Abstract
Purpose
Glucocorticoids are commonly prescribed to treat a number of diseases including the majority of inflammatory diseases. Despite considerable interpersonal variability in response to glucocorticoids, an insensitivity rate of about 30%, and the risk of adverse side effects of glucocorticoid therapy, currently no assay is performed to determine sensitivity.
Patients and methods
Here we propose a whole blood ex vivo stimulation assay to interrogate known glucocorticoid receptor (GR) up- and downregulated genes to indicate glucocorticoid sensitivity. We have chosen to employ real-time PCR in order to provide a relatively fast and inexpensive assay.
Results
We show that the GR-regulated genes, GILZ and FKBP51, are upregulated in whole blood by treatment with dexamethasone and that LPS-induction of cytokines (IL-6 and TNFα) are repressed by dexamethasone in a dose responsive manner. There is considerable interpersonal variability in the maximum induction of these genes but little variation in the EC50 and IC50 concentrations. The regulation of the GR-induced genes differs throughout the day whereas the suppression of LPS-induced cytokines is not as sensitive to time of day.
Conclusion
In all, this assay would provide a method to determine glucocorticoid receptor responsiveness in whole blood.
Introduction
Glucocorticoids have been used in the treatment of many inflammatory diseases, such as rheumatoid arthritis, asthma, multiple sclerosis, and inflammatory bowel disease since the 1940s. Glucocorticoids are one of the most widely prescribed drugs. It has been suggested that 1%–3% of the adult population currently uses glucocorticoids.Citation1
Glucocorticoids are potent regulators of various aspects of immunity.Citation2 They function through a cytosolic receptor, the glucocorticoid receptor (GR), which is a member of the nuclear hormone receptor superfamily. GR binds to DNA to modulate gene transcription.Citation3 Upon ligand binding, GR dissociates from a protein complex that includes heat shock proteins and immunophilins. It then dimerizes and translocates to the nucleus where it binds to specific DNA sequences called glucocorticoid response elements to activate gene transcription.Citation4 GR also interferes with other signaling pathways, such as activator protein-1 and nuclear factor kappa B (NFκB)Citation5 to repress gene transcription. It is by this repression of these pro-inflammatory pathways that many of the anti-inflammatory actions of glucocorticoids are mediated.
Despite their usefulness, glucocorticoid treatment is not without risk. Serious side effects include osteoporosis, adrenal insufficiency, glaucoma, cataracts, and hypertension.Citation6 Glucocorticoid use has also been associated with increased risk of some cancers,Citation7 cardiovascular disease,Citation8 fractures,Citation9 and infections.Citation10 Glucocorticoid resistance, inherited or acquired, is a major problem in the treatment of many diseases including asthma, ulcerative colitis, systemic lupus erythematosus, and rheumatoid arthritis, with patients exhibiting glucocorticoid resistance being reported as high as 30%.Citation11–Citation16 In addition, it is also estimated that 30% of a normal healthy population is glucocorticoid nonresponsive.Citation17
Clinical tests are typically not performed prior to prescription of glucocorticoids. Most clinical decisions are based on cortisol measurements as is used in the treatment of sepsis.Citation18 However, studies have suggested that steroid bioavailability does not predict clinical outcomes.Citation19 Assays to determine GR function have been suggested in the literature. These include the dexamethasone suppression test,Citation20–Citation26 inhibition of peripheral blood mononuclear cell (PBMC) proliferation,Citation20,Citation27–Citation30 quantification of number and affinity of GRs in PBMCs,Citation20,Citation30,Citation31 glucocorticoid suppression of lipopolysaccharide (LPS)-induced genes (primarily cytokines) in alveolar macrophages and PBMCs or blood,Citation21,Citation24–Citation26,Citation30,Citation32–Citation37 genome-wide promoter-based bioinformatics analysis of transcriptional activity,Citation38 glucocorticoid-induced cutaneous vasoconstrictor (skin blanching),Citation21,Citation39 and glucocorticoid-induced gene expression.Citation26,Citation29,Citation30
Here we propose an assay to test GR function particularly for the use of glucocorticoids in the treatment of inflammatory diseases. We propose interrogation of both known GR upregulated as well as downregulated genes. Although many anti-inflammatory actions of glucocorticoids are mediated through their ability to downregulate immune factors, they also upregulate genes that are involved in the regulation of inflammation such as glucocorticoid inducible leucine zipper (GILZ), which inhibits the NFκB pathway,Citation40–Citation43 and mitogen activated protein kinase phosphatase 1 (MKP-1).Citation44 We have proposed to use whole nonseparated blood and real-time polymerase chain reaction to make the assay relatively quick and affordable.
