Abstract
Spinal cord injury (SCI) is a catastrophic condition with high morbidity and mortality that still lacks effective therapeutic strategies. It is well known that the most important stage in SCI pathogenesis is secondary injury, and among the involved mechanisms, the inflammatory cascade is the main contributor and directly influences neurological function recovery. In recent years, increasing evidence has shown that mesenchymal stem cells (MSCs) transplantation is a promising immunomodulatory strategy. Transplanted MSCs can regulate macrophage-, astrocyte-, and T lymphocyte-mediated neuroinflammation and help create a microenvironment that facilitates tissue repair and regeneration. This review focuses on the effects of different types of immune cells and MSCs, specifically the immunoregulatory capacity of MSCs in SCI and repair. We will also discuss how to exploit MSCs transplantation to regulate immune cells and develop novel therapeutic strategies for SCI.
Introduction
Spinal cord injury (SCI) is a potentially devastating event in the central nervous system (CNS) that can lead to the loss of sensory and motor functions below the damaged segment with huge burdens on patients, their families, and society due to the high treatment cost.Citation1,Citation2 The global rate of traumatic SCI ranges from 250,000 to 500,000 people annually and will gradually climb with the growing number of people using modern transportation along with aging populations.Citation3,Citation4 SCI triggers a sequential set of pathophysiological processes that can be classified as primary and secondary injury.Citation5 Acute primary injury commonly occurs due to the mechanical insult, which can be caused by laceration, contusion, compression, or transection.Citation6,Citation7 These events severely disrupt neuronal pathways and axonal networks, cause hemorrhage, and compromise the blood-spinal cord barrier (BSCB).Citation8,Citation9 It is widely believed that the most important phase is secondary injury, which develops a few minutes after the initial injury. It consists of a series of auto-destructive cellular and molecular changes, such as inflammatory response, glial scar formation, edema, thrombosis, free radical release, and apoptotic and necrotic cells death.Citation10,Citation11 Among the mechanisms of secondary injury, a robust and chronic inflammatory response has been observed at the injury epicenter and in surrounding areas.Citation12,Citation13 Studies have demonstrated that this response can contribute to a wide range of inflammatory and autoimmune disorders that exacerbate lesion progression and hamper neurological function recovery.Citation13,Citation14 Unfortunately, there is still a lack of therapeutic methods targeting neuroinflammation; only methylprednisolone has shown efficacy and was approved by the US Food and Drug Administration for clinical treatment.Citation15,Citation16 However, its use has gradually declined over the past few decades due to the serious complications related to glucocorticoid therapy, such as gastrointestinal bleeding, wound infection, pulmonary embolism, and sepsis.Citation17,Citation18 Despite the controversies concerning the glucocorticoid treatment, its effects suggest that suppressing the inflammatory response after injury is a viable approach to improve the condition of SCI patients.Citation19,Citation20 It is therefore imperative to investigate the roles of different types of immune cells on excessive neuroinflammation in order to develop new therapeutic strategies targeting neuroinflammation in the setting of SCI.
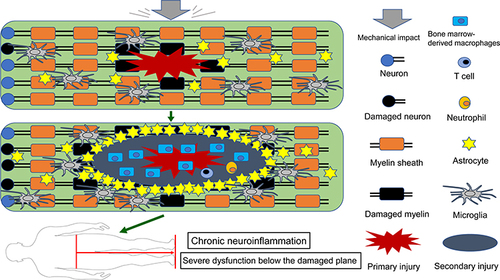
Figure 1 Schematic depicting the activation and migration of resident and peripheral immune cells following SCI. After primary injury, resident astrocytes, microglia, and other glial cells are immediately activated and migrate to the injury site (top). Subsequently, peripheral inflammatory cells including neutrophils, bone marrow-derived macrophages, and lymphocytes infiltrate into the epicenter of the injured spinal cord, and these activated immune cells can exacerbate damage, causing a wider range of secondary injury. Glial cells (mainly astrocytes) form glial scar to seclude the damaged area, and microglia are mainly present around the injury site (middle). These persistent pathophysiological changes ultimately result in severe dysfunction below the damaged segment (bottom).
Over the past few decades, stem cell-based therapy has provided new hope for treating SCI patients.Citation20 Mesenchymal stem cells (MSCs) have attracted increasing attention due to their remarkable advantages of self-renewal, differentiation potential, and immunoregulation.Citation21 The International Society for Cellular Therapy sets a minimum standard of MSCs as follows: cells that are positive for the surface markers of cluster of differentiation (CD)73, CD90, CD29, and CD105, but not with CD14, CD45, CD34, CD11b, or human leukocyte antigen-DR; are plastic-adherent and fibroblast-like under standard culture conditions; and can differentiate into chondrocytes, adipocytes and osteoblasts in vitro.Citation22 Due to the low expression of costimulatory molecules and class II major histocompatibility complex, these pluripotent stem cells do not trigger an obvious immune response after transplantation.Citation22,Citation23 To date, MSCs have been isolated from distinct adult tissues (eg, bone marrow, peripheral blood, adipose tissue) and neonatal tissues (eg, umbilical cord and placenta).Citation24,Citation25 MSCs from different sources have been demonstrated to be effective in animal models of numerous inflammatory diseases as well as in ongoing clinical trials including SCI.Citation26–29 Growing evidence suggests that these multipotent cells can induce an immunosuppressive and reparative microenvironment through cell–cell interaction and a paracrine effect, thereby promoting anatomical and functional recovery after SCI.Citation30,Citation31
In this review, we summarize the roles of immune cells and transplanted MSCs with a focus on the immunomodulatory effects of MSCs in SCI and repair. We will pay special attention to the fact that preconditioning may further promote the effects of MSC-based therapy in SCI models. Adequately clarifying the contributions of different immune cells, MSCs, and their reciprocal interactions to SCI pathogenesis and repair will be of great value for developing new therapeutic approaches for SCI.
