&E (BioVitrum, Saint Petersburg, Russia).
Isolation and cultivation of splenic cells
For the isolation of splenic cells, a piece of tissue was aseptically removed from each rat, placed in Potter homogenizer containing the Roswell Park Memorial Institute (RPMI)-1640 medium (PanEco, Moscow, Russia) and single-cell suspensions were prepared. The red blood cells were lysed by distilled water. To activate cytokine synthesis and secretion, we cultivated 106/mL spleen cells in 1 mL of culture medium with concanavalin A (ConA) (5 µg/mL) for 20 hours at 37°C and 5% CO2 in 24-well cultured plates. The culture medium consisted of RPMI-1640, 5% inactivated FBS, 2 mM glutamine and 50 µg/mL gentamicin.Citation48 The cell viability was determined by the trypan blue exclusion test.
Enzyme-linked immunosorbent assay
We estimated the concentrations of IL-1β, C-reactive protein (CRP; Cloud-Clone Corp., Wuhan, China) and TGF-β (eBioscience, San Diego, CA, USA) in the serum by ELISA. In the culture fluid of splenic cells, we measured the concentrations of IL-10 by ELISA test systems from Cloud-Clone Corp. For determination of the intensity of the color reaction, a micro-plate analyzer ANTHOS 2010 (Anthos Labtec Instruments, Vienna, Austria) was used.
Morphological study
The evaluation of histological slides was randomized and blinded. Using the light microscopic method, the number of neutrophils in the interalveolar septa of the lungs was counted in ten high-power fields of view (25,000 µm2) per section, and the average number of neutrophils per slide was determined.Citation49
Statistical analysis
Digital data were tested for normality using the Kolmogorov–Smirnov test in Statistica 8.0. To isolate the group or groups that differed from others, we used the nonparametric Mann–Whitney U test and multiple comparison procedures. In cases when P<0.05, multiple comparison procedures were performed by the Kruskal–Wallis method. The median and IQR (Me, Low–High) were calculated for values of the measured parameters. The differences were considered statistically significant when P<0.05. At least five observations were presented in each group. Data are represented graphically using box-and-whisker plots, which demonstrate the median, IQR, lower extreme (25%) and upper extreme (75%) of the data.
Results
Expression of Hif-1a and Nf-kb in the liver
Three and six hours after the administration of LPS, the expression level of Nf-kb in the liver increased only in the rats susceptible to hypoxia. In the animals tolerant to hypoxia, the increase was not significant ().
Figure 1 Expression of Nf-kb in the liver of rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%).
Notes: In all groups there were 5 observations except the tolerant group after 24 hours of LPS injection, in which were 8. Statistical significance of differences (P-value) is determined by the Kruskal–Wallis method.
Abbreviation: LPS, lipopolysaccharide.
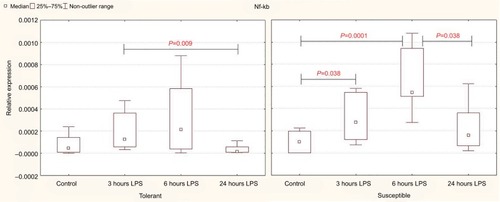
After 24 hours, the level of expression of Nf-kb was normal, but in the susceptible rats, it was higher than in the animals tolerant to hypoxia ().
Table 2 The expression level of mRNA Nf-kb and Hif-1α in the liver of rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%)
Expression of Hif-1a in the control group of rats was significantly higher in the animals susceptible to hypoxia (). Six hours after the injection of LPS, Hif-1a expression increased in both tolerant and susceptible rats (), and in susceptible animals, it was two times higher than in tolerant ones (). After 24 hours, the expression of Hif-1α returned to normal levels; but in the susceptible animals, it remained higher than in tolerant rats.
Figure 2 Expression of Hif-1α in the liver of rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%).
Notes: In all groups there were 5 observations except the tolerant group after 24 hours of LPS injection, in which were 8. Statistical significance of differences (P-value) is determined by the Kruskal–Wallis method.
Abbreviation: LPS, lipopolysaccharide.