Material and methods
Ex vivo stimulation of whole blood
Normal healthy human blood samples were obtained in lavender Hemogard stopper venous blood collection tubes with spray-dried K2 EDTA (10.8 mg) (ThermoFisher Scientific, Pittsburgh, PA) after informed written consent was obtained, as approved by the Institutional Review Board. The demographics of the subjects are shown in . Three milliliters blood was diluted 1:1 with phosphate buffered saline and triplicates of 500 μL aliquots were either untreated or treated with 100 nM dexamethasone (Sigma- Aldrich, St Louis, MO), 1 ng/mL LPS (Sigma-Aldrich), or a combination of dexamethasone and LPS for 2 hours at 37°C. Following incubation, red blood cells (RBC) were lysed by addition of 3 mL of RBC lysis buffer (5 M NH4Cl, 5 M KHCO3, 1 mM EDTA) for 15 minutes at room temperature and centrifugation at 1400 rpm for 10 minutes. The pellet was washed twice more with 500 μL RBC lysis buffer and centrifuged for 2 minutes at 3000 rpm to ensure removal of all hemes. The pellets were resuspended in 400 μL of Ribozol RNA extraction reagent (ISC BioExpress, Kaysville, UT).
Table 1 Demographics of normal human subjects (n = 12)
Quantification of GR-regulated genes by real-time PCR
Total RNA was extracted using the Ribozol RNA extraction reagent following the manufacturer’s instructions with the following modifications. Volumes were adjusted to fit the 400 μL volume of Ribozol, and the isopropanol precipitation step was allowed to proceed overnight at −20°C. In addition, 75% ethanol was chilled before washing the pellet and an additional 1 minute centrifugation at 7500 × g was added to remove excess ethanol. Five hundred nanograms RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). Twenty microliters of cDNA was diluted by addition of 100 μL DEPC-treated water. Real-time PCR was performed on 8 μL diluted cDNA in a 20 μL reaction volume containing 1X Power SYBR® Green PCR master mix (Applied Biosystems) and 0.125 μM of each primer (). The following protocol was used on the ABI 7300 real-time PCR system (Applied Biosystems): heated to 50°C for 2 minutes, denatured for 10 minutes at 50°C, and then subjected to 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Duplicate cycle threshold (Ct) values of triplicate samples were analyzed using the comparative Ct (ΔΔCt) method (Applied Biosystems). The fold induction (2−ΔΔCt) was obtained by normalizing to two endogenous genes, glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) and cyclic AMP-accessory protein (CAP-1),Citation45 and expressed relative to the amount in nontreated cells (GR-induced genes) or LPS only treated cells (GR repressed genes).
Table 2 Real-time PCR primers
Interleukin 6 (IL-6) enzyme-linked immunosorbent assay (ELISA) methods
Whole blood was stimulated as above for 24 hours. Serum was then separated by centrifugation at 10,000 × g for 10 minutes. Serum was stored at −80°C until assayed. IL-6 was assayed by ELISA using the Human IL-6 ELISA MAX Set Deluxe Kit (Biolegend, San Diego, CA) according to manufacturer’s instructions.
Statistical methods
In this descriptive study summary statistics (coefficient of variation [CV], median, and the interquartile range [IQR]) are presented as box plots in and (). A CV greater than one is considered to have a high degree of variability relative to its mean. For – the median and the IQR are presented as summary statistics (). Random-effect linear regression was used to estimate the slope over time or over pre- and post-treatment. Random effects were used since observations are nested within a particular subject. This method takes into account the variability within and between patients and uses this inherent correlation when estimating the standard errors that are used to test slope coefficients. As this study was descriptive in nature, no inferential test comparing different groups were run. All analyses were run using Stata software (v 12.0; Stata Corporation, College Station, TX).
Figure 1 Whole blood was stimulated with 100 nM dexamethasone (A) or 1 ng/mL LPS with and without 100 nM dexamethasone (B) for 2 hours. RNA was isolated and cDNA transcribed. Real-time PCR was performed to determine expression of the GR upregulated genes GILZ, FKBP51, FLAP, and MKP-1 (A) and the GR downregulated genes IL-6, TNFα, and IFNγ (B). The expression of these genes was normalized to the housekeeping genes GAPDH and CAP-1. ΔΔ Ct was calculated for GILZ, FKBP51, FLAP, and MKP-1 as treatment (dexamethasone) against no treatment (A) and for IL-6, TNFα, and IFNγ as treatment (dexamethasone + LPS) against LPS only.