Inflammation After SCI
Although microglia can be found throughout the CNS and numerous immune cells are found in the meningeal spaces, the absence of peripheral immune cells in the normal CNS implies that the spinal cord is privileged from normal immune surveillance.Citation32 The BSCB consists of three main cellular components: capillary basement membrane, astrocytes, and pericytes.Citation33 Similar to the blood–brain barrier, the BSCB is essential for excluding peripheral immune cells and various inflammatory and toxic metabolic products from the CNS, thereby maintaining microenvironment stability.Citation34,Citation35 Following SCI, cells damaged at the injury site can produce types of intracellular proteins and cell debris known as damage-associated molecular patterns (DAMPs) that act as strong inflammatory stimuli and are responsible for the excessive inflammatory response post-SCI.Citation36,Citation37 DAMPs bind to pattern recognition receptors in resident inflammatory cells, including resident microglia and astrocytes, resulting in the rapid activation of these cells.Citation37,Citation38 In response to neuropathology, astrocytes undergo a suite of molecular, morphological, and functional remodeling, which eventually leads to the acquisition of new functions.Citation39 However, the abilities of astrocyte proliferation and polarization vary with the intensity and type of stimulation after SCI. With the increasing stimulation intensity, cell hyperplasia, proliferation, migration, and alignment occur gradually, and these reactive astrocytes show a gradual up-regulation of glial fibrillary acidic protein (GFAP) and secretion of cytokines (eg, interleukin (IL)-6, transforming growth factor beta (TGF-β), IL-1β) and other molecules (eg, cyclooxygenase (COX)-2, inducible nitric oxide synthase, S100β).Citation39 When the BSCB is damaged, these inflammatory mediators can drive peripheral immune cells to the lesion site; they then polarize towards pro-inflammatory phenotypes and exhibit cytokine expression patterns similar to the resident inflammatory cells, resulting in a more severe local inflammatory response that consequently hinders neurological function recovery ().Citation40,Citation41 A sequential inflammatory cascade is switched on following SCI. Neutrophils are driven to the lesion site within 24 hours and facilitate phagocytosis and the removal of cellular debris; many neutrophils also secrete distinct oxidative and tissue-degrading enzymes, proteases, reactive oxygen species (ROS), and tumor necrosis factor (TNF)-α, creating a harmful microenvironment that is neurotoxicity to neurons.Citation14,Citation42,Citation43 After neutrophil infiltration, blood-derived monocytes are the next inflammatory cells to appear at the lesion site at approximately 2 days and peaking on 5–7 days.Citation44,Citation45 These cells play critical roles in clearing cellular debris, promoting angiogenesis, and modulating cytokine secretion and the activation and proliferation of T lymphocytes.Citation46 Lymphocyte numbers are highest at 9 days and are essential for the progression or resolution of secondary damage by adopting distinct immunophenotypes.Citation47 The three main types of immune cells together with their secretion of various neurotoxic factors including inflammatory mediators, free radicals, matrix metalloproteinases, proteolytic enzymes, ROS, and apoptosis-inducing molecules extend the primary damage to adjacent normal tissues, causing further apoptosis and necrosis of neurons and glial cells.Citation48,Citation49
During the course of skin and muscle wound healing, there is generally a distinct shift in the inflammatory response. Initially, various pro-inflammatory cells such as M1 macrophages and neutrophils predominate the lesion site.Citation50 They play critical roles in removing cell debris and providing a sterile microenvironment for tissue regeneration.Citation51 Following beneficial and transient inflammation, an anti-inflammatory and reparative phase is induced that is mainly regulated by regulatory T cells (Treg) and M2 macrophages, which can promote angiogenesis and extracellular matrix deposition.Citation52,Citation53 However, unlike the cutaneous and muscular healing processes, there is no corresponding anti-inflammatory and remodeling phase after SCI.Citation54 Pro-inflammatory cells persist at the lesion site, resulting in secondary neuronal and glial degeneration, which further exacerbates neurological dysfunction.Citation55 Effective strategies to induce the anti-inflammatory remodeling phase after the acute inflammatory response are extremely important for promoting neurological recovery post-SCI.
Contribution of Distinct Immune Cells to SCI Inflammation
The inflammatory response plays critical roles in all the mechanisms of secondary injury and directly influences the neurological outcome post-injury.Citation56 Neuroinflammation was previously regarded as an adverse consequence after SCI because it led to a broader range of destructive processes including widespread healthy spinal cord tissue damage and further neuronal degeneration. With substantial advances in the understanding of SCI pathophysiology, it was gradually revealed that the early inflammatory response could also generate a permissive microenvironment for the regeneration of damaged neurons and axons, similar to early inflammatory benefits in other tissues.Citation57–59 Neuroinflammation is composed of multifaceted cellular and molecular responses, and the unique effects of immune cells including macrophages, astrocytes, and lymphocytes are essential for the occurrence and progression of inflammatory responses post-SCI.Citation60
Effect of Macrophages on Neuroinflammation
The phase-specific functions of macrophages—ranging from initial neuroinflammation to eventual tissue remodeling and repair—are essential for functional locomotion recovery.Citation55 These cells primarily originate from resident microglia that are activated minutes to hours after SCI, but after 2 days they are mainly from circulating monocytes.Citation61 These monocyte- and microglia-derived macrophages are still hard to distinguish owing to their similar phenotypes and morphologies.Citation60,Citation62 Based on their phenotypic and functional differences, macrophages can be divided into two main subtypes termed M1 and M2.Citation63 T-helper 1 (Th-1) cytokines such as TNF-α and interferon gamma (IFN-γ) induce the polarization of classically activated M1 macrophages characterized by up-regulation of inflammatory cytokines such as IL-23, IL-1β, TNF-α, and IL-12.Citation64,Citation65 These mediators can kill neurons, induce axonal degeneration, and further contribute to the activation of neurotoxic Th1 and Th17 cells.Citation66,Citation67 In contrast, alternatively activated M2 macrophages are the product of exposure to Th2-associated cytokines such as IL-4 and IL-13.Citation68,Citation69 These cells are able to produce high levels of anti-inflammatory cytokines such as IL-10, IL-4, and TGF-β, which are essential for inhibiting excessive inflammatory responses and promoting wound remodeling and repair.Citation69,Citation70 In rat models of SCI, M2 macrophages can be observed early, but dissipate rapidly within 3–7 days after injury, while M1 macrophages remain in the lesion indefinitely.Citation31,Citation71 In SCI patients, by ∼5 days post-injury, activated macrophages are abundant in the spinal cord for up to a year, and these cells could produce potentially destructive oxidative and proteolytic enzymes.Citation45
Effect of Astrocytes on Neuroinflammation
It was previously believed that macrophages were the only cell type involved in neuroinflammation. However, it is now known that astrocytes are able to modulate innate and adaptive immune responses in the CNS by activating diverse pathways.Citation38,Citation72,Citation73 For example, as a critical modulator for neuroinflammation, nuclear factor (NF)-κB signaling is highly activated by its associated gene expression after SCI, suggesting that this pathway is important in the pathophysiological process of CNS injury.Citation74–76 In a mice model of SCI, inhibiting astroglia NF-κB was demonstrated to down-regulate monocyte chemoattractant protein (MCP)-1 expression, inhibit leukocyte recruitment, and promote axonal regeneration and germination, finally facilitating functional locomotion recovery.Citation77–79 Furthermore, inhibiting the activation of astrocytes after SCI has been shown to attenuate inflammation and promote axonal regeneration and motor recovery.Citation80 Paradoxically, Anderson et alCitation81 demonstrated that attenuating scar-forming astrocytes elicited a more severe inflammatory response and prevented axonal regeneration in the CNS, leading to more severe dysfunction. These diverse outcomes are likely to be explained by the induction of an astrocyte reaction that is both phenotypically and functionally heterogeneous. Similar to the situation of M1 and M2 macrophages, study has revealed that astrocytes have more than one type of polarization.Citation44 Inflammation and ischemia induce the pro-inflammatory A1 and anti-inflammatory A2 phenotypes.Citation82 It should be noted that the current nomenclature oversimplifies the astrocyte activation continuum, implying that reactive astrocytes have more than two polarization types. The naming convention of A1/A2 is intended to promote scientific research and academic exchanges.Citation83,Citation84 A1 astrocytes strongly increase the expression of the classical complement cascade genes including complement component1s (C1s), C1r, C3, and C4, which were previously shown to be harmful to synapses, indicating that A1 astrocytes might be the “bad” player in neurological repair and remodeling.Citation85,Citation86 In contrast, A2 astrocytes up-regulate the expression of neurotrophic factors and cytokines such as leukemia inhibitory factor, cardiotrophin-like cytokine factor 1, IL-10, and IL-6 to support neuronal restoration and survival, as well as synaptic repair, suggesting that A2 astrocytes might be the “good” players in neuroinflammation.Citation87–89 Therefore, differentiation of astrocytes into an A2 phenotype may facilitate functional recovery after SCI while inhibiting secondary inflammation-mediated damage. These researches have emphasized the phenotypic and functional heterogeneity of reactive astrocytes. However, current studies mainly focus on the rodent astrocytes that have been shown to be significantly different from human astrocytes in morphology, activation timing, and gene expression.Citation90 Hence, it is unclear whether the gene expression and regulation of reactive astrocyte subtypes observed in rodents are directly applicable to human tissues.Citation90 But researchers can explore more extensively by selecting suitable model species, such as non-human primates, whose astrocytes have a transcription profile similar to that of human astrocytes.
