Abstract
Introduction
The use of bacillus Calmette–Guérin (BCG) has long been considered a stimulus for immune reactivity in leprosy household contacts. Probably, the combination of multidrug therapy with BCG could facilitate the clearance of leprosy bacilli in the host, reduce relapse rates, and shorten the duration of skin-smear positivity.
Methods
To investigate the mechanism of action of BCG, a study involving 19 leprosy patients, eleven multibacillary (MB) and eight paucibacillary, was performed to assess the in vitro production of interleukin (IL)-10, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-6, and IL-17 in the supernatant of peripheral blood mononuclear cells, before and 30 days after inoculation with BCG intradermally (BCG-id). Peripheral blood mononuclear cells isolated by Ficoll–Hypaque gradient were cultivated with Concanavalin-A (Con-A), lipopolysccharides (LPS), or BCG. The supernatant was collected for ELISA quantification of cytokines. The immunohistochemistry of IFN-γ, IL-1, IL-10, IL-12, transforming growth factor (TGF)-β, and TNF-α was carried out in biopsies of skin lesions of leprosy patients before and 30 days after inoculation of BCG-id. These patients were followed up for 5 years to assess the therapeutic response to multidrug therapy, the occurrence of leprosy reactions, and the results of bacterial index and anti-PGL-1 serology after the end of treatment.
Results
The results showed increased production of cytokines after BCG-id administration in MB and paucibacillary leprosy patients. There was statistically higher levels of TNF-α (P = 0.017) in MB patients and of IL-17 (P = 0.008) and IFN-γ (P = 0.037) in paucibacillary patients. Immunohistochemical staining, especially for TNF-α, was more intense in biopsies of MB leprosy patients taken after BCG-id administration, probably for induction of innate human immunity. The clinical evaluation suggests that BCG-id is able to induce a more effective therapeutic response, with reduction of the number and the intensity of leprosy reactions.
Conclusion
These results suggest that BCG-id induces activation of the initial phase of immunocellular activity: innate human immunity (increase in TNF-α, IL-12 and macrophage activation). Therefore, we conclude that the use of BCG-id could be indicated as an adjuvant to multidrug therapy in treatment of leprosy patients.
Keywords:
Introduction
Leprosy is a chronic infectious disease caused by Mycobacterium leprae, which affects the skin and peripheral nerves. Leprosy still remains an important public health problem in Brazil, with a prevalence of 1.56/10,000 inhabitants and detection of new cases in 18.2/100,000 inhabitants in 2010.Citation1,Citation2 Although prevalence has decreased, the new cases of leprosy detected each year have remained relatively static, which impairs the elimination of leprosy as a public health problem in the country. In addition, 2% of the new cases present grade 2 physical disability, suggesting the considerable extent of the problem.Citation2 Thus, the current approaches to leprosy control should be revised, and tools to be used as adjuvants in the treatment and elimination of the infection should be explored.
Bacillus Calmette–Guérin (BCG) was the first vaccine to be considered against leprosy, following the report of FernandezCitation3 in 1939 that showed the BCG ability to cause positivity in the Mitsuda reaction (a skin test that uses killed bacilli to evaluate the delayed type hypersensitivity to M. leprae). Later, four large field trials were started in Uganda,Citation4 India,Citation5 Burma,Citation6 and Papua New GuineaCitation7 to evaluate the efficacy of BCG in leprosy prevention and showed large variation in the protective effect of BCG vaccine, ranging from 20% to 80%. Despite the controversial use of BCG in the world, there is sufficient and convincing evidence of the protective effect of BCG vaccine against leprosy, as shown by a meta-analysis and overall review of 29 studies of BCG vaccination in leprosy.Citation8
In leprosy, BCG was initially used for therapeutic purposes and later for prophylaxis. Thus, there are two kinds of vaccination: immunotherapy and immunoprophylaxis.
Even after multidrug therapy (MDT), multibacillary patients with a high bacterial index continue to load dead and viable, persistent bacilli, which leads to immunological complications, such as recurrent reactions and late relapses. To achieve faster killing of viable bacilli and clearance of dead bacilli, immunotherapeutic agents (vaccines) are being evaluated as an adjunct to MDT. The combination of MDT with vaccination could facilitate the bacteriological clearance in the host, improve the bacterial index, and shorten clinical manifestations through unclear immunomodulatory activity, which results in increased host immunity to M. leprae.Citation9
The objective of this study was to evaluate the role of BCG in combination with MDT in the immunotherapy of leprosy and to investigate the mechanisms of action of the vaccine.