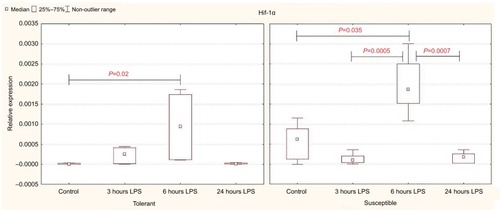
Determination of cytokine content
Based on the results of the enzyme immunoassay, it was demonstrated that the significant increase in the level of the proinflammatory cytokine IL-1β in the blood serum occurred after 3 hours of LPS administration only in the susceptible rats, while in the tolerant animals, the differences were not significant (). After 6 hours in the rats susceptible to hypoxia, the serum levels of IL-1β were normalized; but after 24 hours, they remained significantly higher than in the tolerant rats ().
Table 3 The serum IL-1β and spleen IL-10 levels in rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%)
Figure 3 Serum IL-1β levels in rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%).
Notes: In all groups there were 5 observations except the tolerant group after 24 hours of LPS injection, in which were 8. Statistical significance of differences (P-value) is determined by the Kruskal–Wallis method.
Abbreviation: LPS, lipopolysaccharide.
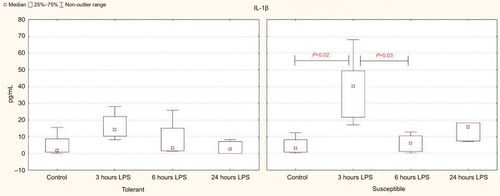
According to our data, only in the rats susceptible to hypoxia, the level of CRP (the marker of inflammation) in the blood was increased from 1,363 (1,128–1,551 pg/mL) in the control group to 2,421 (1,810–2,844 pg/mL) after 24 hours of LPS administration (P=0.04).
In the animals tolerant to hypoxia, after 6 hours of LPS injection, the production of anti-inflammatory cytokine IL-10 in the spleen was significantly reduced, and 24 hours later, it was normalized and was statistically significantly higher than in the susceptible rats (; ).
Figure 4 IL-10 production by spleen cells activated by Concanavalin A in rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%).
Notes: In all groups there were 5 observations except the tolerant group after 24 hours of LPS injection, in which were 8. Statistical significance of differences (P-value) is determined by the Kruskal–Wallis method.
Abbreviation: LPS, lipopolysaccharide.
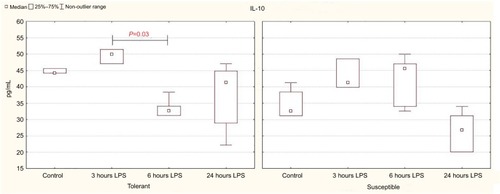
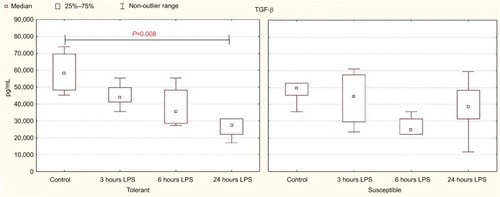
In comparison with the control group, a significant decrease in serum TGF-β level was observed after 24 hours of LPS administration only in the rats tolerant to hypoxia (). In the susceptible rats, this parameter did not change. There were no statistically significant differences in the TGF-β level between the tolerant and susceptible animals ().
Table 4 serum TGF-β level in rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%)
Figure 5 TGF-β serum levels in rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%).
Notes: In all groups there were 5 observations except the tolerant group after 24 hours of LPS injection, in which were 8. Statistical significance of differences (P-value) is determined by the Kruskal–Wallis method.
Abbreviation: LPS, lipopolysaccharide.
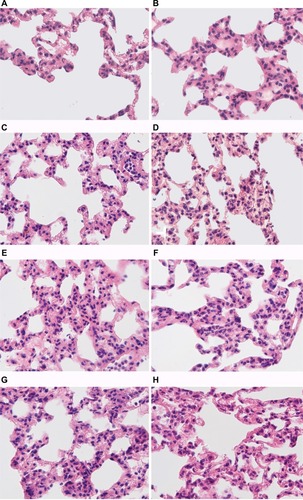
Morphological changes in the lungs
In all the periods, after LPS injection in its target organ – the lungs – both in tolerant and susceptible rats, infiltration of interalveolar septa with neutrophils, hyperemia and intra-alveolar edema was observed ().