Effect of T Lymphocytes on Neuroinflammation
T lymphocytes play important roles in the pathogenesis of neuroinflammation following CNS injury.Citation91 As a key modulator of the adaptive immune response, CD4+ T cells mainly differentiate into four subtypes—Th1, Th2, Th17, and Treg cells—that are essential for affecting the outcome of inflammatory response by restricting or activating other immune cell responses.Citation92,Citation93 Each CD4+ T cell subtype has a specific transcriptional program and cytokine expression pattern that can aggravate or mitigate the degree of secondary injury.Citation94 CNS injury recovery is likely to depend on the balance among these subtypes.Citation95 Th1 cells are the product of exposure to IFN-γ and IL-12 and can induce activation of the Th1-associated specific transcription factor, T-bet, which feeds back to stimulate the expression of additional IFN-γ, IL-12, and TNF-β, thereby promoting macrophage-dependent neuroinflammation and cell-mediated immunity.Citation66,Citation96,Citation97 T-bet-mediated cytokines are also able to induce Th2 phenotype repolarization toward Th1.Citation98 Hence, Th1 might play destructive roles in CNS injury and repair. The key cytokine required for Th2 differentiation is IL-4, which contributes to the expression of the master regulator transcription factor GATA3 of Th2, and itself is released by Th2 in addition to IL-13 and IL-10.Citation99,Citation100 Known effector functions of Th2 include promoting eosinophil accumulation and inducing B cells to produce immunoglobulin (Ig)E and IgG1, as well as inhibiting M1 macrophage activation.Citation66 Some studies have demonstrated that shifting CD4+ T cells to Th2 by potent Th2 inducers like glatiramer acetate can improve the outcome of CNS injury.Citation101 As such, Th2 cells have a greatly protective role. Apart from Th1 and Th2 cells involved in the neuroinflammation, study has shown that both Th17 cells and Treg are essential to regulate the immune response post-SCI.Citation91 Th17 cells are defined by the characteristic production of IL-17A, IL-17F, IL-21, and IL-22 along with the expression of the master transcription factor RORγt.Citation102 Th17 cells are pro-inflammatory and essential to protect against pathogens by recruitment of neutrophil granulocytes.Citation103 This cell type plays a determinant role in various inflammatory diseases, including multiple sclerosis, ankylosing spondylitis, inflammatory bowel disease, and rheumatoid arthritis, as well as SCI.Citation104,Citation105 In contrast, Treg are characterized by the specific transcription factor Foxp3 and play a significant role in inhibiting immune response-related neuroinflammation by secreting anti-inflammatory cytokines such as TGF-β and IL-10.Citation106,Citation107 Th1 and Th17 cells are considered to be the main drivers of pathogenesis because they predominate at the lesion site where they further induce an inflammatory response by activating macrophages and neutrocytes.Citation108 Accumulating data suggest that correcting the imbalance of Th1/Th2 and Th17/Treg cells could improve the prognosis of SCI.
Contribution of MSCs Transplantation to SCI Prognosis
A premise of effective MSC-based therapy is that MSCs migrate from their sources via the bloodstream and home to the injured site.Citation118,Citation119 Researchers have revealed that MSCs are attracted to the area of damage by various diverse chemokines, such as platelet-derived growth factor-AB, stromal-derived factor-1, and macrophage-derived chemokine.Citation120 Study has uncovered that MSCs express a large amount of CXCR4, which is essential for their homing and migrating.Citation121 However, natural migration into lesion sites is extremely limited.Citation122 A similar situation is observed to be found when MSCs are systemically administered because they are trapped in vascularized tissues, especially the lungs.Citation119 Therefore, an effective and simple MSCs transplantation method is local injection into the damaged area or surrounding healthy tissue.Citation123 Initial attention mainly focused on the differentiation potential of MSCs because they might differentiate into neurons and glial cells to replace the dead cells and reconstruct the integrity of neuronal conductive pathways; however, there remains a lack of related differentiation evidence.Citation112,Citation117 Interestingly, MSCs engraftment can still improve various functional parameters in animal models of SCICitation124 (). Scientists proposed that these results might be largely explained by their paracrine effects or direct interactions with immune cells.Citation125
Table 1 The Immunomodulatory Mechanisms of Transplanted MSCs in Improving the Prognosis of SCI
Angiogenesis is particularly important for the recovery of neurological function, so it is a valuable research direction for wound healing processes.Citation33 Studies have shown that MSCs significantly promote angiogenesis by secreting a series of factors, including vascular endothelial growth factor, platelet-derived growth factor, TGF-β, and IL-6, which promote BSCB repair and neurogenesis after SCI.Citation126–128 Meanwhile, an increasing body of evidence shows that MSCs transplanted into SCI models release many neurotrophic factors such as glial cell-derived neurotrophic factor, BDNF, neurotrophin-3, and basic fibroblast growth factor; the production of these soluble factors contributes to the inhibition of cell apoptosis and necrosis and regeneration of axons and myelin sheaths.Citation129–131 Apart from these reparative properties, MSCs also have robust anti-inflammatory roles.Citation130,Citation132 For example, bone marrow-derived mesenchymal stem cells (BM-MSCs) transplanted into a rat model of SCI can reduce the infiltration of neutrophil and significantly down-regulate the expression of pro-inflammatory cytokines.Citation133 In addition to neutrophil, MSCs can also skew the balance of inflammatory cytokines in an anti-inflammatory direction by modulating the state of macrophages, astrocytes, and T cells.Citation130 Harnessing the ability of MSCs to regulate neuroinflammation might be a powerful tool to inhibit secondary injury, which is potentially good news for SCI patients ().