Patients and methods
Patient selection
The study was approved by the Research Ethics Committee of HCFMRP-USP and FMRP-USP (protocol number 11,183/2003).
Nineteen untreated (new cases) leprosy patients were followed up by the Division of Dermatology and National Leprosy Reference Center, University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo, Brazil. They were classified according to the spectrum of the diseaseCitation10 and separated into multibacillary (MB) patients, all of whom had a positive bacterial index (BI); and paucibacillary (PB) patients, all of whom had a negative BI.
The inclusion criteria were: untreated leprosy patients (new cases); without leprosy reactions; taking no anti-inflammatory or immunosuppressive drugs; and age ranging from 12 to 69 years. All included patients agreed to participate and signed the approved consent form to provide informed consent.
All patients received the BCG vaccination before the beginning of MDT. The patients selected did not present any other mycobacterial infection or have a scar from previous BCG vaccination, since in Brazil it is accepted practice to administer one dose of BCG in the first month after birth to prevent severe forms of tuberculosis.
The patients were followed up for 5 years, and the post-treatment therapeutic response to MDT, the occurrence of leprosy reactions, and the results of BI and anti-PGL-1 serology were assessed.
Measurement of the BI
A reasonably quantitative sample of M. leprae was obtained from slit-skin smears for BI measurement. To determine BI, the sample was first stained by the Ziehl–Neelsen method; then, the count of acid-fast bacilli was determined on a logarithmic scale ranging from 0–6+ after examining 25–100 fields, according to a standard method for determining the number of bacilli/field. Citation11
Detection and quantification of anti-PGL-1 in the serum
Ninety-six-well polystyrene plates were coated with 2 μg/mL antigen (PGL-1) in sodium carbonate buffer (pH 9.6) and stored at 4°C overnight until use. Serum from each patient was diluted 1:100 in 15 mM Tris-Tween (20 mM Tris, 150 mM NaCl, and 0.1% Tween®) buffer containing 5% sheep serum and 10 μL was added to each well, and the plate was incubated for 1 hour at 37°C in a humid chamber. After 1 hour, the samples were washed with 15 mM Tris-Tween buffer, and antihuman IgM β-galactosidase conjugate, diluted 1:600 in 15 mM Tris-Tween buffer containing 5% sheep serum, was added. The plates were then incubated at 37°C for 1 hour. Then, 10 μL fluorogenic substrate (4-methylumbelliferyl β-D-galactopyranoside) was added to the samples, and the material was incubated at 37°C for 30 minutes. The plate was read with a multiscan ELISA reader. Sera with an absorbance at 450 nm greater than 0.028 (the mean absorbance plus three standard deviations in 35 healthy Brazilian control subjects) were considered to be positive. Each serum was tested in duplicate. The difference between these duplicates was about 3% to 5%. All assays were carried out at the same time.Citation12
BCG vaccination
Leprosy patients were vaccinated intradermally with 0.1 mL (0.1 mg) of BCG, in the right arm.Citation13
Culture of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll–Hypaque gradient from leprosy patients before and 30 days after vaccination with BCG intradermally (BCG-id). The cell suspension was diluted with culture medium (RPMI 1640) containing 10% fetal calf serum. The adherent cells (enriched monocytes) (2.5 × 106 cells/mL) were cultured in the presence of lipopolysaccharides (LPS) from Salmonella typhymurium (L7261; Sigma-Aldrich, St Louis, MO) (5 μg/mL), at 37°C and 5% CO2, in a humid incubator for 24 hours. Nonadherent cells (enriched lymphocytes) (1.5 × 106 cells/mL) in the presence of Concanavalin-A (Con-A C2631 111H7140; Sigma-Aldrich) (50 μg/mL) were cultured under the same conditions as described above for 96 hours. The supernatants of these cultures were collected and maintained at −20°C for cytokine measurement.Citation14
Detection and quantification of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-10, IL-6, and IL-17 in the supernatant of peripheral blood mononuclear cells
IFN-γ, TNF-α, IL-10, IL-6, and IL-17 were measured by ELISA (sandwich or capture) according to the instructions of the method. The antibodies used were from BD Biosciences Pharmingen (San Diego, CA): IFN-γ: Cat. 51-26131E clone NIB42/Cat. 51-26132E clone 4SB3; TNF-α: Cat. 51-26371E clone MAb1/Cat. 51-26372E clone MAb11; IL-10: Cat. 51-26171E clone JES3-19F1/Cat. 51-26172E clone JES3-12G8; IL-6: Cat. 554543 clone MQ2-13A5/Cat. 554546 clone MQ2-39C3; IL-17A: Cat# 39-8179–60. The plate was read with a multiscan ELISA reader using absorbance at 450 nm.Citation15
Histology and immunohistochemistry
The damaged-skin biopsies, obtained from patients before and 30 days after vaccination with BCG-id, were submitted to routine histopathological analysis, staining with hematoxylin-eosin, and to immunohistochemistry.