Abbreviations: LPS, lipopolysaccharide; PCR, polymerase chain reaction; GR, glucocorticoid receptor; GILZ, glucocorticoid inducible leucine zipper; FKBP51, FK506 binding protein 51; FLAP, arachidonate 5-lipoxygenase-activating protein; MKP- 1, mitogen activated protein kinase phosphatase 1; IL-6, interleukin 6; TNFα, tumor necrosis factor α; IFNγ, interferon γ; IQR, interquartile range; CAP-1, cyclic AMPaccessory protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
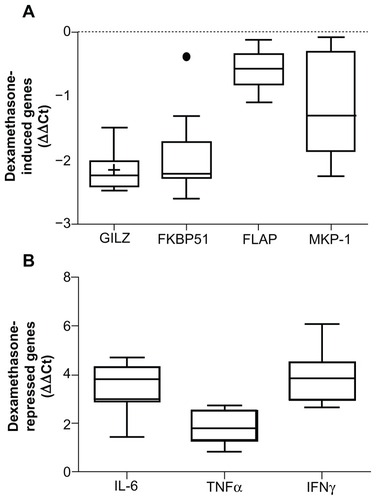
Figure 2 Whole blood was stimulated with increasing amounts of dexamethasone (A) or 1 ng/mL LPS with and without increasing concentrations of dexamethasone (B) for 2 hours. RNA was isolated and cDNA transcribed. Real-time PCR was performed to determine expression of the GR upregulated genes GILZ and FKBP51 (A) and the GR downregulated genes IL-6 and TNFα (B). The expression of these genes was normalized to the housekeeping genes GAPDH and CAP-1. Dose response curves were drawn and the EC50 (A) and IC50 (B) values were calculated.
Abbreviations: LPS, lipopolysaccharide; PCR, polymerase chain reaction; GR, glucocorticoid receptor; GILZ, glucocorticoid inducible leucine zipper; IL-6, interleukin 6; TNFα, tumor necrosis factor α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CAP-1, cyclic AMP-accessory protein; EC50, half maximal effective concentration; IC50, half maximal inhibitory concentration; IQR, interquartile range.
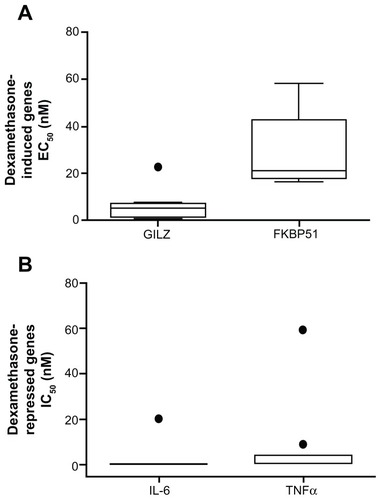
Figure 3 Whole blood was drawn from 10 individuals at 8:30 am, 12:30 pm, 4:30 pm, and 8:30 pm and was stimulated with 100 nM dexamethasone (A and B) or 1 ng/mL LPS with and without 100 nM dexamethasone (C and D) for 2 hours. RNA was isolated and cDNA transcribed. Real-time PCR was performed to determine expression of the GR upregulated genes GILZ (A) and FKBP51 (B) and the GR downregulated genes IL-6 (C) and TNFα (D).
Abbreviations: LPS, lipopolysaccharide; PCR, polymerase chain reaction; GR, glucocorticoid receptor; GILZ, glucocorticoid inducible leucine zipper; FKBP51, FK506 binding protein 51; IL-6, interleukin 6; TNFα, tumor necrosis factor α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CAP-1, cyclic AMP-accessory protein; ΔΔCt, comparative cycle threshold.
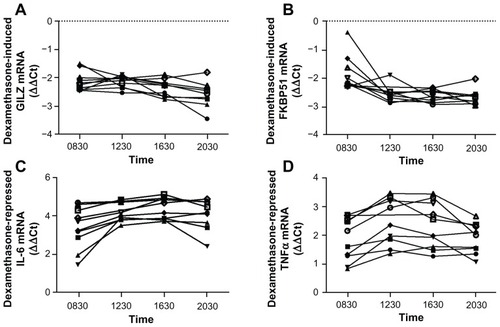
Figure 4 Whole blood from 10 individuals was either treated immediately (closed square) or stored in the EDTA tube at 4°C for 24 hours (open square) prior to stimulation with 100 nM dexamethasone (A) or 1 ng/mL LPS with and without 100 nM dexamethasone (B) for 2 hours. RNA was isolated and cDNA transcribed. Real-time PCR was performed to determine the expression of the GR upregulated genes GILZ and FKBP51 (A) and the GR downregulated genes IL-6 and TNFα (B).