Table 2 Completed Clinical Trials of MSCs in the Treatment of SCI
MSCs for SCI: Macrophage-Mediated Neuroinflammation
Macrophages are the most common immune cells at the lesion site and exert key roles in mediating the neuroinflammation during distinct periods post-SCI. Polarized macrophages can help facilitate angiogenesis and modulate connective tissue synthesis, which are the crucial elements of the damaged spinal cord repair, but can also cause deterioration of extracellular matrix and damage neurons and glia.Citation134 The change of macrophages from a pro-inflammatory to an anti-inflammatory, remodeling phenotype is considered to support the recovery of nerve function and the integrity of the injured tissue.Citation31 Numerous studies have reported that macrophages co-cultured with MSCs have a cytokine secretion pattern similar to M2 macrophages, which are characterized by the up-regulation of IL-4 and IL-10 along with down-regulation of TNF-α, IL-1β, and IL-12.Citation135–138 A similar situation can also be observed in M0 macrophages co-cultured with MSC-conditioned medium (MSC-CM), indicating that soluble factors are essential for their immunomodulatory properties.Citation139 These previous studies in vitro have raised our attentions about the polarization of macrophage in MSCs engraftment following SCI. Nakajima et alCitation112 reported that after MSCs transplantation in the injured spinal cord, M2 macrophages and their associated cytokines IL-13 and IL-4 were significantly increased, while M1 macrophages and their associated cytokines TNF-α and IL-6 were significantly decreased, resulting in axonal regeneration, inhibition of glial scar formation, and increased myelin sparing. Subsequently, it was shown in rat models of SCI that exosome (exo) derived from adipose-derived MSCs could also shift the macrophage phenotype from M1 to M2, accompanied by down-regulation of the pro-inflammatory cytokines IFN-γ and TNF-α, and this promoted a pro-regenerative environment.Citation140 To date, the accumulated evidence confirms that MSCs can skew the balance between M1/M2 macrophages towards the M2 phenotype, thereby facilitating functional neurological improvement post-SCI.Citation141,Citation142
Study investigating the mechanism involved found that this effect was associated with some soluble factors secreted by MSCs.Citation134 Stimulated MSCs can significantly secrete numerous cytokines including TGF-β, indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), IL-4, and IL-6 following stimulation by inflammatory mediators. These diverse soluble factors play an essential role in shifting the macrophage phenotype from M1 to M2.Citation134,Citation143–150 Notably, we previously showed that inflammatory macrophages could activate the NF-κB pathway of peripheral blood-derived mesenchymal stem cells (PB-MSCs) by releasing TNF-α and IL-1β, resulting in the up-regulation of IL1-RA of PB-MSCs, which could induce the macrophage polarization towards M2 phenotype.Citation151 In response to inflammatory stimuli, MSCs up-regulated the expression levels of tumor necrosis factor-induced protein 6, which could reduce the nuclear translocation of NF-κB by binding to the resident macrophages’ CD44 receptor, thereby weakening the macrophages-mediated inflammatory cascade.Citation143,Citation152 Moreover, IL-10 secreted by MSCs is likely to shift the macrophage phenotype towards anti-inflammatory M2 by activating Janus kinase (JAK)/signal transducer and activator of transcription (STAT)3 signaling.Citation153–155
In summary, MSCs have emerged as promising immune-modulators of macrophages polarization by modulating the production of distinct cytokines. Identifying the regulatory factors and key pathways of MSCs-mediated macrophage polarization is the key to shifting the macrophage phenotype from M1 to M2, thus creating an anti-inflammatory microenvironment for axonal extension and functional recovery. However, the specific effects of different subtypes on functional recovery should be fully considered when designing macrophage polarization as an immunomodulatory strategy.
MSCs for SCI: Astrocyte-Mediated Neuroinflammation
Astrocytes are the most abundant glial cells in the CNS, and they are essential for the homeostasis of the CNS.Citation156 As mentioned above, reactive astrocytes are highly heterogeneous after SCI and have been identified in two distinct categories, A1 and A2. Liddelow et alCitation157 have reported that classically activated neuroinflammatory microglia shift astrocytes to the A1 phenotype by releasing TNF-α, IL-1α, and C1q, which together are sufficient and necessary. A1 astrocytes lose most of their original functions and show new features, including rapidly killing the neurons and mature differentiated oligodendrocytes.Citation158 Therefore, efforts should be focused on inhibiting the activation of A1 astrocytes for the treatment of SCI. In response to lipopolysaccharide (LPS), A1-related cytokine, IL-1β and TNF-α, is significantly up-regulated, but this is inhibited when astrocytes are pre-treated with MSC-CM.Citation159 Recently, study has shown that BMMSC-exo transplanted into contusive SCI rat models could significantly reduce the number of A1 neurotoxic astrocytes by inhibiting the activation of inflammatory macrophages, thereby effectively promoting angiogenesis and axonal regeneration, and reducing neuronal apoptosis and inflammatory response. Furthermore, Wang et al found that BM-MSCs and BMMSC-exo could both directly hinder astrocytes from polarizing toward the pro-inflammatory A1 phenotype after SCI by inhibiting the nuclear translocation of NF-κb p65, thereby reducing the lesion area and levels of IL-1β, TNF-α, and IL-1α, as well as increasing the expression of myelin basic protein, marker of neuronal nuclei, and synaptophysin.Citation160,Citation161 These results suggest that MSCs and MSC-exo can not only directly inhibit the formation of A1 astrocytes after SCI, but also indirectly suppress the polarization of A1 astrocytes by preventing the activation of inflammatory macrophages.
JAK/STAT3 pathway activation is critical for astrocyte proliferation, polarization, and growth.Citation84,Citation89,Citation162 After CNS injury, IL-6 secreted by transplanted MSCs might mediate the polarization of A2 astrocytes by activating JAK/STAT3 signaling, thus significantly improving the neurological recovery.Citation163 Besides, in models of systemic LPS activation, IL-10 secreted by M2 macrophages can induce astrocytes polarization toward the anti-inflammatory A2 phenotype, accompanied by significant up-regulation of TGF-β, which could also greatly shift the macrophage phenotype towards M2, with lower expression of IL-6 and IL-1β and high levels of CX3CR1 and IL-4Rα. Hence, the interaction between M2 macrophages and A2 astrocytes is beneficial to generate an anti-inflammatory and reparative microenvironment.Citation164 As mentioned above, MSCs and MSC-exo can drive macrophage toward an M2 phenotype. Taken together, it is possible that MSCs and MSC-exo exert effects on A2 astrocytes not only in a direct way but also mediated through the activation of M2 macrophages, thereby exerting a powerful immunosuppressive function and breaking the inflammatory cascade reaction after SCI. Other studies have revealed that in response to TGF-β, PI3K/AKT signaling in astrocytes modulates various physiological events, such as activation, proliferation, growth, and survival. PI3K/AKT pathway activation also induces astrocyte polarization toward the A2 phenotype; it is well known that MSCs can constitutively produce TGF-β.Citation165–169 Taken together, the evidence suggests that TGF-β secreted by MSCs may mediate astrocyte polarization in the A2 phenotype through the PI3K/AKT pathway. However, more specific studies are needed to clarify this issue.
MSCs for SCI: T Lymphocyte-Mediated Neuroinflammation
The excessive inflammatory Th1 and Th17 phenotypes observed following SCI tilt the scale toward the pro-inflammatory environment, which exacerbates the damage to neural tissue within weeks or even months.Citation49 We have recently demonstrated that after direct culture of CD4+ T cells and PB-MSCs in vitro, the Th17/Treg ratio and levels of pro-inflammatory cytokines IL-17 and IL-6 were significantly down-regulated, while the levels of anti-inflammatory cytokines TGF-β, IL-10 and Foxp3 were significantly up-regulated.Citation135 Moreover, BM-MSCs could also suppress the proliferation, activation, and differentiation of Th17 and Th1 cells and induce Treg polarization in vitro.Citation170 After interacting with dendritic cells, MSCs shift from the Th1 to Th2 subtype, and this is accompanied by higher levels of anti-inflammatory cytokines.Citation171 MSC-exo is also reported inhibiting T cell proliferation and activation and shift their phenotype towards the anti-inflammatory Treg, with a corresponding beneficial change in the cytokine profile.Citation92,Citation172,Citation173 Altogether, these results suggest that MSCs can inhibit Th1 and Th17 cell differentiation and induce Th 2 and Treg cells in vitro. Notably, our team has found that PB-MSCs transplanted into rat models of SCI caused decreases in CD4 + IL17 + Th17 cells along with their associated cytokines IL-6 and IL-21, and increases in the numbers of CD4 + CD25 + Foxp3 + Treg cells and their associated cytokines Foxp3, TGF-β, and IL-10; these changes ultimately contributed to improved functional recovery.Citation117 Furthermore, various activated CD4+ T cell subtypes can form a complex network regulatory system by coordinating and antagonizing other immune cell types. For example, Th1 cells can induce the differentiation of macrophages into M1 phenotype, while Th2 cells can induce the polarization of M2 macrophages that are involved in the induction of Treg. Therefore, in addition to directly regulating each immune cell, the application of MSCs may provide an anti-inflammatory and stable environment for nerve tissue repair by breaking this inflammatory cascade reaction.