Four-micrometer paraffin sections were subjected to antigen retrieval using a pressure cooker, in sodium citrate (pH 6.0), for 4 minutes for IL-1 (1:200), IL-12 (1:300), transforming growth factor (TGF)-β (1:1500), and TNF-α (1:150) antibodies. Tris- EDTA was used for INFγ (1:200) and IL-10 (1:150). Endogenous peroxidase was blocked with 3% hydrogen peroxide (H2O2) in phosphate-buffered saline (PBS) and methanol for 10 minutes followed by nonspecific blocking with 2% PBS + bovine serum albumin (BSA) for 15 minutes. The sections were incubated with the primary antibody overnight at 4°C. The antibodies used were all from Santa Cruz Biotechnology Inc (Santa Cruz, CA). The sections were then incubated with Novocastra Post Primary Block (Leica Biosystems, Wetzlar, Germany) for 30 minutes followed by the Novocastra Novolink Polymer (Leica Biosystems) for 30 minutes. The color reaction was developed using DAB (3,3′ diaminobenzidine tetrahydrochoride (Sigma-Aldrich). The sections were counterstained with Mayer’s hematoxylin (Sigma-Aldrich) and mounted in Entellan ® (Merck Millipore, Billerica, MA). The intensity of the reaction observed on the slides was qualitatively analyzed.
Statistical analysis
Data were analyzed statistically by the paired t-test. A P value < 0.05 was considered to be statistically significant.
Results
Nineteen leprosy patients, most of them Caucasian (83%), with mean age 45.7 years (range: 21 to 69 years), 63% male, were classified into two groups: 1) PB (eight patients) who were classified as having tuberculoid leprosy (TT) (three patients) or borderline-tuberculoid leprosy (BT) (five patients); and 2) MB (eleven patients) who were classified as having lepromatous leprosy (LL) (six patients) or borderline-lepromatous leprosy (BL) (five patients), using clinical, bacilloscopic, and histopathological criteria from the Ridley and Jopling classification. All PB patients presented negative BI, whereas the eleven MB patients presented positive BI ranging from 1+ to 5+ ().
Table 1 Clinical and laboratory data of leprosy patients before, during, and 5 years after MDT
In the 5-year follow-up period, six (54%) MB patients developed leprosy reactions, 18% with reverse reaction and 36% with erythema nodosum, during the beginning of the MDT. Four (36%) patients developed neuritis without permanent disability. Two (25%) PB patients developed reverse reaction and one (12.5%) patient developed neuritis, also without permanent disability (). Except for one patient, all the others successfully completed the treatment with lower levels of anti-PGL-1 and BI (). One polar lepromatous patient developed several episodes of erythema nodosum after the end of MDT. This patient was prescribed an alternative scheme of treatment with clofazimine, ofloxacin, and minocycline during 24 months, with control of the reactions.
Analysis of the cytokine levels in the supernatants of PBMC cultures from MB patients revealed the results shown in .
Table 2 Quantification of IL-10, IFN-γ, TNF-α, and IL-6 (pg/mL) in PBMC supernatants from leprosy MB patients before and 30 days after BCG-id
In cultures of enriched lymphocytes developed in the presence of Con-A (50 μg/mL), IL-10 levels measured before and after BCG-id were similar: 1047.35 pg/mL and 1296.05 pg/mL, respectively (P = 0.339) (). IFN-γ levels tended to be reduced after BCG-id: 7896.50 pg/mL and 3385.30 pg/mL, respectively (P = 0.789) ().
