Abstract
Neonates are known to exhibit increased susceptibility to bacterial and viral infections and increasing evidence demonstrates that the increased susceptibility is related to their attenuated immune response to infections. The lung is equipped with an innate defense system involving both cellular and humoral mediators. The present study was performed to characterize the expression of inflammatory mediators in the lung of neonatal rats in comparison with older animals. Rats at postnatal day 1 (P1), P21, and P70 were treated with saline or 0.25 mg/kg lipopolysaccharide (LPS) via intraperitoneal injection. Two hours later, animals were sacrificed and the transcriptional response of key inflammatory mediators and enzyme activity of myeloperoxidase (MPO) in the lung of these animals were examined. LPS-induced messenger RNA (mRNA) expression of pro-inflammatory cytokines, namely interleukin (IL)-1β, IL-6, and tumor necrosis factor-α, antiinflammatory cytokines, namely IL-10 and IL-1 receptor antagonist (IL-1ra), and chemokines, namely macrophage inflammatory protein (MIP)-1β, MIP-2, and monocyte chemotactic protein-1, in P1 lung was much reduced compared to that in P21 and P70 animals at 2 hours postinjection. These data suggest that LPS-induced transcriptional response of cytokines and chemokines was much reduced in P1 lung even though the protein levels of these genes were not ascertained and mRNA levels of these genes may not reflect their final protein levels. MPO activity in LPS-treated P1 lung was also significantly attenuated compared to that in LPS-treated P70 lung, suggesting impaired neutrophil infiltration in P1 lung at 2 hours following LPS treatment. In parallel, the baseline mRNA expression of LPS-binding protein (LBP) in P1 lung was much lower than that in P21 and P70 lungs. While the protein level of LBP was not examined and the mRNA level of LBP may not reflect its final protein level, the reduced transcriptional response of cytokines and chemokines in P1 lung at 2 hours following LPS treatment may be attributed to lower LBP expression in P1 lung as compared to P21 and P70 lungs.
Introduction
To preserve homeostasis and protect itself from injury, the lung is equipped with an innate defense system involving both cellular and humoral mediators. Both leukocytes and epithelial cells present in the lung express an array of pattern recognition receptors such as toll-like receptors (TLRs) and antimicrobial compounds. Upon binding to appropriate pathogen-associated molecular patterns (PAMPs), TLRs transmit signals to the nucleus of these cells and initiate an immune response. For example, TLR-2 associates with TLR-1 or TLR-6 to recognize triacyl- and diacyl-lipopeptides, respectively, TLR-4 recognizes lipopolysaccharides (LPS), and TLR-5 recognizes flagellin ().Citation1
Figure 1 Overview of LPS/TLR-4 signaling pathway.
Abbreviations: LBP, LPS-binding protein; LPS, lipopolysaccharide; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; MyD88, myeloid differentiation marker 88; TLR, toll-like receptor; TNF-α, tumor necrosis factor-α; TRIF, TIR domain-containing adaptor inducing IFN-β.
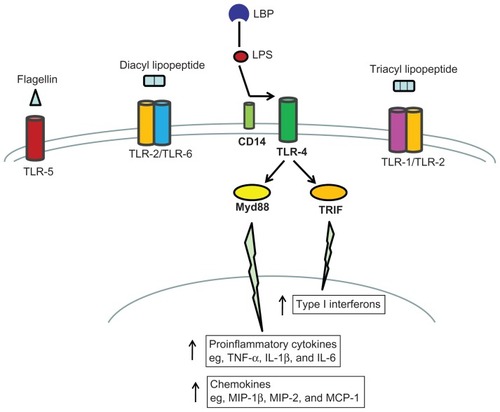
LPS, an endotoxin from the outer membrane of Gram-negative bacteria, first binds to LPS-binding protein (LBP), which facilitates the binding of LPS to membrane CD14 (mCD14).Citation2 mCD14 then transfers LPS to TLR-4, which activates both myeloid differentiation markers (MyD) 88-dependent and MyD88-independent intracellular signaling cascades. The MyD88-dependent pathway ultimately upregulates the production of inflammatory mediators including cytokines and chemokines, and the MyD88-independent pathway ultimately leads to increased production of type I interferons ().Citation3 The proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, are primarily responsible for initiating an effective defense against exogenous pathogens while anti-inflammatory cytokines, such as IL-10 and IL-1 receptor antagonist (IL-1ra), which is a naturally occurring antagonist of IL-1, are crucial for downregulating the inflammatory process.Citation4 Chemokines are directly involved in the recruitment of immune cells, such as neutrophils and T cells, to the site of infection.Citation5
Neonatal sepsis is one of the major health problems throughout the world, with an estimated 30 million newborns acquiring infections every year.Citation6 In the United States, bacterial sepsis affects up to 32,000 live births annually.Citation7 There is evidence that increased neonatal susceptibility to bacterial and viral infections may be related to their attenuated immune response. Studies have found that cord blood monocytes are less responsive to bacterial lipopeptides, double-stranded RNA, and LPS that act through TLR-2, TLR-3, and TLR-4, respectively, than adult peripheral blood monocytes (PBMCs).Citation8–Citation10 Cord blood dendrocytes have also been shown to express lower levels of the gamma-chain of the IL-2 receptor (CD132) and CD86 co-stimulatory molecules, suggesting their relative immaturity as compared to adult peripheral blood dendrocytes.Citation11
It has been reported that TLR-4 expression and cytokine responsiveness of fetal lung to LPS become detectable at embryonic day 17 and further increase after birth in mice.Citation12 Pulmonary neutrophil recruitment following bacterial infections has also been reported to be impaired in neonatal rats compared to adult rats.Citation13,Citation14 Additionally, IL-10 expression is only minimally augmented in neonatal alveolar macrophages compared with those from adult rats.Citation15 These studies suggest that the pulmonary innate immunity in the neonates may be relatively immature.