Further investigations reveal that MSCs stimulated by IFN-γ can significantly up-regulate IDO expression levels; this results in tryptophan degradation that inhibits the allogeneic T cell response, promotes Th2 to secrete IL-4, and prevents Th1 from producing IFN-γ.Citation171,Citation174 MSCs are also able to suppress T cell proliferation and the secretion of related inflammatory cytokines by producing nitric oxide and PGE2, and PGE2 can also prevent CD4+ T cells from differentiating into Th17 cells.Citation175–178 Moreover, studies have demonstrated that MSCs can exert their inhibitory effects on CD4+ T cell differentiation towards Th1 and Th17, possibly due to induction of Treg and IL-10 secretion.Citation170,Citation179 Furthermore, MSC-mediated Treg induction can be inhibited by a TGF-β blocker, indicating that TGF-β secreted by MSCs plays a critical role in the differentiation of CD4+ T cells into Treg.Citation180,Citation181 Despite the fact that soluble factors are involved in Treg induction, ICOSL (inducible T cell costimulatory ligand) expression in MSCs is also essential for contact-dependent modulation of MSC-mediated Treg polarization.Citation182 These results suggest that MSC-mediated CD4+ T cells polarization may be modulated by different mechanisms depending both on soluble factors and cell-surface proteins.
In conclusion, the immunomodulatory role of MSCs in CNS injury involves multiple immune cell types. These immune cells can modulate MSCs gene expression, which subsequently inhibits the immune response of these innate and adaptive immune cells to produce an immunotolerant and permissive micro-environment (). All the achievements in MSCs engraftment bring hope to the successful transformation, but in vivo models of SCI currently used by researchers are mainly small rodents, such as rats and mice. Although this animal model is cost-effective and easy to feed, it is necessary to use larger animal models, such as non-human primates to further confirm the safety and efficacy of MSCs in improving nerve regeneration after nerve injury because their size and neuroanatomical structure are similar to those of human specimens.Citation183 Furthermore, in order to enhance the therapeutic effect of MSCs, more research is needed in the future to determine the ideal number of cells for transplantation, cell source, timing of administration, and route of administration.
Figure 2 MSCs improve SCI prognosis via immunomodulatory effects. These transplanted MSCs inhibit an excessive inflammatory response by up-regulating anti-inflammatory immune cells and associated cytokines and down-regulating the pro-inflammatory immune cells and associated cytokines, thereby promoting anatomical repair and functional recovery.
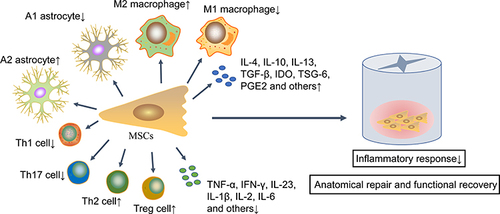
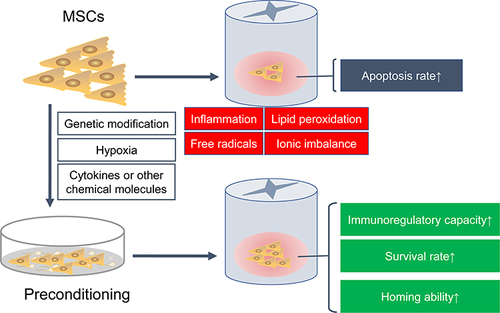
Figure 3 Preconditioning enhances the immunomodulatory ability and survival rate of MSCs in SCI. After SCI, the local harsh microenvironment causes a large amount of transplanted MSCs apoptosis. Various preconditioning strategies, including genetic modification, cytokines, hypoxia or other chemical molecules, can improve the immunomodulatory capacity, survival rate and homing ability of transplanted MSCs.
Prospective
Although transplanted MSCs are able to improve anatomical and locomotor recovery after SCI, the post-injury inflammatory and toxic environment is not suitable for the survival of grafted cells.Citation195,Citation196 Therefore, any strategy that enhances the viability and proliferation of transplanted MSCs is of great value.Citation197,Citation198 It is noteworthy that preconditioning can effectively enhance the immunomodulatory and survival ability of MSCs in vitro and in vivo ()Citation127 (). For example, MSCs pretreated with cobalt chloride could improve their homing ability by up-regulating levels of hypoxia-inducible factor-1α and CXCR4; simultaneously they can decrease their apoptosis rate by down-regulating caspase-3 and Bcl-2 levels. These hypoxic MSCs can also significantly inhibit macrophage polarization toward the pro-inflammatory M1 phenotype, along with lower levels of pro-inflammatory cytokines TNF-α and IL-1β.Citation186 Furthermore, MSC-exo harvested from hypoxic MSCs could significantly shift the macrophage phenotype from M1 to M2 by modulating the TLR4/NF-κB/PI3K/Akt pathway, thereby promoting functional and behavioral recovery.Citation188 Furthermore, moringin-pretreated MSCs significantly down-regulated COX-2 and IL-1β levels in the spinal cord and reduced cellular apoptosis by decreasing the expression levels of Bax, caspase-3, and caspase-9 and increasing levels of the anti-apoptotic protein Bcl-2.Citation185 In another study, it was revealed that the treatment of MSCs with IL-1β and IFN-γ enhanced their ability to induce macrophage polarization towards an anti-inflammatory M2 phenotype in comparison to MSCs treated with nothing.Citation199 These results indicate that hypoxia or cytokine preconditioning has a strong cytoprotective effect, which can help them adapt to the new environment during the acute phase of transplantation.
Table 3 The Immunosuppressive Effect of Pretreated MSCs and Extracellular Vesicles Secreted by MSCs in SCI
Gene modification might serve as another unique way to further improve MSCs immunomodulatory capacity. IL-35 is necessary for Treg to exert the maximum regulatory activity in vivo and in vitro.Citation200,Citation201 MSCs over-expressing IL-35 could significantly increase the percentage of CD4 + CD25 + Treg and suppress the effects of Th1 and Th17 cells. One study showed that compared with an untreated MSCs group, expression of the pro-inflammatory cytokine IL-17A was significantly down-regulated, while IL-10 was significantly up-regulated in the IL-35-transduced MSCs group.Citation201 Additionally, MSCs over-expressing IL-13 could significantly shift the macrophage phenotype to anti-inflammatory M2, thereby promoting the functional and histopathological recovery of SCI mouse models.Citation202 Although genetic modification of MSCs can promote cell survival and immunomodulatory capacity, safety concerns are the main limitations for the future therapeutic application of genetically modified MSCs, as viral vector integration may cause tumorigenesis in recipients after long-term treatment.