Figure 1 Graphs representing the median levels of IL-10, IFN-γ, TNF-α and IL-6 (pg/mL) in PBMC supernatants from leprosy patients before and after BCG-id.
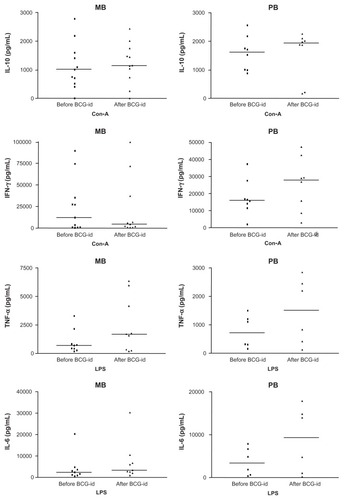
In cultures of enriched monocytes developed in the presence of LPS (5 μg/mL), TNF-α levels were higher after inoculation of BCG-id (1631.75 pg/mL) than before BCG-id (565.60 pg/mL) (P = 0.017) (). IL-6 levels increased after BCG-id (3043.70 pg/mL) when compared with levels before BCG-id (2669.10 pg/mL), although the difference was not statistically significant (P = 0.056) ().
Analysis of the cytokine levels in the supernatants of PBMC cultures from PB patients revealed the results shown in .
Table 3 Quantification of IL-10, IFN-γ, TNF-α, and IL-6 (pg/mL) in PBMC supernatants from leprosy PB patients before and 30 days after BCG-id
In cultures of enriched lymphocytes developed in the presence of Con-A (50 μg/mL), IL-10 levels before (1617.05 pg/mL) and after BCG-id (1933.90 pg/mL) were similar (P = 0.513) (). IFN-γ levels before BCG-id (15,986.35 pg/mL) were lower than after BCG-id (27,967.25 pg/mL) (P = 0.037) ().
In cultures of enriched monocytes developed in the presence of LPS (5 μg/mL), TNF-α levels increased after BCG-id (1516.75 pg/mL) compared to levels before BCG-id (711.20 pg/mL), but the difference was not significant (P = 0.076) (). IL-6 levels, similar to TNF-α levels, were higher after than before BCG-id (9357.85 pg/mL and 3330.30 pg/mL, respectively), but the difference was not significant (P = 0.051) ().
In cultures of enriched lymphocytes, where there was no stimulus of Con-A or LPS, developed in the presence of BCG (20 μg/mL) as a specific stimulus, IL-17 levels measured before BCG-id were lower than after BCG-id (MB: 4.6 and 21.6 pg/mL, respectively; PB: 11.6 and 38.7 pg/mL, respectively), but the difference was significant only in PB patients (P = 0.008) ().
Table 4 Quantification of IL-17 (pg/mL) in PBMC supernatants from leprosy patients before and 30 days after BCG-id
Histopathological analysis of skin biopsies of PB patients, before and after BCG-id, showed increased numbers of epithelioid cells that were present together with Langhans giant cells and lymphocytes in the granuloma. After BCG-id, the granuloma formation became better structured, with epithelioid cells in the center and lymphocytes in the periphery. In MB patients, we also observed increase in number of epithelioid cells after BCG-id and the tendency towards granuloma formation ().
Figure 2 Photomicrograph of a skin biopsy. (A) Infiltrate of histiocytes surrounded by lymphocytes in the dermis in a case of MB leprosy before the BCG-id (HE, ×100). (B) Tendency towards granuloma formation in a case of MB leprosy after the BCG-id (HE, ×100). (C) Immunoreactivity for INFγ in a case of MB leprosy before the BCG-id (NovoLink staining with hematoxylin counterstaining, ×50). (D) Immunoreactivity for INFγ in a case of MB leprosy after the BCG-id (NovoLink staining with hematoxylin counterstaining, ×50). (E) Immunoreactivity for IL-1 in a case of MB leprosy before the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (F) Immunoreactivity for IL-1 in a case of MB leprosy after the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (G) Immunoreactivity for IL-10 in a case of MB leprosy before the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (H) Immunoreactivity for IL-10 in a case of MB leprosy after the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100).
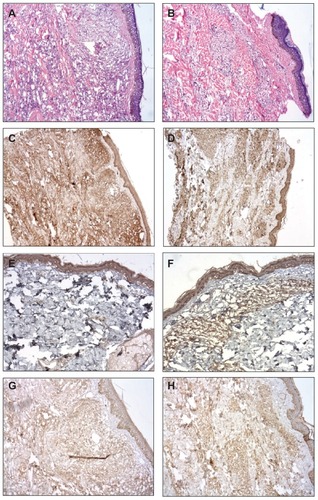
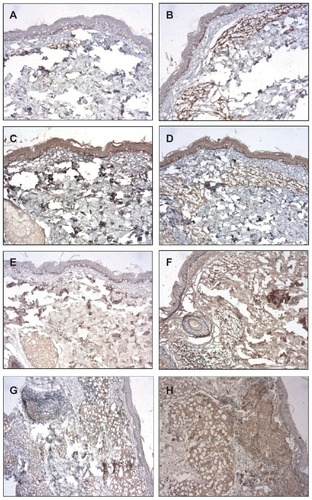
The immunohistochemical (IHC) staining for IL-1, IL-10, IL-12, and TGF-β were higher after BCG-id in the MB group; the IHC staining for TNF-α was increased after BCG-id in both MB and PB groups; the IHC staining for IFN-γ presented no increase after BCG-id ( and ).
Figure 3 Photomicrograph of a skin biopsy. (A) Immunoreactivity for IL-12 in a case of MB leprosy before the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (B) Immunoreactivity for IL-12 in a case of MB leprosy after the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (C) Immunoreactivity for TGF-β in a case of MB leprosy before the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (D) Immunoreactivity for TGF-β in a case of MB leprosy after the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (E) Immunoreactivity for TNF-α in a case of MB leprosy before the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (F) Immunoreactivity for TNF-α in a case of MB leprosy after the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (G) Immunoreactivity for TNF-α in a case of PB leprosy before the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100). (H) Immunoreactivity for TNF-α in a case of PB leprosy after the BCG-id (NovoLink staining with hematoxylin counterstaining, ×100).
Discussion
The intensive use of BCG in some countries has led us to conclude that this vaccination must have had an important role in the decline of leprosy incidence. Several studies obtained reasonable results with use of BCG in leprosy therapeutics, however the side effects, such as leprosy reactions and neuritis, has led to disagreement.Citation16
In this study, we observed a favorable evolution during the treatment in the majority of the patients. Thirty-six percent of MB patients developed erythema nodosum, which is lower than the expected rates described in the literature (50%–60%).Citation17 Considering the seriousness and the morbidity of type 2 leprosy reactions, BCG-id could have an application in patients, in agreement with other reports.Citation18 Thirty-six percent of MB patients developed neuritis, which was easily controlled, without permanent disability, in agreement with the literature.Citation19 Two (25%) PB patients presented type 1 reaction and one (12.5%) patient presented neuritis. All PB patients have completed treatment without skin or nerve lesions and with negative BI and anti-PGL-1 levels.
The species-specific antigen for M. leprae (PGL-1) could be a serological marker for the bacterial load of leprosy patients and so could be used for the classification of patients and for treatment monitoring. Leprosy patients at the lepromatous end of the spectrum produce large quantities of IgM immunoglobulin in response to this antigen (seropositivity of 80%–100%), while patients at the tuberculosis end show much lower levels (seropositivity of 30%–60%).Citation20
In this study there was no control group (MDT without BCG-id) and the conclusions are based on comparison with the literature.
The clinical forms of leprosy constitute a spectrum that correlates closely with the degree of cell-mediated immunity. Patients with the PB forms develop strong cell-mediated response and have only a few, localized lesions, whereas patients with the MB forms are specifically unresponsive to M. leprae and have a large number of disseminated lesions. So, MB patients have not a favorable long-term prognosis. It is known that the MDT is potent in killing M. leprae and in cure of the disease. However, the inflammatory process may persist for a long time after the end of the treatment, causing the leprosy reactions, which are important causes of morbidity in leprosy patients. In this study, lower rates of erythema nodosum were observed in the patients after the vaccination, minimizing the symptoms of the patients.
Therefore, the clinical evaluation suggests that BCG-id is able to induce a more effective therapeutic response, with reduction of the number and the intensity of leprosy reactions.