The present study was undertaken to compare the induction of inflammatory mediators in the lung of animals at different stages of postnatal development following LPS treatment in order to understand the mechanisms underlying increased susceptibility of neonates to infections. We treated animals at postnatal day 1 (P1), P21, and P70 with 0.25 mg/kg LPS and examined the expression of various inflammatory mediators in the lung of these animals. Animals were treated with 0.25 mg/kg LPS for 2 hours based on our previous finding that 0.25 mg/kg LPS was able to induce an inflammatory response in rat liver at P1, P21, and P70 at 2 hours postinjection.Citation16
Materials and methods
Animals
Young adult male and female Sprague–Dawley® rats (Harlan Inc, Indianapolis, IN) were maintained in a temperature- and humidity-controlled facility on a 12 h day/night cycle and fed a standard rat diet with water ad libitum. The female rats were bred with male rats, and the ones with vaginal plugs were moved to separate cages. Animals were treated with either saline or 0.25 mg/kg LPS (Salmonella typhimurium; Sigma Aldrich, St Louis, MO) via intraperitoneal (ip) injection (four animals per group) at P1, P21 (the day of weaning), or P70 (young adult).Citation17 At the end of treatment, the animals were sacrificed and their lung tissues were dissected. Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Seton Hall University.
Total RNA extraction
Total RNA was isolated from the dissected lung tissues using the TRIzol reagent (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions, dissolved in RNase-free water, and stored at −80°C.
cDNA synthesis by reverse transcription (RT)
Total RNA was used in RT reactions with ImProm-II™ reverse transcriptase (Promega, Madison, WI) and oligo (dT)Citation12–Citation18 (Affymetrix, Cleveland, OH) primers. All samples were set up from the same RT master mix and reverse transcribed under the same conditions at 42°C for 60 minutes to minimize differences in RT efficiency.Citation16
Quantitation by real-time polymerase chain reaction (PCR)
To measure the relative mRNA levels of TLR-2, TLR-4, TLR-5, TLR-6, and LBP, real-time PCR was performed in 20 μL reactions with 1X SYBR Green master mix (Applied Biosystems, Foster City, CA) and 1 μL of cDNA diluted 1:2 as PCR template together with 500 μM appropriate specific sense and anti sense primers. β-actin, a housekeeping gene, was used as the control. The sense and antisense primers for rat TLR-2 were 5′-GGA-AGC-AGG-TGA-CAA- CCA-TT-3′ and 5′-CGC-CTA-AGA-GCA-GGA-TCA- AC-3′, respectively; the sense and antisense primers for rat TLR-4 were 5′-TGC-TCA-GAC-ATG-GCA-GTT-TC-3′ and 5′-TCA-AGG-CTT-TTC-CAT-CCA-AC-3′, respectively; the sense and antisense primers for rat TLR-5 were 5′-TCT-TCG- AAC-TTC-GGC-TGT-TT-3′ and 5′-TGT-GAA-TCT-CGT- TGG-CAG-AG-3′, respectively; the sense and antisense primers for rat TLR-6 were 5′-AAG-ATG-ACA-TTC-GGGTTT- GC-3′ and 5′-TCR-GGA-TGA-AGT-GG-GAG-AC-3′, respectively; the sense and antisense primers for rat LBP were 5′-CCG-GGT-TTA-CCA-CCA-GGC-CG-3′, and 5′-TTC-GTG-ACC-ACG-CCA-AGC-CG-3′, respectively; and the sense and antisense primers for rat β-actin were 5′-AGC-CAT-GTA-CGT-AGC-CAT-CC-3′ and 5′-AGC-CAT-GTA- CGT-AGC-CAT-CC-3′, respectively. The PCR reactions were denatured at 95°C for 10 minutes with 40 cycles of amplification and quantification followed by a melting curve program according to the manufacturer’s instructions in a StepOnePlus™ thermal cycler (Applied Biosystems). The generation of only the correct size amplification products was confirmed by agarose gel electrophoresis.
Real-time PCR data were analyzed by the ABI StepOne software supplied by Applied Biosystems. Ct-values of all samples were normalized to the Ct values of β-actin (ΔCt) and ΔCt values of one of the saline control samples (‘baseline’ expression) were subtracted (ΔΔCt). The expression of the control sample used to calculate ΔΔCt was set to 1 and values of other samples were plotted as fold expression of the baseline 2−ΔΔCt.
Quantitation by semi-quantitative PCR
To measure the relative mRNA levels of CD14, MyD88, TNF-α, IL-1β, IL-6, IL-10, IL-1ra, macrophage inflammatory protein (MIP)-1β, MIP-2, and monocyte chemotactic protein (MCP)-1, cDNA samples were used to conduct PCR amplifications using appropriate specific sense and antisense primers.Citation17,Citation18 The sense and antisense primers for rat MyD88 were 5′-CCC-CTG-ACA-TGC-CTA-TCA-CT-3′ and 5′-TGT-CCC-AAA-GGA-AAC-ACA- CA-3′, respectively; the sense and antisense primers for rat IL-10 were 5′-GGG-AAG-CAA-CTG-AAA-CTT-CG- 3′ and 5′-GCT-TTC-GAG-ACT-GGA-AGT-GG-3′, respectively; the sense and antisense primers for rat IL-1ra were 5′-GTG-CTT-TAT-GAG-GGG-AGC-TG-3′ and 5′-GAG-CCA-TGT-TTG-CAC-AGA-GA-3′, respectively; and the sense and antisense primers for rat MCP-1 were 5′-ATG-ATC-CCA-ATG-AGT-CGG-3′ and 5′-ACA-GAA- GTG-CTT-GAG-GTG-3′, respectively. The PCR reactions were carried out by heating the samples to 94°C for 5 minutes, followed by appropriate number of cycles of denaturation at 94°C for 30 seconds, annealing at 57°C for 30 seconds, and extension at 72°C for 30 seconds. After the final cycle, the PCR reactions were extended for another 7 minutes. The optimum number of cycles was 25 cycles for MyD88 and CD14, 26 cycles for TNF-α and MIP-1β, 27 cycles for IL-1β, 28 cycles for IL-6 and MCP-1, 29 cycles for MIP-2, 32 cycles for IL-10 and IL-1ra, and 21 cycles for β-actin. After running PCR products on a 2.0% agarose gel, the gel image was recorded using a UVP GelDoc-It™ imaging system (UVP, Upland, CA) and digitized using VisionWorks™ LS software (UVP). The relative intensities for genes of interest were normalized to the intensity of β-actin in the same sample.Citation16,Citation18
Myeloperoxidase (MPO) activity assay
Fifty micrograms of lung tissue samples from LPS- and saline-treated animals were homogenized in 300 μL of 5 mM potassium phosphate-buffered saline (pH = 6.0) followed by centrifugation at 14,000 rpm for 15 minutes at 4°C. The pellets were then extracted in 300 μL of 50 mM potassium phosphate-buffered saline (pH = 6.0) with 0.5% hexadecyltrimethylammonium bromide followed by 20 seconds of sonication and three cycles of freeze/thaw. The samples were then centrifuged at 14,000 rpm for 15 minutes at 4°C.Citation19 Protein concentrations of the supernatants were determined using bicinchoninic acid (BCA) protein assay reagent from Pierce Biotechnology Inc (Rockford, IL).Citation17 MPO activity was then determined by adding 10 μL of supernatant or equal volume of MPO standard solutions (0, 5, 10, 20, and 40 U/mL) to 990 μL of 50 mM potassium phosphate-buffered saline (pH = 6.0) containing 0.167 mg/mL of o-dianisidine and 0.0005% of H2O2 and recording the absorbance at 460 nm at 2 minutes after initiation of the reaction.Citation20 MPO activity of the lung tissue samples was determined from the standard curve and expressed as unit of MPO activity per mg of protein in the lung tissue extract (U/mg).
Statistical analysis
All data were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni’s post-test (GraphPad Prism, La Jolla, CA). Results of P < 0.05 were considered statistically significant.
Results
Expression of TLRs in the lung of postnatal animals
To investigate how the pulmonary innate immunity develops during the postnatal period, animals at P1, P21, and P70 were treated with saline or 0.25 mg/kg LPS via intraperitoneal (ip) injection. Two hours after the injection, the animals were sacrificed, their lung tissues were dissected, and total RNA was extracted from the lung tissues for determination of expression levels of various immune mediators. The animals stimulated with saline were used as negative controls.
We first examined the relative mRNA levels of TLR-2, TLR-4, TLR-5, and TLR-6 using real-time reverse transcription PCR. While the baseline mRNA levels of TLR-2 and TLR-5 were comparable in the lung of P1, P21, and P70 animals (), TLR-4 and TLR-6 in P1 lung was 3.93- and 2.71-fold lower than that in P70 lung, respectively ( and ). LPS treatment did not significantly alter the mRNA expression of TLR-2, TLR-4, TLR-5, and TLR-6 in P1 lung, but increased the mRNA level of TLR-2 by 6.18-fold in P21 lung without significant effects on the mRNA levels of TLR-4, TLR-5, and TLR-6. In contrast, LPS treatment significantly augmented the mRNA expression of TLR-2 by 8.59-fold, TLR-5 by 1.81-fold, and TLR-6 by 2.56-fold, and attenuated the mRNA expression of TLR-4 by 1.75-fold in P70 lung at 2 hours post-injection (). The relative mRNA levels of TLR-2, TLR-4, TLR-5, and TLR-6 in LPS-treated P70 lung were 16.82-, 2.44-, 2.26-, and 6.88-fold higher than that in LPS-treated P1 lungs, respectively ( and ).
Figure 2 Relative mRNA expression of TLRs in the lung of P1, P21, and P70 animals. P1, P21, and P70 animals were treated with saline (S) or 0.25 mg/kg lipopolysaccharide (L) for 2 hours. Relative mRNA levels of TLR-2 (A), TLR-4 (B), TLR-5 (C), and TLR-6 (D) in the lung of these animals were measured by real-time reverse transcription PCR.
Abbreviations: mRNA, messenger RNA; P1, postnatal day 1; PCR, polymerase chain reaction; TLR, toll-like receptor.
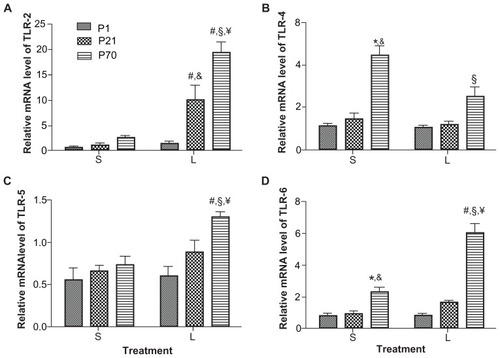
Table 1 Fold changes in the relative mRNA levels of inflammatory genes in the lung of animals at P1, P21, and P70 following treatment with saline (S) or lipopolysaccharide (L)
Expression of upstream TLR-4 signaling mediators in the lung of postnatal animals
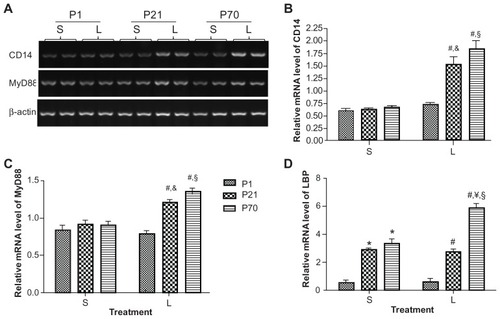
We then examined the mRNA expression of key upstream mediators of the TLR-4 signaling pathway. The relative mRNA levels of CD14 and MyD88 were determined using semi-quantitative reverse transcription PCR and the relative mRNA level of LBP was determined using real-time reverse transcription PCR. The baseline mRNA levels of CD14 and MyD88 were comparable in P1, P21, and P70 lungs, yet the baseline mRNA level of LBP in P1 lung was 5.19 and 5.99-fold lower than that in P21 and P70 lung, respectively ( and ). LPS treatment enhanced the mRNA expression of CD14, MyD88, and LBP in P70 lung by 2.75-, 1.48-, and 1.74-fold, respectively. LPS treatment also enhanced the mRNA expression of CD14 and MyD88 in P21 lung by 2.46- and 1.31-fold, respectively. In contrast, LPS treatment did not significantly affect the mRNA expression of CD14, MyD88, and LBP in P1 lung at 2 hours postinjection ( and ).
Figure 3 Relative mRNA expression of CD14, MyD88, and LBP in the lung of P1, P21, and P70 animals. P1, P21, and P70 animals were treated with saline (S) or 0.25 mg/kg lipopolysaccharide (L) for 2 hours. Relative mRNA levels of CD14 (A and B) and MyD88 (A and C) in the lung of these animals were measured by semi-quantitative reverse transcription PCR and the relative mRNA level of LBP (D) was measured by real-time reverse transcription PCR.
Abbreviations: LBP, lipopolysaccharide binding protein; MyD88, myeloid differentiation marker 88; P1, postnatal day 1; mRNA, messenger RNA; PCR, polymerase chain reaction.