Taken together, the inflammatory cascade is a major contributor to secondary damage and directly affects disease progression. Macrophages, astrocytes, and T cells are the major cell types involved in SCI neuroinflammation, but no existing therapy directly targets neuroinflammation. Over the past few decades, MSCs have emerged as attractive, transplantable, and reparative cells that can not only directly modulate the activation of macrophages, astrocyte, and T cells, but also can break their complex inflammatory cascade reaction, thereby providing an anti-inflammatory and permissive microenvironment for CNS regeneration and repair. Considering that a pro-inflammatory and toxic microenvironment has harmful effects on the survival and immunoregulatory capacity of transplanted MSCs, pretreatment may enhance the ability of MSCs and further improve the ability to inhibit robust inflammation. However, further researches are needed to monitor the tumorigenic risk of engrafted gene-modified MSCs. Despite these promising avenues of research, further work is needed to improve our knowledge of molecular mechanisms and optimal transplantable conditions. Once these issues are addressed, MSCs are likely to be administered in clinical practice to relieve SCI patient suffering and enhance their quality of life.
Disclosure
The authors declare that they have no competing interests.
Acknowledgments
All authors thank Charlesworth Group for English polishing.
Additional information
Funding
References
- Liu WZ, Ma ZJ, Li JR, Kang XW. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021;12(1):102. doi:10.1186/s13287-021-02153-8
- Yang B, Zhang F, Cheng F, et al. Strategies and prospects of effective neural circuits reconstruction after spinal cord injury. Cell Death Dis. 2020;11(6):439. doi:10.1038/s41419-020-2620-z
- Suzuki H, Sakai T. Current concepts of stem cell therapy for chronic spinal cord injury. Int J Mol Sci. 2021;22(14):7435. doi:10.3390/ijms22147435
- Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi:10.2147/CLEP.S68889
- Fakhri S, Abbaszadeh F, Jorjani M. On the therapeutic targets and pharmacological treatments for pain relief following spinal cord injury: a mechanistic review. Biomed Pharmacother. 2021;139:111563.
- Liu X, Zhang Y, Wang Y, Qian T. Inflammatory response to spinal cord injury and its treatment. World Neurosurg. 2021;155:19–31. doi:10.1016/j.wneu.2021.07.148
- Torregrossa F, Salli M, Grasso G. Emerging therapeutic strategies for traumatic spinal cord injury. World Neurosurg. 2020;140:591–601. doi:10.1016/j.wneu.2020.03.199
- Han T, Song P, Wu Z, et al. MSC secreted extracellular vesicles carrying TGF-beta upregulate Smad 6 expression and promote the regrowth of neurons in spinal cord injured rats. Stem Cell Rev Rep. 2021. doi:10.1007/s12015-021-10219-6
- Abbaszadeh F, Fakhri S, Khan H. Targeting apoptosis and autophagy following spinal cord injury: therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol Res. 2020;160:105069. doi:10.1016/j.phrs.2020.105069
- Lin J, Xiong Z, Gu J, et al. Sirtuins: potential therapeutic targets for defense against oxidative stress in spinal cord injury. Oxid Med Cell Longev. 2021;2021:7207692. doi:10.1155/2021/7207692
- Hachem LD, Fehlings MG. Pathophysiology of spinal cord injury. Neurosurg Clin N Am. 2021;32(3):305–313. doi:10.1016/j.nec.2021.03.002
- Piri SM, Ghodsi Z, Shool S, et al. Macrophage migration inhibitory factor as a therapeutic target after traumatic spinal cord injury: a systematic review. Eur Spine J. 2021;30(6):1474–1494. doi:10.1007/s00586-021-06718-2
- Vafaei-Nezhad S, Pour Hassan M, Noroozian M, et al. A review of low-level laser therapy for spinal cord injury: challenges and safety. J Lasers Med Sci. 2020;11(4):363–368. doi:10.34172/jlms.2020.59
- David S, Lopez-Vales R. Bioactive lipid mediators in the initiation and resolution of inflammation after spinal cord injury. Neuroscience. 2021;466:273–297. doi:10.1016/j.neuroscience.2021.04.026
- Chio JCT, Xu KJ, Popovich P, David S, Fehlings MG. Neuroimmunological therapies for treating spinal cord injury: evidence and future perspectives. Exp Neurol. 2021;341:113704. doi:10.1016/j.expneurol.2021.113704
- Takami T, Shimokawa N, Parthiban J, Zileli M, Ali S. Pharmacologic and regenerative cell therapy for spinal cord injury: WFNS spine committee recommendations. Neurospine. 2020;17(4):785–796. doi:10.14245/ns.2040408.204
- Sandean D. Management of acute spinal cord injury: a summary of the evidence pertaining to the acute management, operative and non-operative management. World J Orthop. 2020;11(12):573–583. doi:10.5312/wjo.v11.i12.573
- Canseco JA, Karamian BA, Bowles DR, et al. Updated review: the steroid controversy for management of spinal cord injury. World Neurosurg. 2021;150:1–8. doi:10.1016/j.wneu.2021.02.116
- Sultan I, Lamba N, Liew A, et al. The safety and efficacy of steroid treatment for acute spinal cord injury: a Systematic Review and meta-analysis. Heliyon. 2020;6(2):e03414. doi:10.1016/j.heliyon.2020.e03414
- Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15(3):541–553. doi:10.1007/s13311-018-0631-6
- Herbert FJ, Bharathi D, Suresh S, David E, Kumar S. Regenerative potential of Stem cell-derived extracellular vesicles in spinal cord injury (SCI). Curr Stem Cell Res Ther. 2021. doi:10.2174/1574888X16666210923113658
- Ong WK, Chakraborty S, Sugii S. Adipose tissue: understanding the heterogeneity of stem cells for regenerative medicine. Biomolecules. 2021;11(7):918. doi:10.3390/biom11070918
- Hu C, Li L. The immunoregulation of mesenchymal stem cells plays a critical role in improving the prognosis of liver transplantation. J Transl Med. 2019;17(1):412. doi:10.1186/s12967-019-02167-0
- Iwatani S, Yoshida M, Yamana K, et al. Isolation and characterization of human umbilical cord-derived mesenchymal stem cells from preterm and term infants. J Vis Exp. 2019;143:e58806.