BCG protection is described as an improvement of the immunological Th1 response, due especially to its improvement of IFN-γ production, which is necessary for the control of mycobacterial infections.Citation21 IFN-γ is associated with macrophage cell activation, increase of cell mediated immunity, and M. leprae destruction and achieves this by increasing the production of intermediate reactive oxygen and nitrogen inside the macrophages.Citation22
In this study, we observed that the production of IFNγ by PBMC of MB patients, in the presence of Con-A, tended to be reduced after BCG-id. These apparently contradictory data could be related to the modulation of the immune response induced by BCG-id, leading to local IFN-γ action that could have increased the pathogen recognition, resulting in macrophage stimulation in peripheral lesions. Probably, Th1-lymphocytes concentrated in peripheral lesions and were reduced in peripheral blood.Citation22–Citation24 Also, the interaction of the Con-A with the monocyte favors its differentiation to the M2 phenotype, with higher production of IL-10, which downregulated the IFN-γ production in this culture.Citation25,Citation26 In skin biopsies, the IHC staining for IFN-γ wasn’t augmented after BCG-id, probably because this cytokine had been consumed by the previously established lesions.
Comparing the values of IFN-γ before and after BCG-id in PB patients, we observed that the production of IFN-γ by PBMC increased after the vaccination (P = 0.037). These data agree with those reported by Marchant et al,Citation21 who showed the development of a Th1-cell response in vaccinated children with an increase in the production of IFN-γ, and support the described protection by BCG-id.
In this study, we observed increased production of IL-17 in the supernatants of PBMC cultures from MB and PB patients after BCG-id, but the difference was significant only in PB patients (P = 0.008). Gopal et alCitation27 demonstrated that BCG was capable of inducing IL-23 and Th-17-cell differentiation. Umemura et alCitation28 showed that IL-17 was an important cytokine in the induction of optimal Th1 response and protective immunity against mycobacterial infection. These data suggest that BCG induces Th1-cell responses, which is required for immunity against intracellular bacteria.
The IHC staining for IL-12 was higher in the MB group after BCG-id. IL-12 and IL-23 are pivotal cytokines in the generation of protective Th1/Th17-type immune responses upon infection with intracellular pathogen, which support the described protection by BCG-id.
Both IL-12/IFN-γ and IL-23/IL-17 pathways should be activated in response to BCG-id inoculation. The resident macrophages initiate the immune response by secreting IL-12 and IL-23. The macrophages activate the natural killer cells. IL-12 mediates natural killer cell activation and IFN-γ production, which further stimulates neutrophil IFN-γ production and infiltration of polymorphonuclear leukocytes and other immune cells (CD4+). IL-23 is also involved in natural killer cell activation. IL-23 with TGF-β and IL-6 is known to induce Th17 cell differentiation and proinflammatory cytokine production (IL-6, IL-1, TNF-α).Citation29
Additionally, increased production of TNF-α by PBMC from MB patients was observed in the presence of LPS after the application of BCG-id (P = 0.017), suggesting a nonspecific immune stimulatory action of BCG on the innate immunity response.Citation30 This is a very interesting response in multibacillary leprosy, where TNF-α acts by inducing macrophage activation, thus favoring destruction of the parasite and the maintenance of IFN-γ production.Citation31 The results for PB patients under the same culture conditions were similar, with no statistical significance, probably due to the smaller number of patients. It is suggested that the use of BCG-id in leprosy, especially in MB patients, could improve the immunological response, and this is reinforced by the IHC results that showed higher staining levels for TNF-α in both MB and PB groups after BCG-id.
The production of IL-10 by PBMC of MB patients in the presence of Con-A was similar before and after BCG-id, as also observed in PB patients, with no statistical significance. The explanation of these results could be related to the fact that purified T cells were not used in this study and so the monocytes may also have produced IL-10, resulting in elevated levels of this cytokine.Citation32 Also, the interaction of the Con-A with the monocyte favors its differentiation to the M2 phenotype, with higher production of IL-10.Citation25,Citation26 BCG-id could not stimulate the production of IL-10 in the cultures. The induction of IL-10 production should be avoided by vaccines since the production of this cytokine is induced by the M. leprae and is involved in the inhibition of T cell production and in the liberation of antibacterial cytokines, downregulating the Th1 response.Citation23
The IHC staining for IL-10 was higher in the MB group after BCG-id. Gopal et alCitation27 showed that BCG promotes the production of IL-10, which limits Th1-cell responses, while simultaneously inducing IL-23 and Th-17-cell differentiation. The ability of IL-17 to downregulate IL-10 and induce IL-12 production allows the generation of subsequent Th1-cell responses.
