Abstract
Background and methods
The role of immunoglobulin (Ig) E in immunity against influenza A H1N1 has not been studied. Total serum IgE and specific IgE and IgG anti-H1N1 virus responses were studied in children and adults (n = 2) who received influenza virus vaccination (Flumist® or Fluzone®) in autumn 2008 and 2009, and then subsequently became infected with the H1N1 virus in spring 2009. Twelve months after infection, antibodies in their serum were compared with those in the serum of subjects who were either vaccinated but not infected (n = 4) or nonvaccinated and noninfected subjects (n = 2), using UniCAP total IgE fluoroenzyme immunoassay, sodium dodecyl sulfate polyacrylamide gel electrophoresis, and Western blotting. Band sizes for the influenza virus (58, 56, 40, 30, 25, and 17 kDa) and H1N1 viral proteins (58, 56, 25, and 17 kDa) were determined, using sodium dodecyl sulfate polyacrylamide gel electrophoresis and Coomassie brilliant blue.
Results
We found that the serum of vaccinated and subsequently infected children and adults contained IgE and IgG antibodies to both H1N1 and influenza virus, with a strong IgE and IgG band intensity at 56 kDa. Interestingly, in subjects who were vaccinated but not infected, band intensity at 56 kDa was lowered by approximately two-fold. Serum of nonvaccinated and noninfected subjects had no detectable IgE or IgG antibodies to influenza virus or H1N1.
Conclusion
This is the first description of IgE anti-influenza A H1N1 antibodies in human serum and the first demonstration of their long-term persistence. The decreased intensity of the 56 kDa band in vaccinated noninfected subjects compared with vaccinated infected subjects suggests augmented IgE and IgG antibody responses to influenza A H1N1.
Introduction
Previous studies in our laboratory have established that immunoglobulin (Ig) E plays a role in the immune response to various viruses, including human immunodeficiency virus-1 (HIV-1) seropositive nonprogressing pediatric patients with decreased numbers of peripheral blood CD4+ T cells,Citation1,Citation2 parvovirus B19 in children,Citation3 and varicella zoster virusCitation4,Citation5 in both children and adults with a past history of chicken pox infection or varicella zoster virus vaccination.Citation6 Recent studies in the laboratory have described the presence and long-term persistence of IgE anti-influenza virus antibodies in the serum of IgE positive and negative vaccinated pediatric and adult subjects, approaching 2 years since vaccination.Citation7 The presence of IgE anti-influenza virus antibodies several months following vaccination may have biological significance. However, the exact role of IgE in influenza virus infection remains to be elucidated.
Outbreaks of annual influenza A virus are normally reported in the winter months, and cause fever, cough, and fatigue.Citation8 However, the Centers for Disease Control and Prevention identified two cases of human infection with a swine-origin influenza A H1N1 virus on April 15 and April 17, 2009, which was characterized by a combination of gene segments not previously identified among human or swine influenza A viruses.Citation9 By May 2009, the new H1N1 virus infected humans in Mexico, Canada, and elsewhere in the US,Citation9 and spread to other parts of the world, resulting in the World Health Organization declaring the infection a global pandemic.Citation10
The aim of this study was to assess for the presence of IgE anti-influenza A H1N1 antibodies in human serum. We found lower intensity of the 56 kDa band only in serum from vaccinated subjects compared with vaccinated subjects who were subsequently infected with H1N1, suggesting augmented IgE and IgG antibody responses to influenza A H1N1.
Materials and methods
Characterization of patients
Peripheral blood (3 mL total) was obtained from both pediatric (male and female, aged 1–18 years) and adult (male and female, aged 40–59 years) Caucasian or Hispanic subjects from an outpatient pediatric practice in Brooklyn, NY, and from adults who worked in the same practice, as previously described.Citation7 Briefly, one child and one adult subject (n = 2) received influenza virus vaccination (Flumist® or Fluzone®) in the autumn of 2008, and then subsequently became infected with H1N1 virus in the spring of 2009 (H1N1 influenza pandemic 2009). At 12 months after immunization, IgG and IgE anti-H1N1 antibodies in their serum were compared with those in subjects who were either vaccinated and infected (n = 4) or nonvaccinated and noninfected (n = 2). Subjects were both atopic and nonatopic, with normal (<100 IU/mL) or elevated (>100 IU/mL) serum IgE levels. The study was approved by the institutional review board of the SUNY Downstate Medical Center, Brooklyn, NY.