Expression of pro-inflammatory cytokines in the lung of postnatal animals
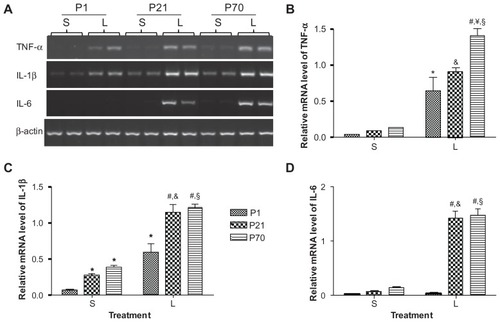
We then examined the mRNA expression of pro-inflammatory cytokines, namely TNF-α, IL-1β, and IL-6, in the lung of P1, P21, and P70 animals using semiquantitative reverse transcription PCR. Baseline expression of TNF-α and IL-6 was hardly detectable in P1 lung and remained low in P21 and P70 lung (). While the baseline level of IL-1β was also low in P1 lung, it was elevated by 4.38- and 6.31-fold in the P21 and P70 lung, respectively ( and ). LPS treatment significantly enhanced the mRNA expression of TNF-α by 19.06-, 10.21-, and 10.75-fold, and upregulated the mRNA expression of IL-1β by 9.54-, 4.28-, and 3.17-fold in P1, P21, and P70 lungs, respectively, compared with their saline controls. LPS treatment also enhanced the mRNA expression of IL-6 by 21.71- and 11.15-fold in P21 and P70 lungs, respectively, without significant effects on P1 lung. Additionally, the mRNA expression of IL-1β and IL-6 in LPS-treated P1 lung was 1.97- and 43.09-fold lower than that in LPS-treated P21 lung, respectively, and the mRNA expression of TNF-α, IL-1β, and IL-6 in LPS-treated P1 lung was 2.19-, 2.10-, and 45.56-fold lower than that in LPS-treated P70 lung, respectively, at 2 hours postinjection ( and ).
Figure 4 Relative mRNA expression of proinflammatory cytokines in the lung of P1, P21, and P70 animals. P1, P21, and P70 animals were treated with saline (s) or 0.25 mg/kg lipopolysaccharide (L) for 2 hours. Relative mRNA levels of TNF-α (A and B), IL-1β (A and C), and IL-6 (A and D) in the lung of these animals were measured by semiquantitative reverse transcription PCR.
Abbreviations: IL, interleukin; P1, postnatal day 1; PCR, polymerase chain reaction; TNF, tumor necrosis factor.
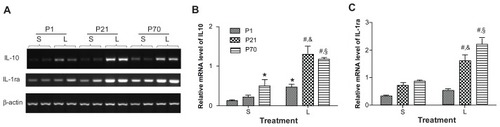
Expression of anti-inflammatory cytokines in the lung of postnatal animals
We then analyzed the transcriptional response of anti-inflammatory cytokines, namely IL-10 and IL-1ra, in the lung of P1, P21, and P70 animals using semiquantitative reverse transcription PCR. Both IL-10 and IL-1ra were found to be constitutively expressed in the lung of animals at different ages and the baseline mRNA expression of IL-10 in P1 lung was 3.98-fold lower than that in P70 lung ( and ). LPS treatment significantly enhanced the mRNA level of IL-10 in the lung of P1, P21, and P70 animals by 3.76-, 5.85-, and 2.35-fold, respectively, and augmented the mRNA level of IL-1ra in the lung of P21 and P70 animals by 2.30- and 2.57-fold, respectively, as compared with their saline controls. The mRNA expression of IL-10 in LPS-treated P1 lung was 2.77- and 2.49-fold lower than that in LPS-treated P21 and P70 lung, respectively, and the mRNA expression of IL-1ra in LPS-treated P1 lung was 3.07- and 4.18-fold lower than that in LPS-treated P21 and P70 lung, respectively, at 2 hours postinjection ( and ).
Figure 5 Relative mRNA expression of anti-inflammatory cytokines in the lung of P1, P21, and P70 animals. P1, P21, and P70 animals were treated with saline (S) or 0.25 mg/kg lipopolysaccharide (L) for 2 hours. Relative mRNA levels of IL-10 (A and B) and IL-1ra (A and C) in the lung of these animals were measured by semiquantitative reverse transcription PCR.
Abbreviations: IL-10, interleukin-10; IL-1ra, interleukin-1 receptor antagonist; mRNA, messenger RNA; P1, postnatal day 1.
Ratio of proinflammatory to anti-inflammatory cytokines in the lung of postnatal animals
We also calculated the ratios of relative mRNA levels of proinflammatory to anti-inflammatory cytokines in the lung of P1, P21, and P70 animals following treatment with saline or LPS. The ratios of TNF-α:IL-10, TNF-α:IL-1ra, IL-1β:IL-10, IL-1β:IL-1ra, IL-6:IL10, and IL-6:IL-1ra in the lung of P1, P21, and P70 animals treated with saline were not significantly different (). However, the ratio of IL-6:IL-10 in P21 (1.14) and P70 lung (1.28) was significantly higher than that in P1 lung (0.07) at 2 hours following LPS treatment. The ratio of IL-6:IL-1ra in P21 (0.99) and P70 lung (0.71) was also significantly higher than that in P1 lung (0.06) at 2 hours following LPS treatment. However, the ratio of TNF-α:IL-10 in P1 lung (1.46) was slightly higher than that in P21 (0.75) and P70 lung (1.20) and the ratios of TNF-α:IL-1ra, IL-1β:IL-10, and IL-1β:IL-1ra were not significantly different in P1, P21, and P70 lung at 2 hours following LPS treatment ().
Table 2 Ratios of the relative mRNA levels of proinflammatory to anti-inflammatory cytokines in the lung of P1, P21, and P70 animals following treatment with saline (S) or lipopolysaccharide (L)
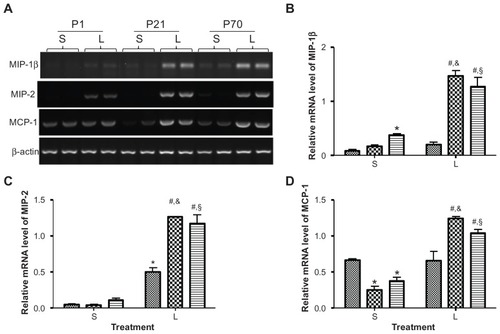
Expression of chemokines in the lung of postnatal animals
We then analyzed the relative mRNA levels of chemokines, namely MIP-1β, MIP-2, and MCP-1, in the lung of P1, P21, and P70 animals using semi-quantitative reverse transcription PCR. The baseline mRNA expression of MIP-2 was barely detectable in the lung of animals at the different ages examined (). The baseline mRNA expression of MCP-1 in P1 lung was 2.63- and 1.79-fold higher than that in P21 and P70 lung, respectively, while the baseline mRNA level of MIP-1β in P1 lung was 4.49-fold lower than that in P70 lung ( and ). LPS treatment significantly enhanced the mRNA expression of MIP-1β by 8.98- and 3.44-fold, and increased the mRNA expression of MCP-1 by 4.94- and 2.78-fold, respectively, in P21 and P70 lung while the mRNA levels of MIP-1β and MCP-1 in P1 lung were not significantly affected at 2 hours following LPS treatment. LPS treatment also upregulated the mRNA level of MIP-2 in P1, P21, and P70 lung by 11.79-, 31.89-, and 11.04-fold, respectively. The mRNA expression of MIP-1β, MIP-2, and MCP-1 in LPS-treated P21 lung was higher than that in LPS-treated P1 lung by 7.31-, 2.52-, and 1.88-fold, respectively, and the mRNA expression of MIP-1β, MIP-2, and MCP-1 in LPS-treated P70 lung was higher than that in LPS-treated P1 lung by 6.32-, 2.33-, and 1.57-fold, respectively ( and ).
Figure 6 Relative mRNA expression of chemokines in the lung of P1, P21, and P70 animals. P1, P21, and P70 animals were treated with saline (S) or 0.25 mg/kg lipopolysaccharide (L) for 2 hours. Relative mRNA levels of MIP-1β (A and B), MIP-2 (A and C), and MCP-1 (A and D) in the lung of these animals were measured by semiquantitative reverse transcription PCR.
Abbreviations: MCP-1, monocyte chemotactic protein-1; MIP, macrophage inflammatory protein; P1, postnatal day 1.
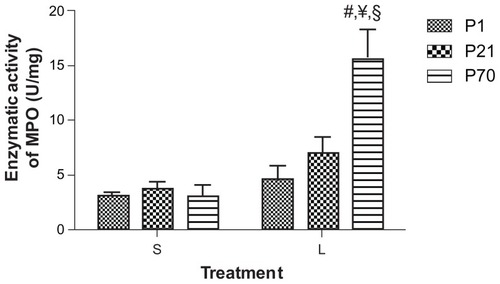
Enzymatic activity of myeloperoxidase in the lung of postnatal animals
Myeloperoxidase (MPO) is a peroxidase enzyme most abundantly expressed in neutrophils. In order to assess the infiltration of neutrophils into the lung, we extracted proteins from the lung tissues and performed MPO enzymatic activity assay. The basal MPO activity was comparable in the lung of P1 (3.19 ± 0.34 U/mg), P21 (3.64 ± 0.63 U/mg), and P70 (3.09 ± 1.18 U/mg) (). LPS treatment significantly increased the MPO activity in P70 lung (15.9 ± 2.42 U/mg) as compared to its saline controls. LPS treatment also appeared to increase the MPO activity in P1 (4.64 ± 1.43 U/mg) and P21 lung (5.62 ± 1.34 U/mg), yet the increase was not statistically significant. Additionally, MPO activity in LPS-treated P70 lung was significantly higher than LPS-treated P1 and P21 lungs ().
Figure 7 Enzymatic activity of myeloperoxidase (MPO) in the lung of P1, P21, and P70 animals. P1, P21, and P70 animals were treated with saline (S) or 0.25 mg/kg lipopolysaccharide (L) for 2 hours. MPO activity in the lung of these animals was then measured by enzymatic assay.
Abbreviation: P1, postnatal day 1.
Discussion
Bacterial sepsis is a significant cause of neonatal morbidity and mortality, which is in large part secondary to developmentally immature host defense mechanisms.Citation21,Citation22 The main objective of the present study was to examine the postnatal development of TLR-4-mediated innate immunity in the lung. Our study showed that LPS-induced transcriptional response of pro-inflammatory cytokines, namely IL-1β, IL-6, and TNF-α, and anti-inflammatory cytokines, namely IL-10 and IL-1ra, in P1 lung, was much reduced compared to that in P21 and P70 lung. Consistently, LPS-induced cytokine response in cord blood has a different profile from the adult venous blood.Citation23 Cord blood monocytes have been shown to be less responsive to LPS by producing diminished levels of cytokines than adult human peripheral blood mononuclear cells (hPBMCs).Citation9,Citation24 Neonatal macrophages have also been reported to produce less IL-1β and TNF-α than adult macrophages from mice.Citation25 Furthermore, our study found that while the ratio of relative mRNA levels of TNF-α:IL-10 in P1 lung was slightly higher than that in P21 or P70 lung following LPS stimulation, the ratio of IL-6:IL10 and IL-6:IL-1ra in P1 lung was significantly lower than that in P21 and P70 lung at 2 hours following LPS stimulation, which may contribute to their increased susceptibility to neonatal infections. Consistently, the IL-6:IL-10 ratio has been reported to be significantly higher in septic patients with multiple organ failure in comparison with patients without multiple organ failure.Citation26
Our study also showed that LPS-induced transcriptional response of chemokines, namely MIP-1β, MIP-2, and MCP- 1, in P1 lung was much reduced compared to that in P21 and P70 lung. MPO activity in LPS-treated P1 lung was also significantly attenuated compared to that in LPS-treated P70 lung, suggesting that neutrophil recruitment into neonatal lung was impaired as compared to that in older animals.
In parallel, our study showed that the basal and LPS-induced mRNA level of LBP in P1 lung was significantly lower than that in P21 and P70 lung. LBP knockout mice have been reported to experience increased mortality from Gram-negative pneumonia compared with their wild-type controls, and this mortality difference is abolished with systemic LBP gene therapy.Citation27 Total lung myeloperoxidase activity is also diminished significantly in LBP knockout mice.Citation28 Therefore, a low level of LBP expression in P1 lung may contribute to its reduced induction of cytokines and chemokines following LPS stimulation as compared to older animals.
It is of interest to note that treatment with LPS, a PAMP for TLR-4, augmented the mRNA expression of TLR-2, TLR-5, and TLR-6 in P70 lung at 2 hours postinjection, suggesting that expression of different TLRs in the lung may be cross-regulated. Consistently, stimulation of TLR-5 by bacterial flagellin has been reported to upregulate TLR-2 and TLR-4 mRNA and result in sensitization of mouse intestinal epithelial cell line mICcl2 for subsequent TLR-2 and TLR-4 stimulation.Citation29 LPS treatment has also been reported to increase TLR-2 but decrease TLR-4 mRNA in rat liver.Citation30 While Matsumura et al reported that LPS stimulation increases TLR-4 expression in murine lung,Citation31 Bozinovski et al reported a decline of TLR-4 mRNA at 2 hours following LPS stimulation and an increase at 4 hours and 24 hours post-LPS injection.Citation32 Consistently, TLR-4 mRNA expression was found to be attenuated in P70 lung at 2 hours post-LPS injection in our studies. It is also important to point out that in our study the levels of inflammatory genes were measured at the mRNA level, and that these measurements may not reflect their final protein levels.
Conclusion
In summary, our study showed that LPS-induced transcriptional response of inflammatory mediators as well as the ratios of relative mRNA levels of IL6:IL-10 and IL6:IL-1ra in the lung of P1 animals were much reduced in magnitude as compared to those in older animals, at 2 hours following LPS treatment, suggesting that LPS-induced inflammatory response in P1 lung was significantly attenuated. In parallel, mRNA expression of LBP in P1 lung was much lower than that in older animals, suggesting that a lower level of LBP may contribute to the attenuated inflammatory response in P1 lung following LPS stimulation as compared to older animals. These studies help to understand the molecular mechanisms underlying the increased susceptibility of neonates to bacterial infections, and may contribute to designing new therapeutic approaches to control infections in neonates.
Acknowledgments
This work was supported in part by the National Institute of Health Grant R15-HD065643 to HZ.
Disclosure
The authors report no conflicts of interest in this work.