The production of IL-6 in PBMC of leprosy patients in the presence of LPS after the application of BCG-id was higher than that before vaccination in both MB and PB patients, but the difference was not statistically significant. Taking into account the function described for IL-6 of increasing the specific destructive capacity of phagocyte cells (NK cells and cytotoxic T cells),Citation33 it is possible that increased IL-6 levels may induce greater destruction of the parasite and control of the infection, as observed by Rada et al.Citation34–Citation36
The clinical forms of leprosy constitute a spectrum that correlates closely with the degree of cell-mediated immunity. Patients with the PB forms develop strong cell-mediated response whereas patients with the MB forms are specifically unresponsive to M. leprae. The effect of BCG in this study was analyzed in the spectrum of leprosy, in the PB group and MB group. The cellular response presents large variability from individual to individual, ranging from a marginal to a strong response, even between individuals within the PB and MB groups. The results were not analyzed individually, but in the group. The results are indicative and not conclusive, because certainly, this would necessitate a larger group of patients.
Indeed, taken together, these results indicate that it is possible to suggest that BCG-id improves the immunological response of leprosy patients by inducing the activation of the initial phase of immunocellular activity, innate human immunity (increase in TNF-α, IL-12 and macrophage activation), and probably also increases the efficacy of the multidrug therapy (especially in multibacillary leprosy), possibly favoring the reduction of reaction episodes and relapse of the disease. The results suggest a potential for BCG as an adjuvant in the treatment of multibacillary leprosy patients.
Acknowledgment
This research was supported by FAEPA, CNPq, and Fundação Paulista Contra Hanseníase.
Disclosure
The authors report no conflicts of interest in this work.
References
- World Health OrganizationLeprosy update, 2011Wkly Epidemiol Rec2011863638940021887885
- Ministério da SaúdeCoordenação Geral do Programa Nacional de Controle da Hanseníase – CGPNCHHansen’s Disease Control National ProgramBrasíliaMinistério da Saúde2011 Available from: http://www.google.ca/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=1&ved=0CCMQFjAA&url=http%3A%2F%2Fwww.ilep.org.uk%2Ffileadmin%2Fuploads%2FCountry_Pages%2FBrazil%2FReport_NHDCP_2011_web.pdf&ei=AEl6UKO0E6azyQG8iYDQAg&usg=AFQjCNEbaMFiccRpuCOrBiBcuhHs8D1iMg&sig2=de23c3KFH09VSRJ6R2a7pg&cad=rjaAccessed October 12, 2012 Portuguese
- FernandezJMThe early reaction induced by leprominInt J Lepr Other Mycobact Dis19408114
- StanleySJHowlandCStoneMMSutherlandIBCG vaccination of children against leprosy in Uganda: final resultsJ Hyg (Lond)19818722332487288177
- GupteMDField trials of antileprosy vaccinesIndian J Lepr199870436336710189586
- LwinKSudaresanTGyiMMBCG vaccination of children against leprosy: fourteen-year findings of the trial in BurmaBull World Health Organ1985636106910782940028
- BagshaweAScottGCRusselDAWingleySCMerianosABerryGBCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea, 1963–1979Bull World Health Organ19896743893992680140
- ZodpeySPProtective effect of bacillus Calmette Guerin (BCG) vaccine in the prevention of leprosy: a meta-analysisIndian J Dermatol Venereol Leprol2007732869317456912
- NarangTKaurIKumarBRadotraBDDograSComparative evaluation of immunotherapeutic efficacy of BCG and mw vaccines in patients of borderline lepromatous and lepromatous leprosyInt J Lepr Other Mycobact Dis200573210511416830653
- RidleyDSJoplingWHClassification of leprosy according to immunity. A five-group systemInt J Lepr Other Mycobact Dis19663432552735950347
- RidleyDSTherapeutic trials in leprosy using serial biopsiesLepr Rev1958291455213515393
- ZenhaEMFerreiraMAFossNTUse of anti-PGL-1 antibodies to monitor therapy regimes in leprosy patientsBraz J Med Biol Res2009421096897219784481