Vaccine
Adults received the influenza virus vaccine, Fluzone (inactivated influenza virus vaccine, 2008–2009 formula, Sanofi Pasteur Inc, Swiftwater, PA) and children were vaccinated with Flumist (live attenuated influenza virus vaccine, intranasal, 2008–2009 formula, MedImmune, Gaithersburg, MD). Each 0.25 mL dose of Fluzone vaccine contains 7.5 μg of influenza virus hemagglutinin and each 0.5 mL dose contains 15 μg hemagglutinin from each of the following three viruses: A/Brisbane/59/2007, IVR-148 (H1N1), A/Uruguay/716/2007, NYMC X-175C (H3 N2, an A/Brisbane/10/2007-like virus), and B/Brisbane/60/2008. Each 0.2 mL dose of Flumist intranasal spray contains 10 fluorescent focus units of live attenuated influenza virus reassortants of each of the three strains for the 2008–2009 season: A/California/7/2009, A/Perth/16/2009, and B/Brisbane/60/2008. Time since vaccination for subjects was up to 24 months. Past history of vaccination was confirmed by positive immunoblot for IgG anti-influenza virus.
The influenza A (H1N1) 2009 monovalent vaccine (Sanofi Pasteur Inc) was used for our sodium dodecyl sulfate polyacrylamide gel electrophoresis study, and is defined as follows: H1N1 2009 monovalent vaccine formulated to contain 15 μg hemagglutinin of influenza A/California/07/2009 (H1N1 v-like) virus per 0.5 mL dose. Gelatin 0.05% is added as a stabilizer. Each 0.5 mL dose may contain residual amounts of formaldehyde (not more than 100 μg), polyethylene glycol p-isoctylphenyl ether (not more than 0.02%), and sucrose (not more than 2.0%).
Influenza A H1N1 infection
The diagnosis of influenza in the context of the influenza A H1N1 pandemic (May 2009) was confirmed by an infectious disease specialist based on clinical symptoms (history of flu-like symptoms, fever and/or cough, malaise) according to the case definition provided by public health authorities.Citation10,Citation11 Diagnostic modalities for influenza virus A testing varied (direct fluorescence antibody testing or viral culture). Patients did not require hospital inpatient care and were defined as mild to moderate H1N1 infection, according to accepted Centers for Disease Control and Prevention guidelines.Citation11 It should be noted that, as of October 3, 2009, data from the Centers for Disease Control and Prevention reported that 99% of circulating influenza viruses in the US were H1N1.Citation11
Total serum IgE determination
Blood was collected and IgE levels were detected in serum (Quest Diagnostics Inc, Teterboro, NJ) according to the manufacturer’s recommendation. The reference range for IgE in healthy adult or child serum is 20–100 IU/mL.
Detection of IgG or IgE anti-influenza A H1N1
Serum from subjects vaccinated with influenza virus vaccine and subsequently infected with wild-type H1N1 infection had a positive cross-reaction with nitrocellulose blots coated with H1N1 influenza vaccine, because part of the vaccine contains the regular influenza virus vaccine. In order to differentiate between the two specific viral protein fractions, the influenza vaccine (0.9 μg protein) and H1N1 vaccine (0.3 μg protein, 20 μL/well) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (), as previously described by Laemmli.Citation12 The broad range molecular weight marker (Bio-Rad Laboratories, Hercules, CA) used was 210, 125, 101, 56.2, 35.8, 29, 21, and 6.9 kDa (). The gel was run at 70 V through stacking and then 150 V through resolving. The gel was stained overnight with Coomassie brilliant blue G (Sigma, St Louis, MO), and destained with a methanol-acetic acid-water mixture ().
Figure 1 Polyacrylamide gel electrophoresis of influenza A and H1N1 proteins. Lane 1, influenza A virus vaccine; lane 2, H1N1 virus vaccine; lane 3, broad weight molecular weight marker.
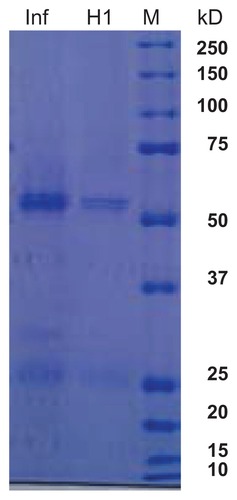
In order to detect H1N1 in our serum samples, the monovalent influenza A H1N1 vaccine was added (sodium dodecyl sulfate polyacrylamide gel electrophoresis, 10%) and the gel was then transferred to 0.2 μm nitrocellulose membrane at 4°C for 4 hours. Membranes were then blocked overnight with 5% milk in the cold. Serum samples were added and either IgG fraction goat anti-human IgG (heavy chain-specific and light chain-specific, ICN/Cappell, West Chester, PA) diluted 1:100 in TBS-Tween 20 and 1% milk in TBS-Tween 20 (1 mL) or IgE fraction goat anti-human IgE (epsilon chain, MP Biomedicals, Solon, OH), diluted 1:40 was added to the membranes and incubated for one hour on a shaker at room temperature. The membranes were then washed three times with TBS-Tween 20.
For detection and development of both IgG and IgE isotypes, nitrocellulose membranes were then incubated with rabbit anti-goat peroxidase-labeled antibody (whole molecule, Cappell), diluted 1:2000 in TBS-Tween 20 and 1% milk for one hour on a shaker, and washed three times with TBS-Tween 20. Bands were visualized using chemiluminescence (2 mL, ECL Detection Reagents RPN3004, GE Healthcare Biosciences, Pittsburgh, PA). Membranes were read, dried, and scanned (Gel Doc 2000 System with specific The Discovery Series: Quantity One software (Bio-Rad)).
Results
Subject characteristics
Total serum IgE levels and IgE anti-influenza A virus and H1N1 virus antibodies were studied in children (male/female, aged 1–18 years) and adults (male/female, aged 41–49 years) approaching 12 months since vaccination, as well as in noninfected and nonvaccinated children (controls, n = 2, males aged one year, ). Total serum IgE levels were either normal or high in adults and children vaccinated for the influenza virus. Children with no history of either influenza virus infection or vaccination had low serum IgE levels (). Total serum IgE levels were low in subjects infected with the H1N1 virus ().
Table 1 Subject characteristics
IgG anti-influenza A H1N1 antibodies
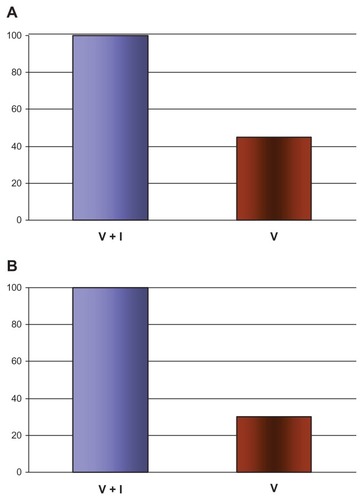
Serum obtained from vaccinated and subsequently infected children and adults contained IgG anti-H1N1 antibodies to both H1N1 () and influenza virus (data not shown), with a prominent band at about 56 kDa. In subjects who were vaccinated against influenza virus, but not subsequently infected with H1N1, the band intensity at 56 kDa was lowered by about 50% (). In contrast, serum from nonvaccinated and noninfected (control) subjects did not contain IgG antibodies to either H1N1 or influenza virus (data not shown).
Figure 2 Immunoblot analysis of IgG and IgE anti-H1N1 virus antibodies.
Abbreviation: Ig, immunoglobulin.

Figure 3 Comparison of IgG and IgE anti-H1N1 virus antibodies after vaccination with subsequent infection.
Abbreviation: Ig, immunoglobulin.
IgE anti-influenza A H1N1 antibodies
Serum obtained from vaccinated and subsequently infected children and adults contained IgE antibodies to both H1N1 () and influenza virus (data not shown), with strong IgE anti-H1N1 band intensity also at about 56 kDa. In subjects who were vaccinated against influenza virus, but not subsequently infected with H1N1, the band intensity at 56 kDa was lowered to about 35% (). In contrast, sera from nonvaccinated and noninfected (control) subjects did not contain IgE antibodies to either H1N1 (data not shown).
Discussion
The present study is the first to describe the existence and persistence of IgE anti H1N1 influenza virus antibodies in human sera obtained during and after the pandemic influenza H1N1 phase. Here we report the presence of IgE anti-H1N1 antibodies (as well as IgG anti-H1N1) antibodies in the serum of patients infected with H1N1 during the 2009 pandemic and in serum from the same patients up to 12 months following infection (Western blotting). Because our infected H1N1 patients were also previously vaccinated with influenza A virus vaccine, the H1N1 enzyme-linked immunosorbent assay which we prepared displayed pre-existing cross-reactive influenza A virus-specific responses with the H1N1 virus antigen, thus necessitating us to separate (and compare) the viral protein components from H1N1 influenza virus and seasonal influenza A virus on polyacrylamide gel. Earlier studies reported by other investigatorsCitation13 have used polyacrylamide gel electrophoresis to compare the structural proteins of the A/Havana/1292/78 national strain with the proteins of three international strains in relation to protein structure (surface antigens).Citation13 The authors reported that the most abundant protein in the four strains was M protein, while other differences between the Cuban strain and the three international strains were not observed.Citation13 In our study, we found that the serum of vaccinated and infected children and adults contained IgE and IgG antibodies to both H1N1 and influenza virus, with strong IgE and IgG band intensity at 56 kDa. Interestingly, in subjects who were vaccinated but not infected, band intensity at 56 kDa was lowered by about 35%–50%. These results suggest augmented IgE and IgG antibody responses to influenza A H1N1 with respect to the vaccinated and infection states. Although there may exist antigenic mimicry to certain components of the virus which are shared in the immune response to vaccination as well as infection, the demonstration of increased virus-specific responses following vaccination in the presence of infection compared with vaccination alone, in the presence of our fractionated protein assay, suggests unique responses to each of these viral strains in their own right. Because the laboratory diagnosis of clinical H1N1 infection differs between laboratories, the uniqueness of epitopes among influenza strains and preparations remains unclear. Future studies utilizing manufacturer’s preparations are warranted to elucidate precisely which antigens are more versus less immunogenic with respect to isotype as well as idiotype.
However, of notable, interest, is the fact that both H1N1-infected patients had low serum IgE levels. Taken together, these results suggest that the total serum IgE level is not as important as the specific fraction of IgE anti-H1N1 influenza antibodies representing a percentage of the total IgE responsible for mediating IgE anti-viral immune responses, and levels of serum IgE do not necessarily correlate with virus-specific IgE. The active role of IgE in viral disease is unknown but in earlier preliminary observationsCitation14 and other studiesCitation1–Citation5 we have suggested that the IgE molecule has evolved to have other beneficial functions, including those of an antiviral nature.
Other studies in our laboratory have investigated the role of IgE in other disease states, ie, anticancer antibodies, which were found in patients with normal and high serum IgE levels and had the ability to mediate antibody-dependent cell-mediated cytotoxicity against cancer cells in vitro.Citation15 Similarly, in the present study, these specific immune responses did not correlate with total serum IgE levels.Citation15
Some limitations in the design of this study should be borne in mind, including its small sample size and ethnic homogeneity, and any generalizations should be made cautiously. However, the strengths of this research include demonstration of the selectivity and specificity of antiviral IgE responses to viruses which maintain some degree of shared antigenicity (influenza versus H1N1) and further supports the uniqueness of antiviral IgE responses. To this end, the distinct and independent regulation of IgE in viral pathogenesis (ie, HIV and varicella zoster virus) as distinct from other immunoglobulin isotypes (IgM, IgG, and IgA) has also been reported.Citation5,Citation16 Studies reported by Ferrazzi et alCitation16 have shown that although adult HIV-1 infected individuals often exhibit hypergammaglobulinemia, the relative increase in serum IgE levels is greater than that of other serum immunoglobulins, with elevation especially apparent in end-stage disease (ie, acquired immune deficiency syndrome) in patients with decreased numbers of blood CD4+ T cells (<200/mm3).Citation16 However, it is well established that T cell responses play a role during the early stage of viral infection.Citation17 Thus, future experiments are warranted to quantify the H1N1 influenza A virus-specific T cell response(s) in combination with humoral responses.
To our knowledge, this study is the first to describe the presence and persistence of IgE anti-H1N1 influenza virus antibodies in serum from human subjects previously infected with H1N1 influenza virus during the 2009 pandemic. Although additional studies are required for further elucidation of the possible molecular mechanisms involved, our results raise the possibility that IgE could be used as a novel biomarker for human viral disease and that IgE may have a possible functional role in virus memory responses and the pathogenesis of viral disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- SecordEAKleinerGIAuciDLIgE against HIV proteins in clinically healthy childen with HIV diseaseJ Allergy Clin Immunol1996989799848939162
- PellegrinoMGBluthMHSmith-NorowitzTAHIV-1 specific IgE in serum of long term surviving children inhibits HIV-1 production in vitroAIDS Res Hum Retroviruses20021836337211897038
- BluthMHNorowitzKBChiceSDetection of IgE anti-parvovirus B19 and increased CD23+ B cells in parvovirus B19 infection: relation to Th2 cytokinesClin Immunol200310815215812921761
- Lev-TovHJosekuttyJKohlhoffSIgE anti-varicella virus (VZV) and other immune responses before, during, and after shinglesJ Allergy Clin Immunol2008121S207
- Smith-NorowitzTAJosekuttyJLev-TovHIgE anti-varicella zoster virus and other immune responses before, during, and after shinglesAnn Clin Lab Sci200939435019201740
- Smith-NorowitzTAJosekuttyJLev-TovHLong term persistence of IgE anti-varicella zoster virus in pediatric and adult serum post chicken pox infection and after vaccination with varicella virus vaccineInt J Biomed Sci2009100105
- Smith-NorowitzTAWongDKusonruksaMLong term persistence of IgE anti-influenza virus in pediatric and adult serum post vaccination with influenza virus vaccineInt J Med Sci2011823924421448311
- PiedraPMajor respiratory viruses of childhood: illness and preventionInfect Dis Child20122534
- NovelSwine-OriginInfluenzaA(H1N1) Virus Investigation TeamEmergence of a novel swine-origin influenza A (H1N1) virus in humansN Engl J Med20093602605261519423869
- Centers For Disease Control and PreventionThe 2009 H1N1 Pandemic: Summary Highlights42009–2010 Updated June 16, 2010. Available from: http://www.cdcgov/H1N1flu/recommendations.htm
- Centers For Disease Control and PreventionUpdated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009–2010 season Updated December 7, 2009. Available from: http://www.cdcgov/H1N1flu/recommendations.htm
- LaemmliUKCleavage of structural proteins during the assembly of the head of bacteriophage T4Nature19702276806855432063
- Perez GuevaraMTSavon ValdesCRivas ArjonaMGoyenechea HernandezAA structural protein study of influenza A (H1N1) virus by polyacrylamide gel electrophoresisRev Cubana Med Trop1992445561 Spanish1344690
- KusonruksaMWongDNorowitzKBLong term persistence of IgE anti-influenza A H1N1 virus antibodies in pediatric and adult serum post influenza A H1N1 infectionJ Allergy Clin Immunol2011127SupplAB229
- FuSPierreJSmith-NorowitzTAIgE antibodies from pancreatic cancer patients mediate antibody-dependent cell mediated cytotoxicity (ADCC) against pancreatic cancer cellsClin Exp Immunol200815340140918803764
- FerrazziMDe RinaldisMLSalottiACirelliASerum IgE levels in human immunodeficiency virus (HIV)-1 infected patients: Correlation between IgE and CD4+ T cellsRiv Eur Sci Med Farmacol19931567707909618
- QiuCWanYZhangWEarly adaptive humoral immune responses and virus clearance in humans recently infected with pandemic 2009 H1N1 influenza virusPLoS One20116e2260321886767