Abbreviations: LPS, lipopolysaccharide; PCR, polymerase chain reaction; GR, glucocorticoid receptor; GILZ, glucocorticoid inducible leucine zipper; FKBP51, FK506 binding protein 51; IL-6, interleukin 6; TNFα, tumor necrosis factor α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CAP-1, cyclic AMP-accessory protein; ΔΔCt, comparative cycle threshold.
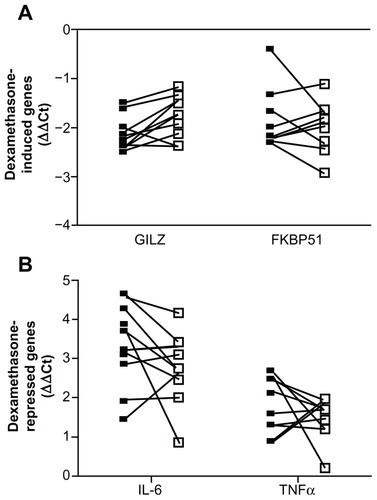
Figure 5 Whole blood was drawn at 8:30 am and stimulated with 1 ng/mL LPS with and without 100 nM dexamethasone for 2 hours for real-time PCR or 24 hours for ELISA.
Abbreviations: LPS, lipopolysaccharide; PCR, polymerase chain reaction; EL ISA, enzyme-linked immunosorbent assay; IL-6, interleukin 6; GAPDH, glyceraldehyde- 3-phosphate dehydrogenase; CAP-1, cyclic AMP-accessory protein; SD, standard deviation.
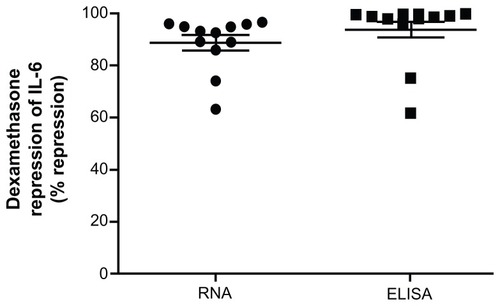
Table 3 Coefficient of variation over gene
Table 4 Descriptive statistics by figure, gene, and time
Results
Interpersonal variations in GR function in whole blood
Whole blood, drawn from 12 normal healthy volunteers at 8:30 am ± 1 hour who had not eaten for 1 hour prior to the blood draw, was stimulated ex vivo with 100 nM dexamethasone () or with 1 ng/mL LPS in the presence and absence of 100 nM dexamethasone () for 2 hours. RNA was isolated and the expression of the known GR upregulated genes – GILZ, FK506 binding protein 51 (FKBP51), arachidonate 5-lipoxygenase-activating protein (FLAP; also known as ALOX5AP), and MKP-1 – and the known GR downregulated genes – IL-6, tumor necrosis factor α (TNFα) and interferon γ (IFNγ) – were determined by real-time PCR. As can be seen in , 100 nM dexamethasone induced GILZ, FKBP51, FLAP, and MKP-1 with considerable interpersonal variation. GILZ was induced 3–6-fold (coefficient of variance [CV] of 0.149), FKBP51 was induced 1–6-fold (CV of 0.306), FLAP was induced 1–2-fold (CV of 0.46) and MKP-1 was induced 1–5-fold (CV of 0.462). In addition, dexamethasone suppression of LPS-induced IL-6, TNFα, and IFNγ also showed considerable interpersonal variation. LPS-induced IL-6 was repressed by 100 nM dexamethasone 63%–96% (CV of 0.113), TNFα by 44%–85% (CV of 0.211) and IFNγ by 84%–98% (CV of 0.053).
In order to test whether the relative efficacy for glucocorticoids was also varied between subjects, whole blood () was stimulated with increasing concentrations of dexamethasone in the presence or absence of 1 ng/mL LPS for 2 hours, RNA was isolated and the expression of GILZ, FKBP51, IL-6, and TNFα was determined by realtime PCR. Dose response curves were drawn and the half-maximal effective concentration (EC50) and half-maximal inhibitory concentration (IC50) values calculated. As can be seen in , the IC50 and EC50 values are tightly clustered with the exception of the odd outlier (observations more than 1.5 times the IQR either above the 3rd quartile or below the 1st quartile). The CV was 1.029 for GILZ, 0.556 for FKBP51, 3.086 for IL-6, and 2.43 for TNFα.
Diurnal variation of GR function
Given the natural diurnal cortisol cycle, we next tested the effect of time of day on this assay. Whole blood from 10 individuals () was drawn at 8:30 am, 12:30 pm, 4:30 pm, and 8:30 pm ± 1 hour and was stimulated with 100 nM dexamethasone in the presence or absence of 1 ng/mL LPS for 2 hours. RNA was isolated and the expression of the GR upregulated genes GILZ () and FKBP51 () and the GR downregulated genes IL-6 () and TNFα () determined by real-time PCR. The GR upregulated genes showed a significant increase in their induction (decrease in ΔΔCt) by 100 nM dexamethasone across the duration of the day (GILZ ΔΔCt slope −0.04, P-value 0.002; FKBP51 ΔΔCt slope −0.06, P-value < 0.001). Dexamethasone suppression of LPS-induced IL-6 significantly increased from 8:30 am to 12:30 pm (ΔΔCt slope 1.69, P-value 0.019) but then did not alter for the remainder of the day. Dexamethasone suppression of LPS-induced TNFα was not significantly altered during the day.
Practicality of the assay
In order to test the practicality of the assay, given that the assay may not be able to be performed immediately, we tested the effect of storing the blood (in the EDTA tube) in a refrigerator for 24 hours prior to performing the assay. Again, two upregulated and two downregulated genes were tested in 10 individuals (). Whole blood was drawn and either the assay was performed immediately (closed squares) or the EDTA tubes were stored at 4°C for 24 hours before performing the assay (open squares). As can be seen in dexamethasone induction of GILZ and FKBP51 and dexamethasone suppression of LPS-induced IL-6 and TNFα was considerably different in the sample that had been stored for 24 hours compared to the sample that was analyzed fresh.
Finally, we compared this assay to the dexamethasone suppression of LPS-induced cytokines analyzed by ELISA, which has been previously used. Whole blood was stimulated with 100 nM dexamethasone in the presence or absence of 1 ng/mL LPS for either 2 or 24 hours. After 2 hours RNA was isolated, cDNA prepared, and the expression of IL-6 mRNA determined by real-time PCR (circles). After 24 hours, serum was separated and IL-6 protein levels were determined by ELISA (squares). As can be seen in the extent of repression of LPS-induced IL-6 production is comparable between these two different assays.
Discussion
We have shown that ex vivo stimulation of whole blood can be used to assess GR function by interrogating known GR up- and downregulated genes. shows that there is considerable interpersonal variation in the maximal induction or repression of these genes. However, there is less variability in the EC50 and IC50 values () suggesting that the relative efficacy for dexamethasone is similar between individuals but the extent of the response is varied. These data also indicate that glucocorticoid induction of GR upregulated genes increases throughout the course of the day, when serum cortisol levels are decreasing, but that glucocorticoid suppression of LPS-induced cytokines is relatively unchanged during the day (). In our studies, the subject had not eaten for 1 hour before the blood draw (self-reported) but food intake, as well as other factors, could affect the outcome of this assay. Therefore, it is necessary to control that as many factors as possible (such as time of day and food intake) remain constant when making comparisons either between different individuals or within the same person. In an attempt to address the practicability of this assay, we assessed if blood could be stored in the collection tubes prior to performing the assay. The data in would suggest that it is not possible and further work would be needed to identify conditions, which would allow for prolonged storage prior to the assay. Indeed, another study suggested that delayed processing decreased glucocorticoid sensitivity.Citation46 Finally, we compared the glucocorticoid suppression of LPS-induced cytokines measured by real-time PCR and ELISA (). Comparable results were found with these two different assays. One pitfall of the current study is that we have used whole blood. Although this provides a fast approach, it does not account for differences in cell populations within the white blood cells, which could play a role in interpersonal variability.
For this assay, we chose to assess both GR up- and downregulated genes. Glucocorticoid suppression of inflammatory- induced genes and/or proteins has been commonly usedCitation21,Citation24,Citation25,Citation32–Citation37,Citation46 but this only assesses one mechanism through which glucocorticoids function. Surprisingly few studies have chosen to use GR upregulated genes.Citation29 In addition, only a couple of groups have incorporated the use of both GR up- and downregulated genes.Citation26,Citation30,Citation47 No correlation was found between glucocorticoid suppression of IL-2 and glucocorticoid induction of GILZ,Citation26,Citation30 agreeing with our suggestion that it would be more appropriate to assess a combination of both up- and downregulated genes as some GR upregulated genes, such as GILZCitation40–Citation43 and MKP-1,Citation44 also play a role in the anti-inflammatory properties of glucocorticoids.
