Abstract
Background
Chemokines contribute to inflammatory responses by inducing leukocyte migration and extravasation. In addition, chemoattractants other than classical chemokines can also be present. Many chemokines have been demonstrated to cooperate, leading to an augmentation in leukocyte recruitment and providing a potential role for the presence of multiple chemoattractants. Extracellular cyclophilins are a group of alternative chemotactic factors, which can be highly elevated during various inflammatory responses and, as we have previously shown, can contribute significantly to neutrophil recruitment in an animal model of acute lung inflammation. In the current studies we investigated whether the most abundant extracellular cyclophilin, CypA, has the capacity to function in partnership with 2 classical chemokines known to be secreted in the same model, macrophage inflammatory protein (MIP)-2/CXCL2 and keratinocyte chemoattractant (KC)/CXCL1.
Methods
Neutrophil migration in response to combinations of CypA and MIP-2 or CypA and KC was measured by in vitro chemotaxis assays. Biochemical responses of neutrophils incubated with the combinations of chemoattractants were determined by changes in chemokine receptor internalization and actin polymerization measured by flow cytometry, and changes in intracellular calcium mobilization measured with a calcium sensitive fluorochrome.
Results
A combination of CypA and MIP-2, but not KC, augmented neutrophil migration. Based on the level of augmentation, the cooperation between CypA and MIP-2 appeared to be synergistic. Evidence that CypA and MIP-2 cooperate at the biochemical level was demonstrated by increases in receptor internalization, calcium mobilization, and actin polymerization.
Conclusion
These findings provide evidence for the capacity of extracellular cyclophilins to interact with classical chemokines, resulting in greater and more efficient leukocyte recruitment.
Keywords:
Introduction
Neutrophils are integral leukocytes in the monitoring of the immune system and the host response to inflammation. The main regulators of leukocyte trafficking are chemokines, a family of chemoattracting cytokines. Chemokines induce cell migration by binding to and signaling through G-protein-coupled receptors (GPCRs) on their target cells. While GPCRs for chemokines are restricted to interaction with specific families of this class of chemoattractants, notably CC versus CXC, many can act as receptors for several different members within a family. The fact that inflammatory processes are usually associated with the production of many different chemokines, some of which target the same leukocyte subset or even the same specific receptors on target cells, suggests a significant overlap and/or redundancy in function by chemokines.
To add to the already complex mix of multiple chemokines is the finding that additional factors with chemoattracting properties can also be present during inflammatory responses, for example, cyclophilins, a family of intracellular proteins that are ubiquitously expressed in all organisms and in all human tissues. Cyclophilins can be secreted by different cell types in response to inflammatory stimuli such as LPSCitation1,Citation2 or reactive oxygen radicals,Citation3 thereby generating extracellular pools of these proteins. Indeed, high levels of extracellular cyclophilins have been reported in several inflammatory diseases, both in humans Citation2,Citation4,Citation5 and mice.Citation6–Citation8 We have previously proposed that extracellular cyclophilins might play a role in inflammatory processes by contributing to the recruitment of leukocytes to sites of ongoing inflammation.Citation9 Indeed, using 3 different animal models of inflammatory disease, our laboratory demonstrated that inhibiting the chemotactic function of cyclophilins using either a nonimmunosuppressive analog of CsACitation6 or an antibody to the principal signaling receptor for extracellular cyclophilins A and B, CD147,Citation6–Citation8 can significantly reduce leukocyte recruitment in vivo.
Studies in which classical chemokines are inhibited also demonstrate significant reductions in leukocyte recruitment. In the case of neutrophils, for example, mice given anti- MIP-2 antibody showed a significantly reduced neutrophil influx into lungs challenged with bacteriaCitation10 and also into LPS-treated air pouches.Citation11 Treatment with a blocking antibody against the CXC chemokine, LIX (CXCL5), significantly decreased neutrophil migration to airways and lungs of mice in an LPS model of acute lung injury.Citation12 Inhibiting the signaling function of another chemokine, CCL2, using mice lacking CCR2 receptors, resulted in a greater than 90% decrease in alveolar neutrophil influx.Citation13 Given that the total contribution of all reported chemokines, as well as non-classical chemokines, produced during an inflammatory response cannot be greater than 100%, the possibility that some of these chemoattracting factors might cooperate has been explored from different mechanistic perspectives.
One such mechanism is chemokine synergy in which the total chemotactic function that is mediated by combining 2 chemokines is greater than their additive individual functions. Therefore, when one chemokine in a cooperative partnership is inhibited, the function of the second chemokine is indirectly impacted, resulting in a greater total reduction in cell recruitment and a potential explanation for the apparent >100% inhibition in leukocyte recruitment when individual effects are added together. Many different chemokines have been shown to work in partnership both in vitro and in vivo, with the potential to amplify inflammatory responses (reviewed inCitation14). Following from our long-standing interest in the contribution of extracellular cyclophilins as chemoattractants during inflammatory responses, we investigated in the current studies whether cyclophilin A (CypA), the most abundant member of the cyclophilin family,Citation15 might have a similar capacity to cooperate with classical chemokines to mediate augmented leukocyte migration. In previous studies we showed that CypA is a potent inducer of neutrophil migration in vitro and that high levels of CypA are present in the airways of mice with ongoing pulmonary neutrophilia induced by intranasal administration of LPS.Citation6 We therefore examined whether combining CypA with other known neutrophil-attracting chemokines would lead to an increase in neutrophil recruitment, and the impact of these combinations on biochemical events associated with cell migration.