Figure 6 Morphological changes in the lungs of male Wistar rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration.
Notes: H&E staining. Original magnification: (A–H) 640×. (A) Rats tolerant to hypoxia, control group – few neutrophils in the field of view. (B) Rats susceptible to hypoxia, control group – few neutrophils in the field of view. (C) Rats tolerant to hypoxia, 3 hours LPS – thickened interalveolar septa with neutrophils, hyperemia. (D) Rats susceptible to hypoxia, 3 hours LPS – pronounced neutrophil infiltration in interalveolar septa, hyperemia. (E) Rats tolerant to hypoxia, 6 hours LPS – neutrophil infiltration in interalveolar septa, hyperemia. (F) Rats susceptible to hypoxia, 6 hours LPS – high number of neutrophils in interalveolar septa, hyperemia. (G) Rats tolerant to hypoxia, 24 hours LPS – interalveolar septa with neutrophils, hyperemia. (H) Rats susceptible to hypoxia, 24 hours LPS – interalveolar septa with neutrophils, hyperemia.
Abbreviation: LPS, lipopolysaccharide.
After 6 hours of LPS injection in the rats susceptible to hypoxia, the number of neutrophils in the interalveolar septa was significantly higher in comparison with the tolerant ones ().
Figure 7 The average number of neutrophils counted in ten high-power fields of view (25,000 µm2) per section in the interalveolar septa of the lungs of rats tolerant and susceptible to hypoxia after 3, 6 and 24 hours of LPS administration (Me; 25%–75%).
Notes: In all groups there were 5 observations except the tolerant group after 24 hours of LPS injection, in which were 8. Statistical significance of differences (P-value) is determined by the Kruskal–Wallis method.
Abbreviation: LPS, lipopolysaccharide.
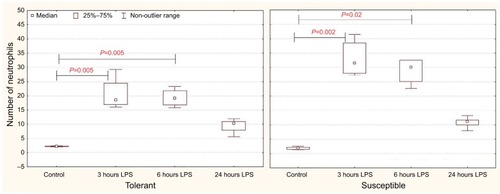
One day after the administration of LPS in animals of both groups, the number of neutrophils in the interalveolar septa did not differ from the control values.
All the results are summarized in .
Table 5 summary of results
Discussion
It is known that with the introduction of endotoxin of gram-negative bacteria LPS, the activation of TLR-4 receptors occurs, which leads to the initiation of IKK and the destruction of IκB in the proteasome, which causes increase in the expression of NF-κB and its translocation into the nucleus.Citation33,Citation37,Citation44,Citation50 NF-κB induces the production of proinflammatory cytokines, IL-1β, TNF-α and IL-6, which stimulate the liver cells to synthesize acute inflammation proteins, including CRP, 4–6 hours after the exposure.Citation51,Citation52 In addition, LPS induces the synthesis of anti-inflammatory cytokines IL-10 as well as IL-4 and IL-13, which block the activation of NF-κB by the negative feedback mechanism.Citation53
In our work, it was demonstrated that in the early periods after the injection of LPS, the increase in Nf-kb expression in the liver was observed only in rats susceptible to hypoxia. In tolerant animals, no significant differences from the control group were found at all the periods after the administration of LPS, and the expression level of Nf-kb after 6 and 24 hours of LPS injection was significantly lower than in susceptible rats. This indicates the development of more pronounced LPS-induced inflammation in rats susceptible to hypoxia. It is manifested by an increase of IL-1β in the blood that could be observed only in susceptible animals 3 hours after the administration of LPS. After 24 hours, its level was normalized in male rats susceptible to hypoxia, but remained higher than in animals tolerant to hypoxia.
According to the literature, in response to the administration of LPS, the level of CRP rises significantly after 10–12 hours and reaches a peak after 24–48 hours.Citation54–Citation57 According to our data, a significant increase in CRP level occurred in the blood serum after 24 hours only in rats susceptible to hypoxia. Probably, it was possible due to the activation of proinflammatory responses, indicated by increase in the level of IL-1β after 3 hours of LPS administration in these rats, since it is known that IL-1β promotes the synthesis of acute inflammation proteins.Citation57,Citation58 As the level of CRP synthesis reflects the intensity of the inflammatory process, it is used as one of the clinical markers of infectious and inflammatory diseases, including sepsis.Citation59,Citation60 It was demonstrated that high concentrations of CRP in the development of sepsis indicate unfavorable prognosis.Citation61 Therefore, rats susceptible to hypoxia are characterized by more pronounced inflammatory reaction in response to the administration of the LPS.