Other assays that have been used include the dexamethasone suppression test,Citation20–Citation25 which measures the response of the adrenal glands to adrenocorticotropic hormone; inhibition of stimulated PBMC proliferation,Citation20,Citation27–Citation30 which correlates with the response to glucocorticoid therapy in some diseases;Citation12,Citation13,Citation48,Citation49 quantification of the number and affinity of GR in the PBMCs;Citation20,Citation30,Citation31 bioinformatics profiling;Citation37,Citation38 and cutaneous vasoconstrictor (skin blanching),Citation21,Citation39 which determines topical potency of glucocorticoids. Attempts to obtain concordance between many of these assays have failed. Chriguer et al compared the dexamethasone suppression test, PBMC proliferation assay, and the numbers of GR in normal healthy volunteers. They found concordance between these assays in the majority of people but in a few there was no concordance suggesting possible tissue-specific glucocorticoid sensitivity.Citation20 Ebrecht et al found no concordance between the dexamethasone suppression of LPS-induced cytokines, skin blanching, and the dexamethasone suppression test in healthy volunteers, again suggesting target tissue specificity.Citation21 Vasiliadi et al also found no concordance between the dexamethasone suppression test and dexamethasone inhibition of LPS-induced TNFαCitation25 and Smit et al found no concordance between the dexamethasone suppression test and expression of GR up- and downregulated genes.Citation26 Concordance between the cutaneous vasoconstrictor assay and glucocorticoid sensitivity in asthma has been varied.Citation39,Citation50
Others have shown that there is considerable interpersonal variability in glucocorticoid responses.Citation23,Citation25,Citation26,Citation29,Citation30,Citation46,Citation51 Interestingly, serum cortisol did not correlate with genome-wide transcriptional analysis of GR and NFκB-regulated genes.Citation38 A few studies have investigated the effect of the diurnal cortisol cycle on these assays but with conflicting results. No difference in dexamethasone suppression of LPS-induced cytokines in the evening or morning was seen in one study.Citation32 In another, increased glucocorticoid inhibition of LPS-induced TNFα was seen in the morning compared to the evening hours.Citation35 However, both these studies pooled several individuals and are confounded by possible interpersonal variations. Recently, a study has described differences in glucocorticoid sensitivity in morning and afternoon samples but comparison within the same individual was not made.Citation46 Again, confounding factors could also affect the outcome of this and other GR functional assays, including stress (social isolation stress),Citation38,Citation52 depression,Citation53,Citation54 exercise,Citation34 and disease state.Citation33
Conclusion
We suggest that this assay could be used to determine glucocorticoid sensitivity. One caveat is that this assay will not be able to determine differences in glucocorticoid sensitivity in a person if it is tissue specific. However, if it is possible for viable cells to be obtained from the tissue of interest, for example by bronchoalveolar lavage,Citation36 then this assay may prove useful in analyzing tissue specific responsiveness.
Acknowledgments/disclosure
The authors report no conflicts of interest in this work. We would like to thank Tim Eubank for assistance with blood draws, Mikhail Gavrilin for the IL-6, IFNγ, and TNFα primers, and Dr Mark Wewers for critically reading this manuscript.