In this report we show that CypA is able to cooperate with MIP-2, but not KC, to induce a synergistic augmentation in neutrophil migration in vitro. Analysis of various parameters associated with cell migration demonstrated that neutrophils stimulated with a combination of CypA and MIP-2 showed greater increases in GPCR (CXCR2) internalization, intracellular calcium mobilization, and actin polymerization, relative to cells stimulated with the individual chemoattractants. These additional increases were not observed in neutrophils stimulated with combinations of CypA and KC. Taken together, these findings provide evidence that CypA has the capacity to interact with selective classical chemokines to mediate an augmented neutrophil migration that is associated with enhanced biochemical events.
Material and methods
Animals
Female C57BL/6 mice, at least 6 weeks of age, were used for these studies and were purchased from the National Cancer Institute (Bethesda, MD). Blood, or bone marrow, was pooled from 2 to 5 age-matched animals for each experiment. All studies were reviewed and approved by the Institutional Animal Care and Use committee at The George Washington University.
Reagents
PE-conjugated anti-mouse Gr-1 was purchased from BD Biosciences (San Jose, CA) and APC-conjugated anti-mouse Gr-1 was purchased from eBioscience (San Diego, CA). PE-conjugated anti-mouse CXCR2 and rat IgG2A isotype were purchased from R&D Systems (Minneapolis, MN). FITC- conjugated rat anti-mouse CD147 monoclonal antibody and IgG1 isotype control were purchased from Abcam (Cambridge, MA). FITC-labeled phalloidin for actin polymerization studies was purchased from Sigma-Aldrich (St. Louis, MO). Anti-human cyclophilin A mAb (cross-reacts with mouse cyclophilin A) was obtained from US Biological (Swampscott, MA), and HRP-conjugated donkey anti-rabbit secondary Ab was purchased from Amersham Biosciences (Piscataway, NJ). fMLP, and BSA Fraction V were obtained from Sigma- Aldrich. Human recombinant CypA, which differs from mouse CypA by only one amino acid residue, was purchased from Calbiochem (San Diego, CA). Recombinant mouse MIP-2 and KC were purchased from PeproTech (Rocky Hill, NJ). FLIPR calcium 3 assay kit component A fluorescent dye for calcium measurements was purchased from Molecular Devices (Sunnyvale, CA) and dATP from Invitrogen (Carlsbad, CA).
Neutrophil enrichment for in vitro assays
For chemotaxis assays: mouse peripheral blood was collected by cardiac puncture. The collected blood was treated with RBC lysis buffer and the neutrophils were then isolated using a MACS Gr-1 positive selection kit (Miltenyi Biotech, Aurora, CA). For actin polymerization and intracellular calcium experiments: bone marrow-derived neutrophils were obtained by flushing tibias and femurs of C57BL/6 mice with HBSS. The isolated bone marrow was washed with HBSS, centrifuged at 300 g for 12 minutes, and resuspended in 6 mL HBSS. The cells were layered on top of a Histopaque 1077/1119 gradient (3 mL Histopaque 1119 overlayed with 3 mL Histopaque 1077) and centrifuged at room temperature for 30 minutes at 700 g. The resulting granulocyte layer was removed, washed, and then used for phalloidin staining or calcium assays. For receptor internalization studies: mouse peripheral blood was collected by cardiac puncture. The collected blood was treated with RBC lysis buffer and the neutrophils were then isolated using an EasySep Mouse Neutrophil Enrichment Kit (Stem Cell Technologies Vancouver, BC, Cananda). Purity for all enriched populations was established to be 80 to 90% by FACS analysis.
Chemotaxis assays
Chemotaxis assays were set up using 48-well modified Boyden chambers (Neuro Probe Inc., Gaithersburg, MD) with the 2 compartments separated by a 5-μm polycarbonate membrane (Neuro Probe). Neutrophils (104 cells/well) in RPMI 1640 culture medium supplemented with 1% BSA were added to the upper chamber wells, while media containing different doses and combinations of chemoattractants (shown in Figures) were added to the lower compartments. In some studies purified mouse neutrophils were preincubated in medium alone (RPMI 1640 + 1% BSA fraction V), or medium containing doses of recombinant CypA or MIP-2 (as indicated in figure legends) for 30 minutes before being washed in PBS, resuspended in fresh medium, and applied to the upper wells of the chamber. A dose of 10−7 M fMLP was used as a positive control for neutrophil migration. The chambers were incubated at 37°C in 5% CO2 for 50 minutes, after which the chamber membrane was removed, non-migrating cells scraped off, and the membrane stained with Wright- Giemsa (Camco, Fort Lauderdale, FL). A chemotactic index was generated for each well by dividing the number of cells counted for that well by the number of cells counted in media wells.
Receptor expression studies
Purified mouse neutrophils were serum starved in RPMI 1640 without BSA for 30 minutes at room temperature. This short starvation step was included to reduce background staining while maintaining maximal cell viability. The cells were then supplemented with an equivalent volume of RMPI 1640 containing 2% BSA fraction V in preparation for stimulation. 1 × 106 neutrophils in 200 μL volume medium (RMPI + 1% BSA fraction V) were set up in a 96-well tissue culture plate. Cells were incubated in medium alone, or with recombinant CypA, MIP-2, or KC, as well as combinations of the various chemoattractants (as indicated in figure legends) for 5 minutes at 37°C in 5% CO2. (Five minutes was chosen based on initial kinetics studies showing optimal changes at this time point). The cells were then removed from wells, washed with PBS, and stained with APC-Gr-1 for gating on neutrophils. The cells were also stained with either FITC anti-CD147, FITC IgG1 isotype control, PE-CXCR2, or PE IgG2A control, and expression of CD147 and CXCR2 receptors on Gr-1 positive cells was determined by flow cytometry.
Intracellular calcium measurements
Intracellular calcium measurements were conducted with bone marrow neutrophils using a Benchtop Scanning Fluorometer and Integrated Fluid Transfer Workstation (FlexStation; Molecular Devices, Sunnyvale, CA). A total of 1 × 106 cells in 100 μL HBSS + 20 mM HEPES buffer was loaded into a 96-well plate together with 100 μL of FLIPR calcium 3 assay kit component A fluorescent dye (Molecular Devices), and then incubated for 30 min at 37°C. A separate plate containing various doses of chemoattractants (shown in figure legends) was set up. Both plates were then loaded into the FlexStation for automated addition of chemoattractants to the fluorescently labeled cells. Baseline fluorescence was established before the chemoattractants were added to cells at 20 seconds. Changes in intracellular calcium concentrations in response to added chemoattractants were recorded as relative fluorescence units over time. dATP (100 μM) was added to all groups of cells at 120 seconds as a positive control for equal capacity to flux calcium.
Actin polymerization
Bone marrow neutrophils were serum starved in 500 μL RPMI 1640 without BSA for 30 minutes at room temperature and then supplemented with 500 μL RMPI 1640 containing 2% BSA fraction V in preparation for stimulation. 5 × 106 neutrophils (now in RMPI 1640 with 1% BSA medium) were incubated at 37°C in 5% CO2 for 5 minutes in medium alone, fMLP as a positive control, CypA, MIP-2, KC alone or various combinations, at doses indicated in figure legends. (Five minutes was chosen based on initial kinetics studies showing optimal changes at this time point). The cells were then immediately fixed by adding 3.7% formalin for 10 minutes at room temperature. Samples were centrifuged for 1 minute at 1400 rpm and supernatants discarded. 100 μL cold 0.1% Triton X-100 was added to each sample and incubated on ice for 30 minutes to permeabilize the cells. Samples were washed with FACS buffer and stained with 6 μg/mL FITC-conjugated phalloidin in FACS buffer at room temperature for 40 minutes. Actin polymerization was determined by the level of phalloidin expression, as measured by flow cytometry.
Statistical analysis
Data are summarized as mean ± SE. Statistical analysis of the results was performed using an unpaired Student t test, with P < 0.05 considered significant.
Supplemental material
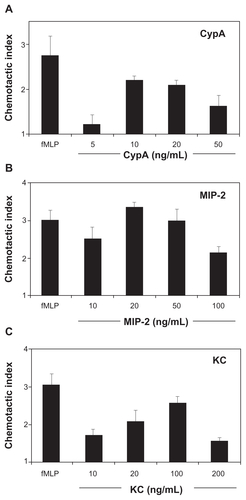
One supplemental figure is provided. shows in vitro neutrophil migration in response to different doses of CypA, MIP-2, and KC. These titration curves were used to select suboptimal doses for each chemoattractant for the combination studies.
Results
CypA can cooperate with MIP-2, but not KC, in vitro
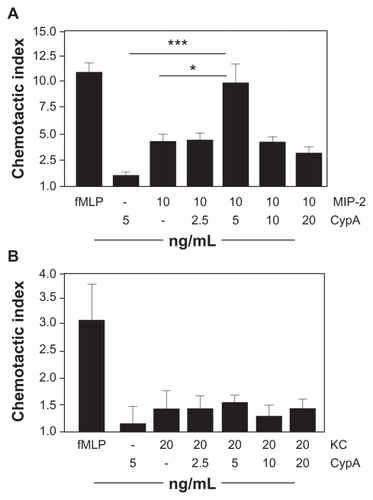
In previous studies we demonstrated the capacity of CypA to induce in vitro migration of humanCitation16 and mouseCitation6 neutrophils. In the current studies we investigated whether CypA might have the capacity to interact with classical chemokines known to be potent inducers of neutrophil migration, notably MIP-2 and KC, to mediate enhanced migration. Based on the findings of others that functional interactions are most likely to occur when suboptimal doses of chemokines are combined,Citation17 we initially established individual dose response curves for all 3 chemoattractants () and then selected doses in the lower range for testing in our combination assays. The data in show results from experiments in which neutrophils were set up with a fixed dose of MIP-2 or KC plus varying doses of CypA. Our findings demonstrate that combining CypA with MIP-2 induces a significant augmentation in neutrophil migration (). Interestingly, this augmentation was greater than the additive migration induced by MIP-2 alone plus CypA alone, suggesting that the 2 chemokines are interacting in a synergistic partnership. No such enhancement in neutrophil migration was observed using various combinations of KC and CypA (). Taken together, these findings demonstrate that the presence of CypA can significantly increase the capacity of MIP-2 to induce neutrophil migration and that this effect is more than just additive.
Figure 1 CypA and MIP-2 cooperate in vitro. In vitro chemotaxis assays were set up using purified mouse neutrophils incubated in the presence of a single dose of fMLP (positive control), a single dose of CypA, MIP-2, or KC, or a fixed dose of MIP-2 or KC plus varying doses of CypA. A chemotactic index was calculated for each group by dividing the number of migrated cells in test wells by the number of cells that migrated to medium alone. A) Mean + SE chemotactic index for neutrophils incubated with fMLP, CypA alone, MIP-2 alone, or combinations of MIP-2 plus CypA. B) Mean + SE chemotactic index for neutrophils incubated with fMLP, CypA alone, KC alone, or combinations of KC plus CypA.
Notes: Statistical significance was determined by Student’s t-test, with n = 6 wells per group. *P < 0.05 and ***P < 0.001. These data are representative of >3 independent experiments.
Abbreviations: See list of abbreviations.
CypA and MIP-2 must be present concurrently for cooperation to occur
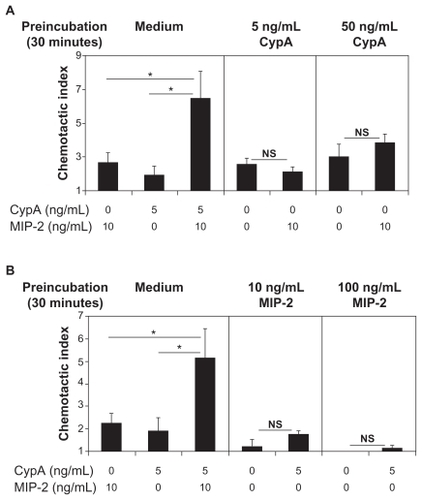
We next examined whether the augmented neutrophil migration observed when CypA and MIP-2 are combined requires that the 2 chemoattractants be present concurrently, or whether the same effect could be mediated by sequential exposure. Thus, in vitro chemotaxis assays were conducted in which neutrophils were preincubated with different doses of one chemoattractant, washed, and then tested for their migratory response to the second chemoattractant. As shown in , neutrophils preincubated with CypA did not respond either additively or synergistically upon subsequent stimulation with MIP-2. Likewise, neutrophils preincubated with MIP-2 did not migrate with either an additive or synergistic response to CypA (). Importantly, in both experiments the concurrent exposure to MIP-2 and CypA led to a greater than additive increase in neutrophil migration, demonstrating the reproducibility of the synergistic interaction when the 2 chemoattractants are present together.
Figure 2 CypA and MIP-2 must be present concurrently for in vitro cooperation to occur. In vitro chemotaxis assays were set up using purified mouse neutrophils preincubated in the presence of chemotaxis medium, medium containing 5 or 50 ng/mL CypA, or medium containing 10 or 100 ng/mL MIP-2, for 30 minutes. The cells were then washed and set up in Boyden chambers with medium alone, 5 ng/mL CypA, or 10 ng/mL MIP-2. Cells preincubated in medium alone were set up with a single dose of MIP-2, CypA, or a combination of both as a positive control for cooperative interaction. A chemotactic index was calculated for each group by dividing the number of migrated cells in test wells by the number of cells preincubated in medium that migrated to medium alone. A) Mean + SE chemotactic index of migration to indicated chemoattractants for neutrophils preincubated with medium alone, 5 or 50 ng/mL of CypA. B) Mean + SE chemotactic index of migration to indicated chemoattractants for neutrophils preincubated with medium alone, 10 or 100 ng/mL doses of MIP-2.
Notes: Statistical significance was determined by Student’s t-test, with n = 6 wells per group. *P < 0.05. These data are representative of >3 independent experiments.
Abbreviations: See list of abbreviations.
Addition of CypA to MIP-2 increases CXCR2 internalization
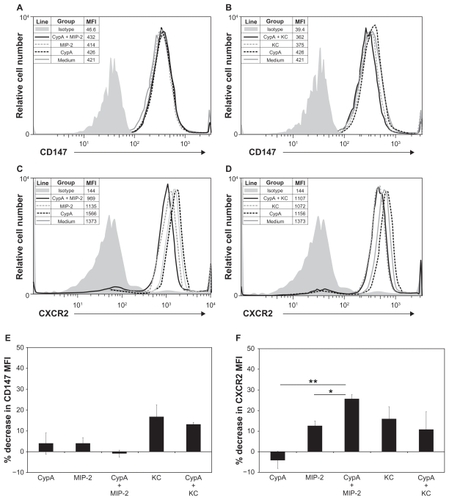
Ligand binding to chemokine receptors triggers a cascade of signaling events that ultimately result in cell polarization and chemotaxis, as well as receptor internalization.Citation18 Increases in receptor internalization are often used as an indicator of increased receptor engagement and/or receptor signaling. In the current studies we investigated the impact of co-incubation with CypA and MIP-2, relative to incubation with the individual chemoattractants, on the internalization of CD147 (receptor for CypACitation19) and CXCR2 (receptor for MIP-2) on neutrophils. It should be noted that the same low doses of CypA and MIP-2, which demonstrated synergistic responses ( and ), were used in the current studies. Combinations of CypA and KC were also included as a comparison for effects mediated by noncooperating chemoattractants. show that CD147 expression was not significantly changed (based on MFI units) after incubation with CypA, MIP-2, or KC alone, or with any of the chemoattractant combinations. In contrast, stimulation with a combination of CypA and MIP-2 resulted in a significantly greater CXCR2 receptor internalization (average of 26% reduction in expression compared with medium alone, based on MFI units), relative to that induced by MIP-2 alone (13% reduction) (). Adding CypA to KC did not significantly augment the degree of CXCR2 internalization mediated by KC alone (11% and 16% reductions, respectively) (). Taken together, these data demonstrate a selective increase in CXCR2 internalization when CypA and MIP-2 are present in combination, suggesting enhanced receptor engagement and/or signaling.
Figure 3 CXCR2 internalization increases when CypA and MIP-2 are combined. Purified neutrophils were incubated in medium alone or with the indicated combination of 5 ng/mL CypA, 10 ng/mL MIP-2, or 20 ng/mL KC, for 5 minutes. Cells were then washed in PBS and stained with either FITC-conjugated anti-CD147 or FITC-conjugated isotype control Ab, or PE-conjugated anti-CXCR2 or PE-conjugated isotype control AB. A) CD147 expression on cells incubated with CypA alone, MIP-2 alone, or a combination of CypA + MIP-2. B) CD147 expression on neutrophils incubated with CypA alone, KC alone, or a combination of CypA + KC. C) CXCR2 expression on neutrophils incubated with CypA alone, MIP-2 alone, or a combination of CypA + MIP-2. D) CXCR2 expression on neutrophils incubated with CypA alone, KC alone, or a combination of CypA + KC. MFI were calculated and are shown for each group in panel legends. Bar graphs showing average decrease in MFI relative to medium for E) CD147 expression and F) CXCR2 expression were calculated using 3 independent sets of data.
Abbreviations: See list of abbreviations.
CypA and MIP-2 combination increases intracellular calcium mobilization
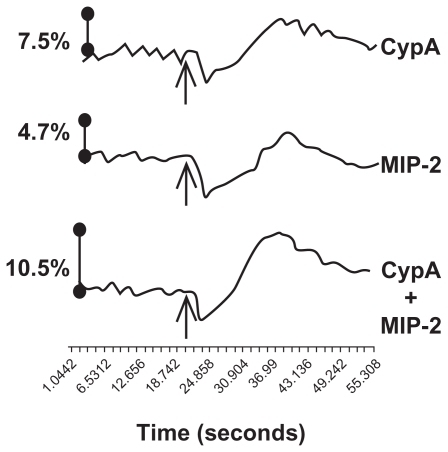
Intracellular calcium release is triggered upon GPCR binding and activation, and therefore increases in intracellular calcium levels are often used as a readout of GPCR activation. Similarly, intracellular calcium release is triggered by CD147 engagement by cyclophilins. Cooperating chemokines have been shown to induce enhanced calcium flux when added in combination.Citation18,Citation20,Citation21 Having demonstrated increased receptor internalization in the presence of CypA and MIP-2, we next conducted calcium flux assays to provide further support for additive signaling between CypA and MIP-2. Calcium release in response to the individual chemoattractants, or their combination, was measured in neutrophils in real time using a Molecular Devices FlexStation. As shown in , the greatest increase in intracellular calcium levels was observed when CypA and MIP-2 were present in combination. Moreover, the increase in calcium flux mediated by the combination appeared to be more rapid and more prolonged, compared with that mediated by MIP-2 and CypA alone. These findings provide additional evidence for the capacity of a MIP-2/CypA combination to mediate augmented signaling.
Figure 4 The combination of CypA and MIP-2 increases neutrophil calcium flux. Neutrophils (106) were incubated for 30 minutes at 37°C in fluorescent dye followed by exposure to chemoattractants (5 ng/mL CypA alone, 10 ng/mL MIP-2 alone, or CypA and MIP-2 combined) at the time indicated by arrows. Changes in intracellular calcium levels were monitored over time by changes in fluorescence intensity using the FlexStation system (Molecular Devices). The % increase in Ca2+ was calculated as the peak fluorescence with the addition of chemoattractants over the average baseline fluorescence of the cells. As a positive control for the ability of cells to flux calcium, dATP was added at 120 seconds, with the following increases in Ca2+ over baseline (CypA = 20%, MIP-2 = 22% and CypA + MIP-2 = 20%). These data are representative of 3 independent experiments.
Abbreviations: See list of abbreviations.
CypA and MIP-2 combination stimulates increased actin polymerization
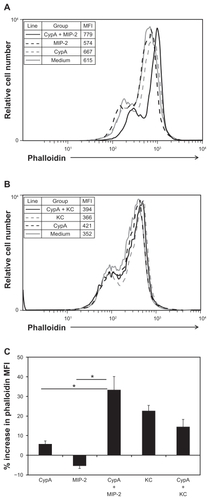
F-actin polymerization occurs at the leading edge of neutrophils in response to chemokine-mediated GPCR stimulation. Cooperating chemokines have been shown to trigger increased actin polymerization when present in combination compared with individual chemokines,Citation22 providing a potential mechanism for enhanced leukocyte migration by synergizing chemoattractants. In the current studies neutrophils were stimulated with CypA, MIP-2, or KC alone, or with combinations of CypA and MIP-2 or CypA and KC, followed by staining with FITC-conjugated phalloidin to measure the extent of actin polymerization. Phalloidin expression was significantly increased in neutrophils incubated with a combination of CypA and MIP-2 (average of 33% increase compared with medium alone, based on MFI units), compared with those incubated with individual chemoattractants (MIP-2 = 5% decrease and CypA = 6% increase) (). No such changes in phalloidin expression were mediated when CypA and KC were combined (KC alone = 23% increase versus KC + CypA = 14% increase) ().
Figure 5 Actin polymerization increases when CypA and MIP-2 are combined. Neutrophils were serum starved in RMPI for 30 minutes at room temperature before BSA Fraction V was added. Cells were then incubated for 5 minutes at 37°C in: A) medium alone, 5 ng/mL CypA alone, 10 ng/ml MIP-2 alone, or a combination of CypA + MIP-2, or B) 20 ng/mL KC alone, 5 ng/mL CypA alone, or a combination of CypA + KC. The cells were immediately fixed in formalin followed by permeabilization in Triton X-100 and staining with FITC-conjugated phalloidin. MFI were calculated and are shown for each group in panel legends. C) Bar graph showing average increases in phalloidin expression relative to medium that were calculated using 2 independent sets of data.
Abbreviations: See list of abbreviations.
Discussion
Neutrophil migration toward sites of infection is integral in the innate immune response. Chemokines secreted by resident cells in response to inflammatory stimuli establish a gradient that induces neutrophils to extravasate and migrate to infected tissues. Although beneficial for pathogen clearance, the neutrophilia that ensues during inflammatory responses often results in extensive bystander tissue damage. Neutrophil accumulation and activation within tissues contributes significantly to the pathology of both autoimmune and non-autoimmune inflammatory conditions (reviewed inCitation23) and can also promote the onset of carcinogenic events (reviewed inCitation24). Therefore, understanding how neutrophil recruitment is regulated, including the factors that contribute to this regulation, has important therapeutic implications for a wide variety of diseases associated with tissue injury.
Many chemokines are secreted during inflammation, suggesting either a high degree of redundancy or an overlap in function. Interestingly, several chemokines have been shown to work in partnership with the potential to amplify inflammatory responses. For example, studies with the CC plasma chemokine, Regakine-1, showed a synergistic enhancement in neutrophil migration when combined with IL-8 (CXCL8), GCP-2 (CXCL6), MCP-3 (CCL7),Citation25 or with NAP-2Citation26 in vitro, and when co-administered with GCP-2 in vivo.Citation27 Interestingly, Regakine-1 was also shown to synergize with a chemotactic anaphylatoxin, C5a,Citation26 suggesting that these types of interactions are not restricted to classical chemokines. Other studies have found that IL-8 cooperates with GM-CSF in the female reproductive tract, resulting in enhanced neutrophil chemotaxis. Citation28 This enhanced recruitment was inhibited by up to 95% when the 2 chemokines were neutralized in combination.Citation28 RANTES (CCL5) and MCP-1 (CCL2) were shown to work together to enhance the migration of PBMCs in vitroCitation18 and the in vivo co-injection of RANTES and IP-10 (CXCL10) was reported to augment T lymphocyte recruitment.Citation29 IP-10 has also been shown to increase the migration of Th1 and Th2 clones when combined with MDC (CCL22) in vitro.Citation22 Extracellular cyclophilins are another family of proteins with chemoattracting properties that are elevated during inflammatory responses. Our laboratory has previously demonstrated that extracellular cyclophilins contribute significantly to neutrophil recruitment in a mouse model of LPS-induced lung inflammation. Citation6 Inhibiting the function of cyclophilins reduced the influx of neutrophils by 40%–50% in this model.Citation6 Given the many other neutrophil-recruiting chemokines known to be present following LPS challenge,Citation12 we investigated in the current studies whether cyclophilins might have the capacity to synergize with MIP-2 and/or KC, the major neutrophil-attracting chemokines in this disease.
In initial in vitro chemotaxis studies we observed a potent functional interaction between CypA and MIP-2, but not with KC, that resulted in augmented neutrophil migration. Although several different doses of KC were tested and failed to cooperate with CypA in the combination assays (data not shown), we cannot rule out the possibility that KC and CypA might be able to function in either an additive or synergistic partnership at doses not tested here. Nevertheless, the current findings suggest that partnerships between chemoattractants are likely selective. Recent studies suggest that the family to which a chemokine belongs is a critical parameter in determining whether cooperative interactions will occur. Specifically, these studies showed that chemokines belonging to the same family (for example CXC or CC) are less likely to interact due to their competing for related receptors and/or using similar signaling pathways.Citation20 Both MIP-2 and KC signal through the same receptor, CXCR2, suggesting that the interaction with CypA may occur at a step preceding receptor binding. A possible mechanism is that interactions between MIP-2 and CypA enhance MIP-2 binding and/or signaling through CXCR2, possibly via the peptidyl-prolyl isomerization activity of CypA.
The principal binding and signaling receptor for CypA is CD147, a type I integral membrane protein carrying 2 immunoglobulin-like domains.Citation30 Since CD147 bears no structural homology with any of the 4 major families of chemokine receptors (CR, CCR, CXCR, and CX3CR), extracellular cyclophilins may be less restricted in their capacity to interact and synergize with other chemokines. However, it should be noted that the interaction between CypA/CypB and CD147 has also been shown to require the presence of cell surface heparans for optimal induction of cyclophilin binding and signaling.Citation15,Citation31 Recent studies have reported that syndecan-1 is the principal heparan co-receptor for CypB, which coordinates with CD147 to activate signaling pathways and induce T cell adhesion and migration.Citation32 Interestingly, KC has also been shown to bind syndecan-1.Citation33 The fact that syndecan-1 is implicated in both cyclophilin and KC signaling pathways could provide an alternative explanation for why we did not observe any cooperation between these 2 chemoattractants in the current studies: CypA and KC may compete for syndecan-1 binding, thus reducing their ability to signal through the corresponding receptor and preventing their capacity to interact.
Of particular interest was the observation that combinations of CypA and MIP-2 mediated increases in neutrophil migration that were greater than additive, suggesting a synergistic interaction. The phenomenon of synergy between chemoattractants in vitro is typically observed only within narrow ranges of concentrations that greatly depend on the cell type.Citation34 Our data fit this criterion in that the synergistic effect was seen only at very specific dose combinations. To provide support that the apparent synergistic interaction between CypA and MIP-2 was also associated with altered signaling events, several biochemical parameters were measured. Combinations of CypA and MIP-2 did indeed lead to an augmented internalization of CXCR2, increased and more prolonged calcium mobilization, and enhanced actin polymerization in neutrophils, although these changes were for the most part additive rather than synergistic. Similar discrepancies in functional outcome versus signaling profiles have been reported during chemokine synergy by other laboratories.Citation35,Citation36 One possibility is that the specific parameters measured in the current studies are not directly associated with the signaling events required to mediate functional synergy between chemokines. However, a more likely explanation is that synergistic outcomes are due to the integration of multiple signaling events. Thus, while changes in one signaling parameter provide evidence for enhanced receptor-mediated events, these events may represent only one arm of an integrated signaling pathway.
Based on our current findings, we propose the following mechanism for the augmented activation of neutrophils by combinations of CypA and MIP-2. When the 2 chemoattractants are present together, CypA may promote greater and/or more efficient binding of MIP-2 to its receptor CXCR2, resulting in increased CXCR2 internalization (). The internalized receptor-chemokine (CXCR2-MIP-2) complexes may then contribute to increased signaling by promoting receptor recycling. Increases in downstream signaling events are evidenced by an augmentation in calcium flux (). The ensuing increase in actin polymerization () results in a more efficient migration by individual cells, or enables a greater total number of cells to migrate, due to previously sub-optimal thresholds of activation becoming optimal. This is supported by in vivo observations that neutrophil numbers are still high as late as 24 hours after LPS delivery, yet inhibiting cyclophilins at this time point (when cyclophilins are at their peak level) reduces neutrophilia by only 40% to 50%.Citation6 Co-inhibiting CypA and MIP-2 (or any additional chemokines found to cooperate with CypA) provides a potential approach whereby late neutrophil recruitment might be further reduced, resulting in a decrease in the amplitude of the response and the collateral tissue damage associated with prolonged neutrophilia. Indeed, such an outcome was reported when the synergizing chemokines, IL-8, and GM-CSF, were co-inhibited,Citation28 providing preliminary support for this type of therapeutic intervention.
Abbreviations
| Ab | = | antibody |
| BAL | = | bronchoalveolar lavage |
| CsA | = | cyclosporine A |
| CypA | = | cyclophilin A |
| dATP | = | deoxyadenosine triphosphate |
| ELISA | = | enzyme-linked immunosorbent assay |
| FITC | = | fluorescein isothiocyanate |
| FLIPR | = | fluorescence imaging plate reader |
| fMLP | = | formyl-methionyl-leucylphenylalanine |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| GCP | = | granulocyte chemoattractant protein |
| GPCR | = | G protein-coupled receptor |
| HRP | = | horseradish peroxidase |
| Ig | = | immunoglobulin |
| IP | = | interferon gamma-induced protein |
| IL | = | interleukin |
| KC | = | keratinocyte chemoattractant |
| LIX | = | lipopolysaccharide-induced CXC chemokine |
| LPS | = | lipopolysaccharide |
| mAB | = | monoclonal antibody |
| MCP | = | monocyte chemotactic protein |
| MDC | = | macrophage-derived chemokine |
| MFI | = | mean fluorescence intensity |
| MIP | = | macrophage inflammatory protein |
| NAP | = | neutrophil activating protein |
| PBMC | = | peripheral blood mononuclear cell |
| PBS | = | phosphate buffered saline |
| PE | = | phycoerythrin |
| RANTES | = | regulated on activation, normally T cell expressed and secreted |
| RBC | = | red blood cell |
| SDS-PAGE | = | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SE | = | standard error |
Acknowledgment
These studies were supported by NIH grant R01AI067254.
Supplementary figure
Figure S1. Dose responses for CypA, MIP-2, and KC-mediated mouse neutrophil chemotaxis. In vitro chemotaxis assays were set up using purified mouse neutrophils incubated in the presence of a single dose of fMLP (positive control) and increasing doses of CypA, MIP-2, and KC. A chemotactic index was calculated for each group by dividing the number of migrated cells in test wells by the number of cells that migrated to medium alone. A) Mean ± SE chemotactic index for neutrophils incubated with fMLP and increasing doses of CypA. B) Mean ± SE chemotactic index for neutrophils incubated with fMLP and increasing doses of MIP-2. C) Mean ± SE chemotactic index for neutrophils incubated with fMLP and increasing doses of KC. N = 6 wells for each group.
Abbreviations: See list of abbreviations.
Disclosure
The authors report no conflicts of interest in this work.