References
- TakedaKAkiraSTLR signaling pathwaysSemin Immunol20041613914751757
- FitzgeraldKAPalsson-McDermottEMBowieAGMal (MyD88- adapter-like) is required for Toll-like receptor-4 signal transductionNature20014136851788311544529
- ZeytunAvan VelkinburghJCPardingtonPECaryRRGuptaGPathogen-specific innate immune responseAdv Exp Med Biol200759834235717892223
- ArendWPInterleukin-1 receptor antagonistAdv Immunol1993541672278379462
- NgPCLiKWongRPProinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infectionsArch Dis Child Fetal Neonatal2003883F209F213
- AfrozaSNeonatal sepsis – a global problem: an overviewMymensingh Med J200615110811416467776
- LukacsSLSchoendorfKCSchuchatATrends in sepsis-related neonatal mortality in the United States, 1985–1998Pediatr Infect Dis J200423759960315247595
- YanSRQingGByersDMStadnykAWAl-HertaniWBortolussiRRole of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharideInfect Immun20047231223122914977922
- HebraAStrangePEgbertJMAliMMullinaxABuchananEIntracellular cytokine production by fetal and adult monocytesJ Pediatr Surg20013691321132611528598
- LevyOZaremberKARoyRMCywesCGodowskiPJWesselsMRSelective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848J Immunol200417374627463415383597
- CrespoIPaivaACouceiroAPimentelPOrfaoARegateiroFImmunophenotypic and functional characterization of cord blood dendritic cellsStem Cells Dev2004131637015068694
- HarjuKOjaniemiMRouniojaSExpression of toll-like receptor 4 and endotoxin responsiveness in mice during perinatal periodPediatr Res2005575 Pt 164464815718365
- LeePTHoltPGMcWilliamASRole of alveolar macrophages in innate immunity in neonates: evidence for selective lipopolysaccharide binding protein production by rat neonatal alveolar macrophagesAm J Respir Cell Mol Biol200023565266111062144
- MartinTRRuzinskiJTWilsonCBSkerrettSJEffects of endotoxin in the lungs of neonatal rats: age-dependent impairment of the inflammatory responseJ Infect Dis199517111341447798654
- LeePTHoltPGMcWilliamASOntogeny of rat pulmonary alveolar macrophage function: evidence for a selective deficiency in il-10 and nitric oxide production by newborn alveolar macrophagesCytokine2001151535711509009
- Le RouzicVCoronaJZhouHPostnatal development of hepatic innate immune responseInflammation201134657658420978830
- SurrigaOOrtegaAJadejaVBellafronteALasalaNZhouHAltered hepatic inflammatory response in the offspring following prenatal LPS exposureImmunol Lett20091231889519428555
- LasalaNZhouHEffects of maternal exposure to LPS on the inflammatory response in the offspringJ Neuroimmunol20071891–29510117706793
- AmbrosiniALouinGCrociNPlotkineMJafarian-TehraniMCharacterization of a rat model to study acute neuroinflammation on histopathological, biochemical and functional outcomesJ Neurosci Methods2005144218319115910976
- SheridanBCMcIntyreRCJrMeldrumDRFullertonDAL-arginine prevents lung neutrophil accumulation and preserves pulmonary endothelial function after endotoxinAm J Physiol19982743 Pt 1L337L3429530168
- ChiricoGCortinovisSFonteCGiudiciGBacterial sepsisJ Chemother200719Suppl 2283018073176
- BogaertDWeinbergerDThompsonCLipsitchMMalleyRImpaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse modelInfect Immun20097741613162219168741
- BelderbosMEvan BleekGMLevyOSkewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of lifeClin Immunol2009133222823719648060
- SadeghiKBergerALanggartnerMImmaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signalingJ Infect Dis2007195229630217191175
- ChelvarajanRLCollinsSMDoubinskaiaIEDefective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigensJ Leukoc Biol200475698299414982942
- LoisaPRinneTLaineSHurmeMKaukinenSAnti-inflammatory cytokine response and the development of multiple organ failure in severe sepsisActa Anaesthesio Scand2003473319325
- HemmilaMRKimJSunJMGene therapy with lipopolysaccharide binding protein for gram-negative pneumonia: respiratory physiologyJ Trauma2006613598605 discussion 616966994
- FanMHKleinRDSteinstraesserLAn essential role for lipopolysaccharide-binding protein in pulmonary innate immune responsesShock200218324825412353926
- van AubelRAKeestraAMKrooshoopDJvan EdenWvan PuttenJPLigand-induced differential cross-regulation of Toll-like receptors 2, 4 and 5 in intestinal epithelial cellsMol Immunol200744153702371417493681
- PengJHHuYYChengYEffect of JIANPI HUOXUE decoction on inflammatory cytokine secretion pathway in rat liver with lipopolysaccharide challengeWorld J Gastroenterol200814121851185718350622
- MatsumuraTItoATakiiTHayashiHOnozakiKEndotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytesJ Interferon Cytokine Res2000201091592111054280
- BozinovskiSJonesJBeavittSJCookADHamiltonJAAndersonGPInnate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4Am J Physiol Lung Cell Mol Physiol20042864L877L88514617520