- Fu Q, Zhang Q, Jia LY, et al. Isolation and characterization of rat mesenchymal stem cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood. Cells Tissues Organs. 2015;201(6):412–422. doi:10.1159/000445855
- Tian J, Hong Y, Zhu Q, et al. Mesenchymal stem cell enhances the function of MDSCs in experimental Sjogren syndrome. Front Immunol. 2020;11:604607. doi:10.3389/fimmu.2020.604607
- Lv X, Wang L, Zou X, Huang S. Umbilical cord mesenchymal stem cell therapy for regenerative treatment of rheumatoid arthritis: opportunities and challenges. Drug Des Devel Ther. 2021;15:3927–3936. doi:10.2147/DDDT.S323107
- Song EM, Joo YH, Choe AR, et al. Three-dimensional culture method enhances the therapeutic efficacies of tonsil-derived mesenchymal stem cells in murine chronic colitis model. Sci Rep. 2021;11(1):19589. doi:10.1038/s41598-021-98711-4
- Xiao X, Li W, Rong D, et al. Human umbilical cord mesenchymal stem cells-derived extracellular vesicles facilitate the repair of spinal cord injury via the miR-29b-3p/PTEN/Akt/mTOR axis. Cell Death Discov. 2021;7(1):212. doi:10.1038/s41420-021-00572-3
- Boido M, Ghibaudi M, Gentile P, Favaro E, Fusaro R, Tonda-Turo C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci Rep. 2019;9(1):6402. doi:10.1038/s41598-019-42848-w
- Maldonado-Lasuncion I, Verhaagen J, Oudega M. Mesenchymal stem cell-macrophage choreography supporting spinal cord repair. Neurotherapeutics. 2018;15(3):578–587. doi:10.1007/s13311-018-0629-0
- Gadani SP, Walsh JT, Lukens JR, Kipnis J. Dealing with danger in the CNS: the response of the immune system to injury. Neuron. 2015;87(1):47–62. doi:10.1016/j.neuron.2015.05.019
- Yao C, Cao X, Yu B. Revascularization after traumatic spinal cord injury. Front Physiol. 2021;12:631500. doi:10.3389/fphys.2021.631500
- Matsushita T, Lankford KL, Arroyo EJ, et al. Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp Neurol. 2015;267:152–164. doi:10.1016/j.expneurol.2015.03.001
- Xin W, Qiang S, Jianing D, et al. Human bone marrow mesenchymal stem cell-derived exosomes attenuate blood-spinal cord barrier disruption via the TIMP2/MMP pathway after acute spinal cord injury. Mol Neurobiol. 2021;58(12):6490–6504. doi:10.1007/s12035-021-02565-w
- Al Mamun A, Wu Y, Monalisa I, et al. Role of pyroptosis in spinal cord injury and its therapeutic implications. J Adv Res. 2021;28:97–109. doi:10.1016/j.jare.2020.08.004
- Fan H, Tang HB, Chen Z, et al. Inhibiting HMGB1-RAGE axis prevents pro-inflammatory macrophages/microglia polarization and affords neuroprotection after spinal cord injury. J Neuroinflammation. 2020;17(1):295. doi:10.1186/s12974-020-01973-4
- Ji H, Zhang Y, Chen C, et al. D-dopachrome tautomerase activates COX2/PGE2 pathway of astrocytes to mediate inflammation following spinal cord injury. J Neuroinflammation. 2021;18(1):130. doi:10.1186/s12974-021-02186-z
- Gaudet AD, Fonken LK. Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics. 2018;15(3):554–577.
- Takata F, Nakagawa S, Matsumoto J, Dohgu S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front Cell Neurosci. 2021;15:661838. doi:10.3389/fncel.2021.661838
- Khadka B, Lee JY, Kim KT, Bae JS. Recent progress in therapeutic drug delivery systems for treatment of traumatic CNS injuries. Future Med Chem. 2020;12(19):1759–1778. doi:10.4155/fmc-2020-0178
- Dolma S, Kumar H. Neutrophil, extracellular matrix components, and their interlinked action in promoting secondary pathogenesis after spinal cord injury. Mol Neurobiol. 2021;58(9):4652–4665. doi:10.1007/s12035-021-02443-5
- Zivkovic S, Ayazi M, Hammel G, Ren Y. For better or for worse: a look into neutrophils in traumatic spinal cord injury. Front Cell Neurosci. 2021;15:648076. doi:10.3389/fncel.2021.648076
- Hassanzadeh S, Jalessi M, Jameie SB, et al. More attention on glial cells to have better recovery after spinal cord injury. Biochem Biophys Rep. 2021;25:100905. doi:10.1016/j.bbrep.2020.100905
- Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129(Pt 12):3249–3269. doi:10.1093/brain/awl296
- Ma SF, Chen YJ, Zhang JX, et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–170. doi:10.1016/j.bbi.2014.11.007
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133(2):433–447. doi:10.1093/brain/awp322
- Anjum A, Yazid MD, Fauzi Daud M, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21(20):7533. doi:10.3390/ijms21207533
- Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflamm. 2016;2016:1–21. doi:10.1155/2016/9476020
- Kiran S, Dwivedi P, Kumar V, Price RL, Singh UP. Immunomodulation and biomaterials: key players to repair volumetric muscle loss. Cells. 2021;10(8):2016. doi:10.3390/cells10082016
- Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi:10.1016/j.brainres.2014.12.045
- Nosbaum A, Prevel N, Truong HA, et al. Cutting edge: regulatory T cells facilitate cutaneous wound healing. J Immunol. 2016;196(5):2010–2014. doi:10.4049/jimmunol.1502139
- Deonarine K, Panelli MC, Stashower ME, et al. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5(1):11. doi:10.1186/1479-5876-5-11
- Nakazaki M, Morita T, Lankford KL, Askenase PW, Kocsis JD. Small extracellular vesicles released by infused mesenchymal stromal cells target M2 macrophages and promote TGF-beta upregulation, microvascular stabilization and functional recovery in a rodent model of severe spinal cord injury. J Extracell Vesicles. 2021;10(11):e12137. doi:10.1002/jev2.12137
- Amo-Aparicio J, Garcia-Garcia J, Puigdomenech M, et al. Inhibition of the NLRP3 inflammasome by OLT1177 induces functional protection and myelin preservation after spinal cord injury. Exp Neurol. 2021;347:113889. doi:10.1016/j.expneurol.2021.113889
- Chio JCT, Wang J, Surendran V, et al. Delayed administration of high dose human immunoglobulin G enhances recovery after traumatic cervical spinal cord injury by modulation of neuroinflammation and protection of the blood spinal cord barrier. Neurobiol Dis. 2021;148:105187. doi:10.1016/j.nbd.2020.105187
- Gaire BP, Choi JW. Critical roles of lysophospholipid receptors in activation of neuroglia and their neuroinflammatory responses. Int J Mol Sci. 2021;22(15):7864. doi:10.3390/ijms22157864
- Francos-Quijorna I, Santos-Nogueira E, Gronert K, et al. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J Neurosci. 2017;37(48):11731–11743. doi:10.1523/JNEUROSCI.1395-17.2017
- Fan R, Zhang Y, Botchway BOA, Liu X. Resveratrol can attenuate astrocyte activation to treat spinal cord injury by inhibiting inflammatory responses. Mol Neurobiol. 2021;58(11):5799–5813. doi:10.1007/s12035-021-02509-4
- Zhang H, Younsi A, Zheng G, et al. Sonic Hedgehog modulates the inflammatory response and improves functional recovery after spinal cord injury in a thoracic contusion-compression model. Eur Spine J. 2021;30(6):1509–1520. doi:10.1007/s00586-021-06796-2
- Brockie S, Hong J, Fehlings MG. The role of microglia in modulating neuroinflammation after spinal cord injury. Int J Mol Sci. 2021;22(18):9706. doi:10.3390/ijms22189706
- Gaojian T, Dingfei Q, Linwei L, et al. Parthenolide promotes the repair of spinal cord injury by modulating M1/M2 polarization via the NF-κB and STAT 1/3 signaling pathway. Cell Death Discovery. 2020;6(1):97. doi:10.1038/s41420-020-00333-8
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. doi:10.1523/JNEUROSCI.3257-09.2009
- Akhmetzyanova E, Kletenkov K, Mukhamedshina Y, Rizvanov A. Different approaches to modulation of microglia phenotypes after spinal cord injury. Front Syst Neurosci. 2019;13:37. doi:10.3389/fnsys.2019.00037
- Milich LM, Ryan CB, Lee JK. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 2019;137(5):785–797. doi:10.1007/s00401-019-01992-3
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi:10.1038/nri2448
- Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–238. doi:10.1038/ni.1990
- Guo J, Wang H, Li L, Yuan Y, Shi X, Hou S. Treatment with IL-19 improves locomotor functional recovery after contusion trauma to the spinal cord. Br J Pharmacol. 2018;175(13):2611–2621. doi:10.1111/bph.14193
- Ghosh M, Xu Y, Pearse DD. Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J Neuroinflammation. 2016;13(1):9. doi:10.1186/s12974-015-0463-9
- Li M, Xu J, Mei X, et al. Regulatory effects of dermal papillary pluripotent stem cells on polarization of macrophages from M1 to M2 phenotype in vitro. Transpl Immunol. 2019;52:57–67. doi:10.1016/j.trim.2018.11.003
- Duan K, Liu S, Yi Z, et al. S100-beta aggravates spinal cord injury via activation of M1 macrophage phenotype. J Musculoskelet Neuronal Interact. 2021;21(3):401–412.