Apparently, greater sensitivity to the development of a systemic inflammatory response in animals susceptible to hypoxia is due to a high level of oxidative stress,Citation28,Citation32 since it is known that it plays an important role in the development of systemic inflammatory response.Citation62 However, HIF-1α may also participate in the development of a systemic inflammatory response and can be connected to its greater sensitivity to rats susceptible to hypoxia. Hyperactivation of the production of inflammatory molecules leads to a disturbance of hemodynamics, activation of coagulation with the increase of NO synthesis, vasoconstriction and the development of hypoxia, which also affects the immune cells.Citation63 In 2006, Frede et al demonstrated that LPS can induce the NF-κB–dependent increase in mRNA and HIF-1α protein levels.Citation11 As it was mentioned before, in systemic infections such as sepsis, the increase in HIF-1α levels results in greater mortality and enhancement in the levels of proinflammatory cytokines (IL-1β, TNFα) in the serum.Citation19 In our study, the level of Hif-1α expression was statistically significantly higher in rats susceptible to hypoxia from the control group than in rats tolerant to hypoxia, consistent with previous studies.Citation27 This may indicate that animals susceptible to hypoxia are more predisposed to the development of inflammatory diseases. As it was discussed earlier, the situation in which HIF-1 is activated (hypoxia or inflammation) influences the result of this process. If HIF-1 is activated due to hypoxia, the transcription of various targeted genes is enhanced, which facilitates adaptation to the lack of oxygen. Being influenced by the TLR-4– and NF-κB–dependent pathways, the genes of proinflammatory cytokines become active.Citation16 In our study, after 6 hours of LPS injection, the level of liver Hif-1α expression increased significantly in both the rats susceptible and tolerant to hypoxia; however, the increase was twice higher in susceptible rats than in tolerant rats. Nevertheless, in tolerant rats, there was no increase in the level of expression of Nf-kb, which indicates that in these animals, HIF is activated independently from the NF-κB pathway and does not lead to a pronounced inflammatory reaction.
It is known that anti-inflammatory cytokines, such as IL-10 and TGF-β, produced by macrophages and peripheral mononuclear cells under the influence of LPS, regulate the development of the inflammatory response. IL-10 limits the inflammatory response, and TGF-β plays an important role in suppressing the functional activity of immune cells. Anti-inflammatory cytokines are able to inhibit the production of IL-1 and TNFα.Citation64,Citation65 However, their excessive secretion can contribute to immunodeficiency, increased sensitivity to infections, subsequent development of secondary infections and chronic inflammation.Citation66–Citation68 We demonstrated that in rats tolerant to hypoxia, there was a significant decrease in the level of IL-10 after 6 hours and TGF-β after 24 hours of LPS administration. According to the literature, the neutralization of IL-10 activity increases the survival rate of animals in the model of sepsis that developed against the background of pneumonias.Citation69 A higher level of IL-10 is also observed in patients with post-burn sepsis and a fatal outcome.Citation70 Apparently, in rats tolerant to hypoxia, decrease in serum IL-10 levels after 6 hours and TGF-β after 24 hours of LPS administration prevents immunosuppression and increases the survival rate of animals. In rats susceptible to hypoxia, the level of anti-inflammatory cytokines did not change, which indicates development of the intensive inflammatory reaction in response to the administration of LPS.
According to the literature, myeloid cells, including neutrophils and monocytes/macrophages, contain a variety of proteolytic enzymes and are able to rapidly generate the ROS to destroy pathogens. Thus, although neutrophils are important for the destruction of pathogens, their activation can cause a hyperimmune response and cellular damage during sepsis and other inflammatory diseases.Citation39,Citation71 Neutrophil-mediated damage to lung, liver and other organs has been manifested in sepsis.Citation72,Citation73 In our study, in comparison with rats tolerant to hypoxia, a higher number of neutrophils was observed in the interalveolar septa of the lungs in susceptible animals after 6 hours of LPS injection, which may indicate more pronounced lung damage in these animals.
In studies of the possibility of using HIF-1 as a marker of inflammatory diseases and as a potential therapeutic target in humans, its expression level was examined, which may differ depending on the resistance to hypoxia. These studies were performed mainly on patients who already developed inflammatory disease, which prevents us from certainly concluding about the connection between inflammatory reactions and the initial resistance to hypoxia. In our experimental study, it was demonstrated that in response to the injection of LPS, HIF-1a expression was more pronounced in animals susceptible to hypoxia, and they had a greater severity of the inflammatory response, as confirmed by higher levels of Nf-kb, CRP and IL-1β. Therefore, modulation of the expression of HIF-1α may have therapeutic value, especially in organisms that are susceptible to hypoxia.
As mentioned above, increased expression of HIF-1 can be both proinflammatory and anti-inflammatory, depending on the context of inflammation. Proinflammatory activation of HIF-1α is demonstrated on the LPS-induced sepsis model,Citation19 and anti-inflammatory activation is demonstrated on the colitis model.Citation18 We first showed that increased expression of HIF-1α in systemic infections leads to a greater severity of the inflammatory response in animals susceptible to hypoxia. However, further studies are essential for the understanding of the possible protective role of increased expression of HIF-1α in animals susceptible to hypoxia on a colitis model.
Along with the main therapy of infectious and inflammatory diseases, antioxidants and antihypoxants are often used, but their effectiveness varies between patients.Citation74,Citation75 Their effectiveness can also be determined by the variability in the individual resistance to hypoxia. Considering our data that rats susceptible to hypoxia have more pronounced systemic inflammatory response, it can be assumed that the use of antihypoxants will reduce the severity of the inflammatory response. Indeed, in experimental studies it has been demonstrated that antihypoxic drugs, when used in combination with nonsteroidal anti-inflammatory drugs, significantly reduce the severity of inflammatory reactions.Citation76,Citation77 However, the effectiveness of such drugs may not be the same in individuals with different resistance to hypoxia. It is likely that the dose and course of treatment with antihypoxants should be selected taking into consideration the individual’s resistance to hypoxia. In our opinion, the use of antihypoxants will be more effective in patients susceptible to hypoxia. Therefore, our data probably will allow to develop new approaches for personalized medicine, taking into consideration the individual’s initial resistance to hypoxia.
Some authors use hypoxic preconditioning in experimental studies to increase the level of HIF-1, which improves adaptation to hypoxia.Citation41,Citation78 However, given the initially high level of expression of HIF-1 in rats susceptible to hypoxia, shown in our work and by other authors,Citation27 its additional increase may, on the contrary, contribute to the predisposition to develop inflammatory processes. Therefore, in such studies, it is also necessary to take into consideration the results obtained in our work.
Conclusion
Thus, for the first time, the various expressions of SIRS in animals with different resistance levels to hypoxia are demonstrated here. Rats susceptible to hypoxia are characterized by a more pronounced inflammatory response induced by LPS, which is revealed by increased expression of Nf-kb and Hif-1α in the liver, a greater number of neutrophils in the interalveolar septa of the lungs and an increased content of IL-1β and CRP. In tolerant animals, after 6 hours of LPS administration, the level of the anti-inflammatory cytokine IL-10 produced by splenic cells was reduced and after 24 hours, its level was higher than in susceptible rats. Twenty-four hours after the administration of LPS, a statistically significant decrease in serum TGF-β level occurred in rats tolerant to hypoxia, which prevents immunodeficiency states and increases the survival rate of animals. The data will be the basis for further experimental investigations and the development of new approaches for treating infectious diseases.
Acknowledgments
We are grateful to Lokhonina AV, Elchaninov AV and Usman NY for their help in PCR.
Disclosure
The authors report no conflicts of interest in this work.
References
- MartinGSManninoDMEatonSMossMThe epidemiology of sepsis in the United States from 1979 through 2000N Engl J Med2003348161546155412700374
- EsperAMMossMLewisCAThe role of infection and comorbidity: factors that influence disparities in sepsisCrit Care Med200634102576258216915108
- MayrFBYendeSAngusDCEpidemiology of severe sepsisVirulence20145141124335434
- SemenzaGLWangGLA nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activationMol Cell Biol19921212544754541448077
- WengerRHStiehlDPCamenischGIntegration of oxygen signaling at the consensus HRESci STKE20052005306re1216234508
- KaelinWGRatcliffePJOxygen sensing by metazoans: the central role of the HIF hydroxylase pathwayMol Cell200830439340218498744
- HirotaKInvolvement of hypoxia-inducible factors in the dysregulation of oxygen homeostasis in sepsisCardiovasc Hematol Disord Drug Targets2015151294025567333
- BonelloSZähringerCBelaibaRSReactive oxygen species activate the HIF-1α promoter via a functional NFκB siteArterioscler Thromb Vasc Biol200727475576117272744
- RiusJGumaMSchachtrupCNF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alphaNature2008453719680781118432192
- van UdenPKennethNSRochaSRegulation of hypoxia-inducible factor-1alpha by NF-kappaBBiochem J2008412347748418393939
- FredeSStockmannCFreitagPFandreyJBacterial lipopoly-saccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-κBBiochem J2006396351752716533170
- Hellwig-BürgelTRutkowskiKMetzenEFandreyJJelkmannWInterleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1Blood19999451561156710477681
- ChandelNSMcclintockDSFelicianoCEReactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensingJ Biol Chem200027533251302513810833514
- CumminsEPBerraEComerfordKMProlyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activityProc Natl Acad Sci USA200610348181541815917114296
- OliverKMTaylorCTCumminsEPHypoxia. Regulation of NFkappaB signalling during inflammation: the role of hydroxylasesArthritis Res Ther200911121519291263
- JantschJWieseMSchödelJToll-like receptor activation and hypoxia use distinct signaling pathways to stabilize hypoxia-inducible factor 1α (HIF1A) and result in differential HIF1A-dependent gene expressionJ Leukoc Biol201190355156221685248
- DevrajGBeerlageCBrüneBKempfVAHypoxia and HIF-1 activation in bacterial infectionsMicrobes Infect201719314415627903434
- KarhausenJFurutaGTTomaszewskiJEEpithelial hypoxia-inducible factor-1 is protective in murine experimental colitisJ Clin Invest200411481098110615489957
- PeyssonnauxCCejudo-MartinPDoedensACutting edge: essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsisJ Immunol2007178127516751917548584
- TextorisJBeaufilsNQuintanaGHypoxia-inducible factor (HIF1α) gene expression in human shock statesCrit Care2012164R12022781303
- LorenzoVFYangYSimonsonTSGenetic adaptation to extreme hypoxia: study of high-altitude pulmonary edema in a three-generation Han Chinese familyBlood Cells Mol Dis200943322122519481479
- KobayashiNHanaokaMDromaYPolymorphisms of the tissue inhibitor of metalloproteinase 3 gene are associated with resistance to high-altitude pulmonary edema (HAPE) in a Japanese population: a case control study using polymorphic microsatellite markersPLoS One201388e7199323991023
- De Carvalho FragaCAAlvesLRMarques-SilvaLHigh HIF-1α expression genotypes in oral lichen planusClin Oral Investig201317920112015
- StraussEWaliszewskiKOszkinisGStaniszewskiRPolymorphisms of genes involved in the hypoxia signaling pathway and the development of abdominal aortic aneurysms or large-artery atherosclerosisJ Vasc Surg20156151105111324657063
- Paradowska-GoryckaAStypinskaBPawlikAHIF-1a gene polymorphisms and its protein level in patients with rheumatoid arthritis: a case–control studyInflamm Res201867542343329411043
- YuZGWangBZChengZZThe association of genetic polymorphisms of hypoxia inducible factor-1 alpha and vascular endothelial growth factor with increased risk of chronic obstructive pulmonary disease: a case–control studyKaohsiung J Med Sci201733943344128865600
- KirovaYIGermanovaELLukyanovaLDPhenotypic features of the dynamics of HIF-1α levels in rat neocortex in different hypoxia regimensBull Exp Biol Med2013154671872223658906
- JainKSuryakumarGPrasadRGanjuLUpregulation of cytoprotective defense mechanisms and hypoxia-responsive proteins imparts tolerance to acute hypobaric hypoxiaHigh Alt Med Biol2013141657723537263
- TregubPKulikovVBespalovATolerance to acute hypoxia maximally increases in case of joint effect of normobaric hypoxia and permissive hypercapnia in ratsPathophysiology201320316517024083870
- LukyanovaLDGermanovaELKopaladzeRADevelopment of resistance of an organism under various conditions of hypoxic preconditioning: role of the hypoxic period and reoxygenationBull Exp Biol Med2009147440040419704933
- SerebrovskayaTVXiLIndividualized intermittent hypoxia training: principles and practicesXiLSerebrovskayaTHypoxia and Human DiseasesLondonSpringer2012
- DzhalilovaDSDiatroptovMETsvetkovISExpression of HIF-1α, NF-κB and VEGF genes in the liver and the content of HIF-1α, erythropoietin, VEGF, TGF-β, 8-isoprostane and corticosterone in the blood serum in tolerant and susceptible to hypoxia Wistar ratsBull Exp Biol Med2018165678178530353332
- ChowJCYoungDWGolenbockDTChristWJGusovskyFToll-like receptor-4 mediates lipopolysaccharide-induced signal transductionJ Biol Chem199927416106891069210196138
- LuYCYehWCOhashiPSLPS/TLR4 signal transduction pathwayCytokine200842214515118304834
- UlevitchRJTobiasPSRecognition of gram-negative bacteria and endotoxin by the innate immune systemCurr Opin Immunol1999111192210047547
- AderemAUlevitchRJToll-like receptors in the induction of the innate immune responseNature2000406679778278710963608
- CinelIOpalSMMolecular biology of inflammation and sepsis: a primerCrit Care Med200937129130419050640
- KosyrevaAMSimonovaEYMakarovaOVGender differences in pulmonary and immune response in acute experimental endotoxicosisBull Exp Biol Med2012153334034222866306
- FangHJiangWChengJBalancing innate immunity and inflammatory state via modulation of neutrophil function: a novel strategy to fight sepsisJ Immunol Res2015201518704826798659
- BhatiaBThomasSPurkayasthaSSSeasonal variations in the survival index of rats at simulated high altitudesInt J Biometeorol196610163696003442
- ShrivastavaKRamMSBansalASinghSSIlavazhaganGCobalt supplementation promotes hypoxic tolerance and facilitates acclimatization to hypobaric hypoxia in rat brainHigh Alt Med Biol200891637518331222
- LukyanovaLDKirovaYIEffect of hypoxic preconditioning on free radical processes in tissues of rats with different resistance to hypoxiaBull Exp Biol Med2011151329229622451869
- GautierELHubyTSaint-CharlesFEnhanced dendritic cell survival attenuates lipopolysaccharide-induced immunosuppression and increases resistance to lethal endotoxic shockJ Immunol2008180106941694618453615
- BlackwellTSYullFEChenCLMultiorgan nuclear factor kappa B activation in a transgenic mouse model of systemic inflammationAm J Respir Crit Care Med20001623 Pt 11095110110988136
- PisarevVBNovochadovVVBogomolovaNVBacterial Endotoxicosis: Pathologist’s ViewVolgogradVolgograd State Medical University2008 Russian
- PfafflMWA new mathematical model for relative quantification in real-time RT-PCRNucleic Acids Res2001299e4511328886
- ParasuramanSRaveendranRKesavanRBlood sample collection in small laboratory animalsJ Pharmacol Pharmacother201012879321350616
- LinK-HLinK-CLuW-JAstaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and IL-2 secretion in primary cultured lymphocytes in vitro and ex vivoInt J Mol Sci201617144
- YamadaMKuboHKobayashiSThe increase in surface CXCR4 expression on lung extravascular neutrophils and its effects on neutrophils during endotoxin-induced lung injuryCell Mol Immunol20118430531421460863
- SeeleyJJGhoshSTolerization of inflammatory gene expressionCold Spring Harb Symp Quant Biol201378697925028399
- CannonJGTompkinsRGGelfandJACirculating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin feverJ Infect Dis1990161179842295861
- EstebanEFerrerRAlsinaLArtigasAImmunomodulation in sepsis: the role of endotoxin removal by polymyxin B-immobilized cartridgeMediators Inflamm201320137112
- WangPWuPSiegelMIEganRWBillahMMInterleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanismsJ Biol Chem199527016955895637721885
- NgPCChengSHChuiKMDiagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infantsArch Dis Child Fetal Neonatal Ed1997773F221F2279462194
- MakhoulIRYacoubASmolkinTValues of C-reactive protein, procalcitonin, and Staphylococcus-specific PCR in neonatal late-onset sepsisActa Paediatr200695101218122316982493
- HoferNZachariasEMüllerWReschBAn update on the use of C-reactive protein in early-onset neonatal sepsis: current insights and new tasksNeonatology20121021253622507868
- SlaatsJTen OeverJvan de VeerdonkFLNeteaMGIL-1β/IL-6/CRP and IL-18/ferritin: distinct inflammatory programs in infectionsPLoS Pathog20161212e100597327977798
- KramerFTorzewskiJKamenzJInterleukin-1beta stimulates acute phase response and C-reactive protein synthesis by inducing an NFkappaB- and C/EBPbeta-dependent autocrine interleukin-6 loopMol Immunol20084592678268918262272
- VigushinDMPepysMBHawkinsPNMetabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and diseaseJ Clin Invest1993914135113578473487
- PóvoaPSouza-DantasVCSoaresMSalluhJFC-reactive protein in critically ill cancer patients with sepsis: influence of neutropeniaCrit Care2011153R12921595932
- BeltempoMViel-ThériaultIThibeaultRJulienASPiedboeufBC-reactive protein for late-onset sepsis diagnosis in very low birth weight infantsBMC Pediatr20181811629382319
- MacdonaldJGalleyHFWebsterNROxidative stress and gene expression in sepsisBr J Anaesth200390222123212538380
- CohenJThe immunopathogenesis of sepsisNature2002420691788589112490963
- SanjabiSZenewiczLAKamanakaMFlavellRAAnti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunityCurr Opin Pharmacol20099444745319481975
- CastellheimABrekkeOLEspevikTHarboeMMollnesTEInnate immune responses to danger signals in systemic inflammatory response syndrome and sepsisScand J Immunol200969647949119439008
- OpalSMDepaloVAAnti-inflammatory cytokinesChest200011741162117210767254
- CavaillonJMAnnaneDCompartmentalization of the inflammatory response in sepsis and SIRSJ Endotoxin Res200612315117016719987
- CouperKNBlountDGRileyEMIL-10: the master regulator of immunity to infectionJ Immunol200818095771577718424693
- van der PollTMarchantAKeoghCVGoldmanMLowrySFInterleukin-10 impairs host defense in murine pneumococcal pneumoniaJ Infect Dis1996174599410008896500
- YehFLLinWLShenHDChanges in circulating levels of an anti-inflammatory cytokine interleukin 10 in burned patientsBurns200026545445910812267
- BrownKABrainSDPearsonJDNeutrophils in development of multiple organ failure in sepsisLancet2006368953015716916829300
- KabayBKocaefeCBaykalAInterleukin-10 gene transfer: prevention of multiple organ injury in a murine cecal ligation and puncture model of sepsisWorld J Surg200731110511517171483
- StehrSNKnelsLWeissflogCEffects of IGM-enriched solution on polymorphonuclear neutrophil function, bacterial clearance, and lung histology in endotoxemiaShock200829216717217666945
- ZarubinaIVYunusovIAMaryshevaVVShabanovPDComparative efficiency of succinate-containing antihypoxants in traumatic toxicosisBull Exp Biol Med2010150221221421240375
- Bar-OrDCarrickMMMainsCWSepsis, oxidative stress, and hypoxia: are there clues to better treatment?Redox Rep201520519319725803628
- FateevaEIMitrochinNMSernovLNStudy protective propertie of mexidol and new derivative 3-hydroxypyridine in combination with NSAIDsKubanskii Nauchnyi Meditsinskii Vestnik200867883
- PogilovaEVInfluence of antihypoxants on the development of carrageenin-induced inflammationI.P. Pavlov Russian Med Biol Herald20142246167
- ShuklaDSaxenaSJayamurthyPHypoxic preconditioning with cobalt attenuates hypobaric hypoxia-induced oxidative damage in rat lungsHigh Alt Med Biol2009101576919278353