References
- van StaaTPLeufkensHGAbenhaimLBegaudBZhangBCooperCUse of oral corticosteroids in the United KingdomQJM200093210511110700481
- WebsterJITonelliLSternbergEMNeuroendocrine regulation of immunityAnnu Rev Immunol20022012516311861600
- ArandaAPascualANuclear hormone receptors and gene expressionPhysiol Rev20018131269130411427696
- SchoneveldOJGaemersICLamersWHMechanisms of glucocorticoid signallingBiochim Biophys Acta20041680211412815488991
- HayashiRWadaHItoKAdcockIMEffects of glucocorticoids on gene transcriptionEur J Pharmacol20045001–3516215464020
- SchäckeHDöckeWDAsadullahKMechanisms involved in the side effects of glucocorticoidsPharmacol Ther2002961234312441176
- JensenAØThomsenHFEngebjergMCUse of oral glucocorticoids and risk of skin cancer and non-Hodgkin’s lymphoma: a population-based case-control studyBr J Cancer2009100120020519034275
- WeiLMacDonaldTMWalkerBRTaking glucocorticoids by prescription is associated with subsequent cardiovascular diseaseAnn Intern Med20041411076477015545676
- SteinbuchMYouketTECohenSOral glucocorticoid use is associated with an increased risk of fractureOsteoporos Int200415432332814762652
- KleinNCGoCHCunhaBAInfections associated with steroid useInfect Dis Clin North Am200115242343211447704
- ButtgereitFSaagKGCutoloMda SilvaJABijlsmaJWThe molecular basis for the effectiveness, toxicity, and resistance to glucocorticoids: focus on the treatment of rheumatoid arthritisScand J Rheumatol2005341142115903020
- CorriganCJBrownPHBarnesNCGlucocorticoid resistance in chronic asthma. Glucocorticoid pharmacokinetics, glucocorticoid receptor characteristics, and inhibition of peripheral blood T cell proliferation by glucocorticoids in vitroAm Rev Respir Dis19911445101610251952426
- HearingSDNormanMProbertCSHaslamNDayanCMPredicting therapeutic outcome in severe ulcerative colitis by measuring in vitro steroid sensitivity of proliferating peripheral blood lymphocytesGut199945338238810446106
- LeungDYde CastroMSzeflerSJChrousosGPMechanisms of glucocorticoid-resistant asthmaAnn N Y Acad Sci19988407357469629300
- SekiMUshiyamaCSetaNApoptosis of lymphocytes induced by glucocorticoids and relationship to therapeutic efficacy in patients with systemic lupus erythematosusArthritis Rheum19984158238309588733
- van SchaardenburgDValkemaRDijkmansBAPrednisone treatment of elderly-onset rheumatoid arthritis. Disease activity and bone mass in comparison with chloroquine treatmentArthritis Rheum19953833343427880187
- HearingSDNormanMSmythCFoyCDayanCMWide variation in lymphocyte steroid sensitivity among healthy human volunteersJ Clin Endocrinol Metab199984114149415410566664
- MarikPEPastoresSMAnnaneDfor American College of Critical Care MedicineRecommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care MedicineCrit Care Med20083661937194918496365
- TurnerDKolhoKLMackDRGlucocorticoid bioactivity does not predict response to steroid therapy in severe pediatric ulcerative colitisInflamm Bowel Dis201016346947319714760
- ChriguerRSEliasLLda SilvaIMJrVieiraJGMoreiraACde CastroMGlucocorticoid sensitivity in young healthy individuals: in vitro and in vivo studiesJ Clin Endocrinol Metab200590115978598416091495
- EbrechtMBuske-KirschbaumAHellhammerDTissue specificity of glucocorticoid sensitivity in healthy adultsJ Clin Endocrinol Metab200085103733373911061532
- FariaCDCobraJFSousaESilvaTA very low dose intravenous dexamethasone suppression test as an index of glucocorticoid sensitivityHorm Res200869635736218504395
- HuizengaNAKoperJWde LangePInterperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individualsJ Clin Endocrinol Metab199883147549435415
- SyedAARedfernCPWeaverJUIn vivo and in vitro glucocorticoid sensitivity in obese people with cushingoid appearanceObesity (Silver Spring)200816102374237818719648
- VasiliadiDGratsiasYTsagarakisSGlucocortidoid sensitivity assessed in peripheral blood cells do not correlate with the feedback sensitivity of the hypothalamo-pituitary adrenal AXISHormones (Athens)20021423323817018452
- SmitPRusscherHde JongFHBrinkmannAOLambertsSWKoperJWDifferential regulation of synthetic glucocorticoids on gene expression levels of glucocorticoid-induced leucine zipper and interleukin-2J Clin Endocrinol Metab20059052994300015728202
- CreedTJLeeRWNewcombPVdi MambroAJRajuMDayanCMThe effects of cytokines on suppression of lymphocyte proliferation by dexamethasoneJ Immunol2009183116417119542427
- Sliwinska-StanczykPPazdurJZiolkowskaMJaworskiJKaminska-TchorzewskaELackiJKThe effect of methylprednisolone on proliferation of PBMCs obtained from steroid-sensitive and steroid-resistant rheumatoid arthritis patientsScand J Rheumatol200736316717117657668
- VermeerHHendriks-StegemanBIVerrijn StuartAAvan Buul-OffersSCJansenMA comparison of in vitro bioassays to determine cellular glucocorticoid sensitivityEur J Endocrinol20041501414714713278
- RusscherHSmitPvan RossumEFStrategies for the characterization of disorders in cortisol sensitivityJ Clin Endocrinol Metab200691269470116317053
- IoannesyantsIAPolevayaEBSensitivity of peripheral blood lymphocytes from breast cancer patients to glucocorticoidsBull Exp Biol Med20061411737616929969
- De RijkRMichelsonDKarpBExercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1 beta (IL-1 beta), IL-6, and tumor necrosis factor-alpha (TNF alpha) production in humans: high sensitivity of TNF alpha and resistance of IL-6JClin Endocrinol Metab1997827218221919215292
- DeRijkRHEskandariFSternbergEMCorticosteroid resistance in a subpopulation of multiple sclerosis patients as measured by ex vivo dexamethasone inhibition of LPS induced IL-6 productionJ Neuroimmunol20041511–218018815145616
- DeRijkRHPetridesJDeusterPGoldPWSternbergEMChanges in corticosteroid sensitivity of peripheral blood lymphocytes after strenuous exercise in humansJ Clin Endocrinol Metab19968112282358550757
- GratsiasYMoutsatsouPChrysanthopoulouGTsagarakisSThalassinosNSekerisCEDiurnal changes in glucocorticoid sensitivity in human peripheral blood samplesSteroids2000651285185611077082
- ArmstrongJSargentCSinghDGlucocorticoid sensitivity of lipopolysaccharide-stimulated chronic obstructive pulmonary disease alveolar macrophagesClin Exp Immunol20091581748319737233
- HakonarsonHBjornsdottirUSHalapiEProfiling of genes expressed in peripheral blood mononuclear cells predicts glucocorticoid sensitivity in asthma patientsProc Natl Acad Sci U S A200510241147891479416203992
- ColeSWHawkleyLCArevaloJMSungCYRoseRMCacioppoJTSocial regulation of gene expression in human leukocytesGenome Biol200789R18917854483
- BrownPHTeelucksinghSMatusiewiczSPGreeningAPCromptonGKEdwardsCRCutaneous vasoconstrictor response to glucocorticoids in asthmaLancet199133787415765801671942
- AyroldiERiccardiCGlucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid actionFASEB J200923113649365819567371
- EddlestonJHerschbachJWagelie-SteffenALChristiansenSCZurawBLThe anti-inflammatory effect of glucocorticoids is mediated by glucocorticoid-induced leucine zipper in epithelial cellsJ Allergy Clin Immunol2007119111512217208592
- CannarileLZolloOD’AdamioFCloning, chromosomal assignment and tissue distribution of human GILZ, a glucocorticoid hormone-induced geneCell Death Differ20018220120311313722
- D’AdamioFZolloOMoracaRA new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/ CD3-activated cell deathImmunity1997768038129430225
- LiLChenSFLiuYMAP kinase phosphatase-1, a critical negative regulator of the innate immune responseInt J Clin Exp Med200921486719436832
- GavrilinMABouaklIJKnatzNLInternalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and releaseProc Natl Acad Sci U S A2006103114114616373510
- CardinalJPretoriusCJUngererJPBiological and diurnal variation in glucocorticoid sensitivity detected with a sensitive in vitro dexamethasone suppression of cytokine production assayJ Clin Endocrinol Metab20109583657366320463096
- MacedoJAHesseJTurnerJDMeyerJHellhammerDHMullerCPGlucocorticoid sensitivity in fibromyalgia patients: decreased expression of corticosteroid receptors and glucocorticoid-induced leucine zipperPsychoneuroendocrinology200833679980918468809
- AlvarezJSursWLeungDYIkléDGelfandEWSzeflerSJSteroid-resistant asthma: immunologic and pharmacologic featuresJ Allergy Clin Immunol19928937147211545093
- KirkhamBWCorkillMMDavisonSCPanayiGSResponse to glucocorticoid treatment in rheumatoid arthritis: in vitro cell mediated immune assay predicts in vivo responsesJ Rheumatol19911868218251895263
- WilsonAMCoutieWJSimsEJLipworthBJThe skin vasoconstrictor assay does not correlate significantly to airway or systemic responsiveness to inhaled budesonide in asthmatic patientsEur J Clin Pharmacol2003581064364712610738
- RohlederNWolfJMKirschbaumCGlucocorticoid sensitivity in humans-interindividual differences and acute stress effectsStress20036320722213129814
- ColeSWSocial regulation of leukocyte homeostasis: the role of glucocorticoid sensitivityBrain Behav Immun20082271049105518394861
- MillerGERohlederNStetlerCKirschbaumCClinical depression and regulation of the inflammatory response during acute stressPsychosom Med200567567968716204423
- ParianteCMGlucocorticoid receptor function in vitro in patients with major depressionStress20047420921916019586