References
- SherryBYarlettNStruppACeramiAIdentification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophagesProc Natl Acad Sci U S A1992898351135151565646
- TegederISchumacherAJohnSElevated serum cyclophilin levels in patients with severe sepsisJ Clin Immunol19971753803869327337
- JinZGMelaragnoMGLiaoDFCyclophilin A is a secreted growth factor induced by oxidative stressCirc Res200087978979611055983
- BillichAWinklerGAschauerHRotAPeichlPPresence of cyclophilin A in synovial fluids of patients with rheumatoid arthritisJ Exp Med199718559759809120404
- JinZGLunguAOXieLWangMWongCBerkBCCyclophilin A is a proinflammatory cytokine that activates endothelial cellsArterioscler Thromb Vasc Biol20042471186119115130913
- AroraKGwinnWMBowerMAExtracellular cyclophilins contribute to the regulation of inflammatory responsesJ Immunol2005175151752215972687
- GwinnWMDamskerJMFalahatiRNovel approach to inhibit asthma-mediated lung inflammation using anti-CD147 interventionJ Immunol200617774870487916982929
- DamskerJMOkwumabuaIPushkarskyTAroraKBukrinskyMIConstantSLTargeting the chemotactic function of CD147 reduces collagen-induced arthritisImmunology20091261556218557953
- BukrinskyMICyclophilins: unexpected messengers in intercellular communicationsTrends Immunol200223732332512103338
- GreenbergerMJStrieterRMKunkelSLNeutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumoniaJournal of Infectious Diseases199617311591658537653
- McCollSRClark-LewisIInhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonistsJ Immunol199916352829283510453028
- JeyaseelanSChuHWYoungSKWorthenGSTranscriptional profiling of lipopolysaccharide-induced acute lung injuryInfect Immun200472127247725615557650
- MausUvon GroteKKuzielWAThe role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact miceAm J Respir Crit Care Med2002166326827312153956
- GouwyMStruyfSProostPVan DammeJSynergy in cytokine and chemokine networks amplifies the inflammatory responseCytokine Growth Factor Rev200516656158016023396
- SaphireACBobardtMDGallayPAHost cyclophilin A mediates HIV-1 attachment to target cells via heparansEmbo J199918236771678510581250
- YurchenkoVZybarthGO’ConnorMActive site residues of cyclophilin A are crucial for its signaling activity via CD147J Biol Chem200227725229592296511943775
- GessnerPKIsobolographic analysis of interactions: an update on applications and utilityToxicology19951052–31611798571354
- MelladoMRodriguez-FradeJMVila-CoroAJChemokine receptor homo- or heterodimerization activates distinct signaling pathwaysEmbo J200120102497250711350939
- YurchenkoVO’ConnorMDaiWWCD147 is a signaling receptor for cyclophilin BBiochem Biophys Res Commun2001288478678811688976
- GouwyMStruyfSNoppenSSynergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated eventsMol Pharmacol200874248549518469140
- KuscherKDanelonGPaolettiSSynergy-inducing chemokines enhance CCR2 ligand activities on monocytesEur J Immunol20093941118112819291700
- SebastianiSDanelonGGerberBUguccioniMCCL22-induced responses are powerfully enhanced by synergy inducing chemokines via CCR4: evidence for the involvement of first beta-strand of chemokineEur J Immunol200535374675615714581
- SegelGBHaltermanMWLichtmanMAThe paradox of the neutrophil’s role in tissue injuryJ Leukoc Biol201189335937221097697
- LonkarPDedonPCReactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fatesInt J Cancer201112891999200921387284
- StruyfSProostPLenaertsJPStoopsGWuytsAVan DammeJIdentification of a blood-derived chemoattractant for neutrophils and lymphocytes as a novel CC chemokine, Regakine-1Blood20019782197220411290579
- GouwyMStruyfSMahieuFPutWProostPVan DammeJThe unique property of the CC chemokine regakine-1 to synergize with other plasma-derived inflammatory mediators in neutrophil chemotaxis does not reside in its NH2-terminal structureMol Pharmacol200262117318012065768
- StruyfSGouwyMDillenCProostPOpdenakkerGVan DammeJChemokines synergize in the recruitment of circulating neutrophils into inflamed tissueEur J Immunol20053551583159115827963
- ShenLFaheyJVHusseySBAsinSNWiraCRFangerMWSynergy between IL-8 and GM-CSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxisCell Immunol20042301233215541716
- StanfordMMIssekutzTBThe relative activity of CXCR3 and CCR5 ligands in T lymphocyte migration: concordant and disparate activities in vitro and in vivoJ Leukoc Biol200374579179912960247
- YurchenkoVConstantSBukrinskyMDealing with the family: CD147 interactions with cyclophilinsImmunology2006117330130916476049
- DenysAAllainFCarpentierMSpikGInvolvement of two classes of binding sites in the interactions of cyclophilin B with peripheral blood T-lymphocytesBiochem J1998336Pt 36896979841882
- PakulaRMelchiorADenysAVanpouilleCMazurierJAllainFSyndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxisGlycobiology200717549250317267519
- LiQParkPWWilsonCLParksWCMatrilysin shedding of syndecan- 1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injuryCell2002111563564612464176
- GouwyMStruyfSVerbekeHCC chemokine ligand-2 synergizes with the nonchemokine G protein-coupled receptor ligand fMLP in monocyte chemotaxis, and it cooperates with the TLR ligand LPS via induction of CXCL8J Leukoc Biol200986367168019451399
- El-AsmarLSpringaelJYBalletSAndrieuEUVassartGParmentierMEvidence for negative binding cooperativity within CCR5-CCR2b heterodimersMol Pharmacol200567246046915509716
- GouwyMStruyfSCatusseJProostPVan DammeJSynergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migrationJ Leukoc Biol200476118519415075362