- Hart CG, Karimi-Abdolrezaee S. Recent insights on astrocyte mechanisms in CNS homeostasis, pathology, and repair. J Neurosci Res. 2021;99(10):2427–2462. doi:10.1002/jnr.24922
- Ahmed A, Patil AA, Agrawal DK. Immunobiology of spinal cord injuries and potential therapeutic approaches. Mol Cell Biochem. 2018;441(1–2):181–189. doi:10.1007/s11010-017-3184-9
- Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. doi:10.3389/fimmu.2019.00085
- Tang R, Botchway BOA, Meng Y, et al. The inhibition of inflammatory signaling pathway by secretory leukocyte protease inhibitor can improve spinal cord injury. Cell Mol Neurobiol. 2020;40(7):1067–1073. doi:10.1007/s10571-020-00799-1
- Xu L, Botchway BOA, Zhang S, Zhou J, Liu X. Inhibition of NF-κB signaling pathway by resveratrol improves spinal cord injury. Front Neurosci. 2018;12:690. doi:10.3389/fnins.2018.00690
- Brambilla R, Bracchi-Ricard V, Hu W-H, et al. Inhibition of astroglial nuclear factor κB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202(1):145–156. doi:10.1084/jem.20041918
- Brambilla R, Hurtado A, Persaud T, et al. Transgenic inhibition of astroglial NF-κB leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009;110(2):765–778. doi:10.1111/j.1471-4159.2009.06190.x
- Khorooshi R, Babcock AA, Owens T. NF-κB-driven STAT2 and CCL2 expression in astrocytes in response to brain injury. J Immunol. 2008;181(10):7284–7291. doi:10.4049/jimmunol.181.10.7284
- Yuan J, Liu W, Zhu H, et al. Curcumin inhibits glial scar formation by suppressing astrocyte-induced inflammation and fibrosis in vitro and in vivo. Brain Res. 2017;1655:90–103. doi:10.1016/j.brainres.2016.11.002
- Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi:10.1038/nature17623
- Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32(18):6391–6410. doi:10.1523/JNEUROSCI.6221-11.2012
- Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24(3):312–325. doi:10.1038/s41593-020-00783-4
- Li X, Li M, Tian L, Chen J, Liu R, Ning B. Reactive astrogliosis: implications in spinal cord injury progression and therapy. Oxid Med Cell Longev. 2020;2020:9494352. doi:10.1155/2020/9494352
- Zou HJ, Guo SW, Zhu L, Xu X, Liu JB. Methylprednisolone induces neuro-protective effects via the inhibition of A1 astrocyte activation in traumatic spinal cord injury mouse models. Front Neurosci. 2021;15:628917. doi:10.3389/fnins.2021.628917
- Lana D, Ugolini F, Nosi D, Wenk GL, Giovannini MG. The emerging role of the interplay among astrocytes, microglia, and neurons in the hippocampus in health and disease. Front Aging Neurosci. 2021;13:651973. doi:10.3389/fnagi.2021.651973
- Kisucka A, Bimbova K, Bacova M, Galik J, Lukacova N. Activation of neuroprotective microglia and astrocytes at the lesion site and in the adjacent segments is crucial for spontaneous locomotor recovery after spinal cord injury. Cells. 2021;10(8):1943. doi:10.3390/cells10081943
- Liu LR, Liu JC, Bao JS, Bai QQ, Wang GQ. Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. 2020;11:1024. doi:10.3389/fimmu.2020.01024
- Liddelow SA, Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46(6):957–967. doi:10.1016/j.immuni.2017.06.006
- Boghdadi AG, Teo L, Bourne JA. The neuroprotective role of reactive astrocytes after central nervous system injury. J Neurotrauma. 2020;37(5):681–691. doi:10.1089/neu.2019.6938
- Xu L, Ye X, Wang Q, et al. T‐cell infiltration, contribution and regulation in the central nervous system post‐traumatic injury. Cell Prolif. 2021;54(8):e13092. doi:10.1111/cpr.13092
- Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2(4):34. doi:10.1186/scrt75
- Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine. 2015;72(2):146–153. doi:10.1016/j.cyto.2014.12.027
- Monahan R, Stein A, Gibbs K, Bank M, Bloom O. Circulating T cell subsets are altered in individuals with chronic spinal cord injury. Immunol Res. 2015;63(1–3):3–10. doi:10.1007/s12026-015-8698-1
- Hu JG, Shi LL, Chen YJ, et al. Differential effects of myelin basic protein-activated Th1 and Th2 cells on the local immune microenvironment of injured spinal cord. Exp Neurol. 2016;277:190–201. doi:10.1016/j.expneurol.2016.01.002
- Chaudhry SR, Kahlert UD, Kinfe TM, et al. Differential polarization and activation dynamics of systemic T helper cell subsets after aneurysmal subarachnoid hemorrhage (SAH) and during post-SAH complications. Sci Rep. 2021;11(1):14226.
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi:10.1016/S0092-8674(00)80702-3
- Butcher MJ, Zhu J. Recent advances in understanding the Th1/Th2 effector choice. Fac Rev. 2021;10:30. doi:10.12703/r/10-30
- Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi:10.1016/j.cyto.2014.09.011
- Rahimi K, Ahmadi A, Hassanzadeh K, et al. Targeting the balance of T helper cell responses by curcumin in inflammatory and autoimmune states. Autoimmun Rev. 2019;18(7):738–748. doi:10.1016/j.autrev.2019.05.012
- Hendrix S, Nitsch R. The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol. 2007;184(1–2):100–112. doi:10.1016/j.jneuroim.2006.11.019
- Qu N, Xu M, Mizoguchi I, et al. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin Dev Immunol. 2013;2013:968549. doi:10.1155/2013/968549
- Liu D, Liu B, Lin C, Gu J. Imbalance of peripheral lymphocyte subsets in patients with ankylosing spondylitis: a meta-analysis. Front Immunol. 2021;12:696973. doi:10.3389/fimmu.2021.696973
- Tuzlak S, Dejean AS, Iannacone M, et al. Repositioning TH cell polarization from single cytokines to complex help. Nat Immunol. 2021;22(10):1210–1217. doi:10.1038/s41590-021-01009-w
- Carvalheiro T, Rafael-Vidal C, Malvar-Fernandez B, et al. Semaphorin4A-plexin D1 axis induces Th2 and Th17 while represses Th1 skewing in an autocrine manner. Int J Mol Sci. 2020;21(18):6965. doi:10.3390/ijms21186965
- Lin W, Chen W, Liu W, Xu Z, Zhang L. Sirtuin4 suppresses the anti-neuroinflammatory activity of infiltrating regulatory T cells in the traumatically injured spinal cord. Immunology. 2019;158(4):362–374. doi:10.1111/imm.13123
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi:10.1038/nri2785
- