Abstract
Background
Eosinophilic inflammatory phenotype was thought to be the most common phenotype of cough variant asthma (CVA), nevertheless other phenotypes were also reported.
Purpose
The study aimed to analyze the inflammatory phenotypes of CVA in relation to treatment response to the stepwise anti-asthmatic treatment.
Patients and Methods
The study included 45 patients with chronic cough (CC) and suspicion of CVA (normal chest X-ray, presence of bronchial hyperresponsiveness and no history of wheezing or dyspnea) in whom induced sputum was successfully collected. Based on the cellular composition of the sputum, patients were divided into major inflammatory phenotypes: eosinophilic, neutrophilic, paucigranulocytic or mixed granulocytic. A stepwise treatment, including inhaled corticosteroids with long-acting β2-agonist, montelukast and short-term therapy with prednisone was initiated. Good treatment response was defined as the reduction in cough severity at least 20 mm from the baseline in visual analogue scale and improvement in cough-related quality of life assessed by the Leicester cough questionnaire at least 1.3 points after any of three steps.
Results
Finally, 40/45 (88.9%) patients improved after therapy. Eosinophilic asthma was found in 13/40 (32.5%) patients, neutrophilic in 6/40 (15.0%) and paucigranulocytic pattern in 21/40 (52.5%) patients. No one demonstrated a mixed granulocytic phenotype. The response to the treatment was similar in all groups. However, the reduction in cough severity was inversely related to the percentage of sputum neutrophils (r = −0.44, P = 0.003). We showed that the percentage of neutrophils in sputum >46% may be considered as a predictor of poor response to anti-asthmatic therapy.
Conclusion
The diversity of inflammatory phenotypes with paucigranulocytic preponderance was found in subjects with CVA. The response to anti-asthmatic treatment in patients with CVA was not related to the inflammatory phenotype. High neutrophil count in sputum may predict poor response to anti-asthmatic therapy in patients with CC and bronchial hyperresponsiveness.
Introduction
Cough variant asthma (CVA) is a form of asthma reported in patients with cough as a sole symptom and is a common cause of chronic cough (CC) in non-smoking patients.Citation1,Citation2 Four inflammatory phenotypes of asthma have been proposed according to the sputum cytology (ie, eosinophilic, neutrophilic, paucigranulocytic and mixed granulocytic).Citation3 In CVA, comparably to the classic form of asthma, eosinophilic phenotype is indicated as the most frequent phenotype, but other phenotypes have been also reported.Citation4–7 Although eosinophilic phenotype of classic asthma responds better to anti-inflammatory treatment (ie, inhaled corticosteroids, ICS),Citation8–10 only one real-life study assessing treatment of CVA in relation to inflammatory phenotypes has been published so far.Citation7
As the response to ICS seems to be better in the eosinophilic asthma phenotype, this study was performed to evaluate the relationship between the cellular phenotypes of CVA and response to anti-asthmatic therapy.
Materials and Methods
General Study Design and Patients
This prospective, single-center cohort study (ClinicalTrials.gov NCT03363698) was performed between 2016 and 2020 in the Department of Internal Medicine, Pulmonary Diseases and Allergy of at the Medical University of Warsaw, Poland. Consecutive non-smoking patients referred for the management of CC were initially enrolled. All subjects underwent the diagnostic work-up of CC according to current recommendations. Patients suspected of having CVA were further selected to form a proper study group. These patients received anti-asthmatic treatment and the proof of its effectiveness was considered as the confirmation of the CVA diagnosis.Citation2,Citation11
The specific inclusion criteria were as follows: 1) age 18–85 years, 2) presence of CC (over 8 weeks), 3) no history of wheezing or dyspnea, 4) normal or near-normal spirometry, 5) presence of bronchial hyperresponsiveness (BHR, provocative concentration of methacholine causing 20% fall in FEV1 (PC20) below 16 mg/mL) in provocation challenge, 6) effective collection of induced sputum. Exclusion criteria included: 1) acute respiratory tract infection within previous 6 weeks, 2) inhaled corticosteroids (ICS) or long-acting β2-mimetics (LABA) or leukotriene receptor antagonist (LTRA) or oral corticosteroids (OCS) or proton pump inhibitor (PPI) or antihistamine or intranasal corticosteroids therapy within 4 weeks before the onset of the diagnostic work-up, 3) abnormal chest X-ray, 4) active smoking (within 12 months before enrollment).
The study was performed in accordance with the Declaration of Helsinki and the study protocol was approved by the Institutional Review Board of the Medical University of Warsaw (KB/222/2016) and all participants signed written informed consent.
Diagnostic Work-Up and Treatment Protocol
All patients underwent diagnostic procedures, including pulmonary function testing, sputum induction, methacholine and capsaicin challenge prior to treatment administration.Citation12 This step-wise diagnostic algorithm was based on European Respiratory Society and American College of Chest Physicians guidelines.Citation11,Citation13 Methacholine challenge was performed according to the American Thoracic Society guidelines, in a 2-minute tidal breathing protocol.Citation14 Capsaicin cough challenge was performed as previously recommended (Koko Digidoser, nSpire Health Inc., Longmont, USA) using a single-breath method.Citation11 Cough reflex sensitivity was measured before treatment initiation and during the patient’s final evaluation after treatment and was expressed as the lowest capsaicin concentrations evoking two (C2) and five (C5) coughs in the first 15 seconds after inhalation. Reduction in cough hypersensitivity was defined as elevation of capsaicin concentration threshold in C2 or C5.
Sputum induction was performed as described elsewhere.Citation15 Cells were counted manually based on the morphology of 300 influx cells from various fields. Inflammatory phenotypes were determined based on the cellular composition of sputum: 1) eosinophilic: sputum eosinophils ≥3% of cells, 2) neutrophilic: sputum neutrophils ≥61% of cells, 3) paucigranulocytic: sputum eosinophils <3% and sputum neutrophils <61% of cells, 4) mixed granulocytic: sputum eosinophils ≥3% and sputum neutrophils ≥61% of cells.Citation16 The atopy was defined as a presence of positive skin prick test (a mean wheal diameter ≥3 mm) or presence of serum-specific IgE antibody for at least one allergen.Citation17
CVA therapy included stepwise asthma treatment: 1st step - 4 weeks of moderate dose ICS+LABA, 2nd step - 4 weeks add-on montelukast (10 mg), 3rd step – 10 days weeks of add-on prednisone (0.5 mg/kg).Citation18 Each step of the treatment was followed by the measurement of cough severity in visual analogue scale (VAS) and quality of life assessment using the Leicester cough questionnaire (LCQ). The criteria for improvement were as follows: the reduction in VAS (ΔVAS) at least 20 mm and improvement of quality of life in LCQ (ΔLCQ) at least 1.3 points after any step of three-step therapy.Citation11 The diagnosis of CVA was established in all patients who met the above criteria of the post-treatment improvement.Citation1 Significance of BHR as a predictor of response to this stepwise approach of anti-asthmatic treatment in non-smoking adults with CC was analyzed in our previous study.Citation19
Statistical Analysis
The data were analyzed using Statistica 13.3 software package (StatSoft, Tulsa, USA) and presented as median and interquartile range or numbers and percentages. The differences between patients with different inflammatory phenotypes of CVA were tested using Kruskal–Wallis or χ2 tests. Correlations between variables were calculated using Pearson’s correlation coefficient. The receiver operating characteristic (ROC) curve with the Youden index was established to evaluate the cut-off point of neutrophil percentage in induced sputum in the prediction of treatment response in patients with BHR. A P value lower than 0.05 was considered statistically significant.
Results
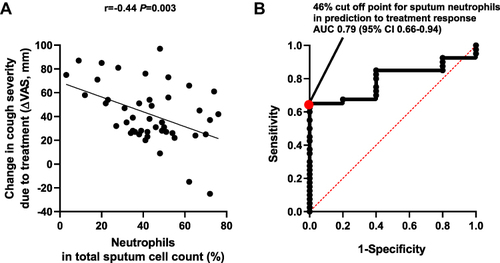
Forty-five CC patients with suspicion of CVA were selected from 250 patients with CC (characteristics of this group is given in Supplementary Table 1). Among all patients with suspicion of CVA modest negative correlation between sputum neutrophil percentage and the reduction of cough measured in VAS (r = - 0.44, P = 0.003) was found (). The area under the ROC curve for sputum neutrophils percentage in predicting lack of treatment response was 0.79 (95% CI 0.66–0.94 Youden index 0.65) with the cut-off point at 46% of neutrophils ().
Figure 1 Relationship between the percentage of neutrophils in induced sputum and treatment response in patients with chronic cough and suspicion of cough variant asthma.
The diagnosis of CVA was finally established in 40/45 (88.9%) patients in whom significant reduction in cough was documented. The patients were characterized with a median age of 62.0 years (56.5–69.5), female predominance 33/40 (82.5%), long-lasting [median duration of 4 years (2–10)] and severe cough [70.0 mm (43–80) in VAS] with substantially impaired QoL in LCQ [10.6 points (8.1–13.4)].
Eosinophilic asthma was found in 13/40 (32.5%) patients, neutrophilic in 6/40 (15.0%) and paucigranulocytic pattern was shown in 21/40 (52.5%) patients, while no one demonstrated mixed granulocytic phenotype (). The CVA patients with different cellular phenotypes (ie, eosinophilic, neutrophilic and paucigranulocytic) did not differ in terms of demographic data, clinical characteristics [cough duration, severity of BHR, co-morbidities (upper airway cough syndrome, gastroesophageal reflux), cough severity, LCQ score or intensity of anti-asthmatic treatment], as well as in the degree of improvement after applied treatment, either in ΔVAS, or ΔLCQ or percentage of subjects with reduction in cough hypersensitivity (see ).
Table 1 Overall Characteristics of Patients with Chronic Cough Due to Cough Variant Asthma (CVA) and Comparison Between CVA Patients with Different Inflammatory Phenotypes
Discussion
This study showed a relatively low prevalence of eosinophilic phenotype in patients with CC due to CVA. Paucigranulocytic phenotype was the most common inflammatory phenotype. Surprisingly, response to anti-asthmatic therapy was similar in patients with eosinophilic phenotype and other CVA phenotypes. Moreover, there was a negative correlation between reduction in CC severity and the percentage of neutrophils in sputum. Furthermore, the percentage of neutrophils in induced sputum over 46% may be considered as a predictor of poor treatment response in adults with CC and suspicion of CVA.
To our knowledge, only few reports were published so far describing inflammatory phenotypes of CVA.Citation4,Citation6,Citation7 Even though eosinophilic pattern has been reported as a frequent inflammatory phenotype,Citation4,Citation6,Citation7 Matsuoka et al showed similar prevalence of eosinophilic, neutrophilic and paucigranulocytic inflammation.Citation7 Intriguingly, the female preponderance in neutrophilic inflammation, which was the highest in previously reported study,Citation7 what was not observed in our study. Comparably to Matsuoka et al, our analysis showed no differences between eosinophilic, neutrophilic and paucigranulocytic phenotypes in terms of disease duration, atopy or bronchial hyperresponsiveness.Citation7 In regard to treatment efficacy in CVA, there has been only one real-life study that assessed relation of inflammatory phenotypes to treatment effects in CVA.Citation7 In this study, Matsuoka et al, proved that patients with elevated airway eosinophils (eosinophilic or mixed granulocytic phenotypes) required higher doses of ICS to obtain good asthma control. However, in the present study, we did not find any significant differences in reduction of cough or improvement in cough-related quality of life after anti-asthmatic treatment among patients with different inflammatory phenotypes. In our previous study, we did not observe correlation between PC20 and response to anti-asthmatic therapy in patients with CVA either.Citation19
Moreover, there is paucity of data on noneosinophilic phenotype of CVA so far. Gao et al compared classic asthma and CVA, showing more severe BHR, a lower percentage of eosinophils and higher neutrophils in induced sputum cell count in CVA, which probably indicated different airway inflammatory subtypes.Citation4 However, in contrast to our results, Gao et al showed that patients with eosinophilic CVA were characterized with more severe BHR compared to those with noneosinophilic phenotype. Even though paucigranulocytic phenotype is considered to be the most common asthma phenotype in patients with stable asthma, there is a paucity of studies on this phenotype.Citation20 Similarly, the paucigranulocytic phenotype in CVA had been barely diagnosed before. In this context, we believe that the results of this study are value-adding to the present knowledge on CVA.
As noneosinophilic asthma is thought to be less responsive to ICS, it frequently requires other forms of therapies (dedicated to neutrophilic inflammation, airway smooth muscle changes or neuronal dysfunction).Citation21 Therefore, we assumed this might also refer to noneosinophilic phenotype of CVA. However, we did not find any differences in response to classical anti-asthmatic therapy between phenotypes of asthma in our study, which might result from using both, ICS and LABA as the first step of treatment.
There are several limitations of this study that need to be considered. Firstly, this was a single-center, cohort study with a limited number of participants. Secondly, as the study group was recruited from patients referred to the cough center (ie, patients with long-lasting, severe refractory CC) it cannot be excluded that such distribution of inflammatory phenotypes does not refer to all patients with CVA, but those diagnosed in cough center. Thirdly, due to the lack of a cough monitor system, the study endpoint relied on the subjective cough assessment, ie, VAS and LCQ scores. Another limitation of the study could be the lack of sputum cytology analysis after the treatment. Despite these limitations, we believe that the results of this study present important additions to knowledge and warrant further research on the field of the pathophysiology of CVA.
Conclusion
In conclusion, the diversity of inflammatory phenotypes with paucigranulocytic preponderance was found in subjects with CVA. The response to anti-asthmatic treatment in patients with CVA was not related to any inflammatory phenotype. The sputum neutrophil count >46% may be considered as a predictor of poor response to anti-asthmatic therapy in patients with CC and BHR.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure
Dr Aleksandra Rybka-Fraczek reports conference fee ERS 2019 from Polpharma, outside the submitted work. Dr Marta Dabrowska reports personal fees from Merck for lectures on chronic cough, outside the submitted work. Dr Elzbieta M Grabczak reports personal fees from MSD for lecture, outside the submitted work. Dr Rafal Krenke reports personal fees from MSD, Roche, AstraZeneca and Polpharma, outside the submitted work. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors thank Aleksandra Szubert-Franczak, MD and Katarzyna Mycroft, MD for language editing. Part of this study was presented at the 11th International Cough Symposium, 21–22 January 2021.
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2021. Available from: https://ginasthma.org/wp-content/uploads/2021/04/GINA-2021-Main-Report_FINAL_21_04_28-WMS.pdf. Accessed May 01, 2021.
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55:1901136. doi:10.1183/13993003.01136-2019
- Gibson PG. Inflammatory phenotypes in adult asthma: clinical applications. Clin Respir J. 2009;3:198–206. doi:10.1111/j.1752-699X.2009.00162.x
- Gao J, Wu F, Wu S, Yang X. Inflammatory subtypes in classic asthma and cough variant asthma. J Inflamm Res. 2020;13:1167–1173. doi:10.2147/JIR.S269795
- Malerba M, Ragnoli B, Azzolina D, Montuschi P, Radaeli A. Predictive markers of bronchial hyperreactivity in a large cohort of young adults with cough variant asthma. Front Pharmacol. 2021;12:630334. doi:10.3389/fphar.2021.630334
- Gao J, Wu H, Wu F. Small airway dysfunction in patients with cough variant asthma: a retrospective cohort study. BMC Pulm Med. 2021;21:49.
- Matsuoka H, Niimi A, Matsumoto H, et al. Inflammatory subtypes in cough-variant asthma: association with maintenance doses of inhaled corticosteroids. Chest. 2010;138:1418–1425. doi:10.1378/chest.10-0132
- Pavord ID. Blood eosinophil-directed management of airway disease. the past, present, and future. Am J Respir Crit Care Med. 2020;202:637–639. doi:10.1164/rccm.202004-1013ED
- Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56:985–1003. doi:10.1258/0007142001903490
- Martin RJ, Szefler SJ, King TS, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007;119:73–80. doi:10.1016/j.jaci.2006.10.035
- Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–1276.
- Rybka-Fraczek A, Dabrowska M, Grabczak EM, et al. Blood eosinophils as a predictor of treatment response in adults with difficult-to-treat chronic cough. ERJ Open Res. 2021;7:00432–02021. doi:10.1183/23120541.00432-2021
- Dicpinigaitis PV. Chronic cough due to asthma: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:75s–79s. doi:10.1378/chest.129.1_suppl.75S
- Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161:309–329.
- Paggiaro PL, Chanez P, Holz O, et al. Sputum induction. Eur Respir J Suppl. 2002;37:3s–8s. doi:10.1183/09031936.02.00000302
- Taylor SL, Leong LEX, Choo JM, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol. 2018;141(94–103):e115. doi:10.1016/j.jaci.2017.03.044
- Johansson SG, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–824. doi:10.1034/j.1398-9995.2001.t01-1-00001.x
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2016. Available from: https://ginasthma.org/wp-content/uploads/2019/01/2016-GINA.pdf. Accessed May 5, 2021.
- Rybka-Fraczek A, Dabrowska M, Grabczak EM, et al. Does bronchial hyperresponsiveness predict a diagnosis of cough variant asthma in adults with chronic cough: a cohort study. Respir Res. 2021;22:252. doi:10.1186/s12931-021-01845-2
- Tliba O, Panettieri RA Jr. Paucigranulocytic asthma: Uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol. 2019;143:1287–1294. doi:10.1016/j.jaci.2018.06.008
- Thomson NC. Novel approaches to the management of noneosinophilic asthma. Ther Adv Respir Dis. 2016;10:211–234. doi:10.1177/1753465816632638