- Ministério da Saúde, Secretaria de Políticas de Saúde, Departamento de Atenção BásicaControle da Hanseníase Na Atenção Básica: Guia Prático Para Profissionais da Equipe de Saúde Da FamíliaLeprosy Control in Primary Care: A Practical Guide for Professionals in Family Health TeamBrasíliaMinistério da Saúde2001 Portuguese
- FossMCFossNTPaccolaGMSilvaCLSerum levels of tumor necrosis factor in insulin-dependent diabetic patientsBraz J Med Biol Res19922532392421341919
- BonatoVLDCorrelação dos Anticorpos Anti-PGL-I com o Índice Baciloscópico, a Reação De Mitsuda, o Tratamento Poliquimioterápico e as Interleucinas nas Diferentes Formas da HanseníaseCorrelation of Antibodies Ant-PGL-1 with Bacterial Index, the Mitsuda Reaction from Chemotherapy Treatment and Interleukins in Different Forms of Leprosy [Master’s thesis]São PauloUniversidade de São Paulo1995 Portuguese
- ChenZLTangQGWangZMChenJPilot study to determine acceptability and ability of heat-killed Mycobacterium leprae plus BCG (HKML + BCG) vaccine to induce skin test conversionLepr Rev19936421171278341114
- Beck-BleuminkMBerheDOccurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in EthiopiaInt J Lepr Other Mycobact Dis19926021731841522359
- ZaheerASMisraRSSharmaAKImmunotherapy with Mycobacteruim w vaccine decreases the incidence and severity of type 2 (ENL) reactionsLepr Rev19936417148464320
- SharmaPKarHKMisraRSReactional states and neuritis in multibacillary leprosy patients following MDT with/without immunotherapy with Mycobacterium w antileprosy vaccineLepr Rev200071219320510920614
- MouraRSCaladoKLOliveiraMLBührer-SékulaSLeprosy serology using PGL-1: a systematic reviewRev Soc Bras Med Trop200841Suppl 2S11S18
- MarchantAGoetghebuerTOtaMONewborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guérin vaccinationJ Immunol199916342249225510438968
- MurrayHWRubinBYRothermelCDKilling of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokineJ Clin Invest1983724150615106415111
- SielingPAModlinRLCytokine patterns at the site of mycobacterial infectionImmunobiology19941914–53783877713551
- MillsKHMcGuirkPAntigen-specific regulatory T cells – their induction and role in infectionSemin Immunol200416210711715036234
- ApteRSRegulation of angiogenesis by macrophagesAdv Exp Med Biol2010664151920237997
- Mahdavian DelavaryBvan der VeerWMvan EgmondMNiessenFBBeelenRHMacrophages in skin injury and repairImmunobiology2011216775376221281986
- GopalRLinYObermajerNIL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccinationEur J Immunol201242236437322101830
- UmemuraMYahagiAHamadaSIL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guérin infectionJ Immunol200717863786379617339477
- LiLHuangLVergisALIL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injuryJ Clin Invest2010120133134220038794
- Conti-FreitasLCFoss-FreitasMCMamedeRCFossNTEffect of BCG stimulus on proinflammatory cytokine production in laryngeal cancerCancer Immunol Immunother2009581252918421458
- SilvaCLFossNTTumor necrosis factor in leprosy patientsJ Infect Dis198915947877902926170
- CastilhoMLORCorrelação Entre BCG Intradérmico e Linfoproliferação, Produção e Expressão de Rnam das Citocinas Ifnγ, IL-12, IL-10 E IL-4, Níveis de Anti-PGL-1 em Pacientes com Hanseníase e em Seus ComunicantesCorrelation Between BCG and Lymphoproliferation, Production and mRNA Expression of Cytokines IFNg, IL-12, IL-10 and IL-4, Levels of Anti-PGL-1 in Leprosy Patients and their Contacts [Master’s thesis]São PauloUniversidade de São Paulo2001 Portuguese
- SenguptaUCell-mediated immunity in leprosy: an updateInt J Lepr Other Mycobact Dis19936134394548228444
- RadaESantaellaCAranzazuNConvitJPreliminary study of cellular immunity to Mycobacterium leprae protein in contacts and leprosy patientsInt J Lepr Other Mycobact Dis19926021891941522361
- RadaEUlrichMAranzazuNA longitudinal study of immunologic reactivity in leprosy patients treated with immunotherapyInt J Lepr Other Mycobact Dis19946245525587868953
- RadaEUlrichMAranzazuNA follow-up study of multibacillary Hansen’s disease patients treated with multidrug therapy (MDT) or MDT + immunotherapy (IMT)Int J Lepr Other Mycobact Dis19976533203279401484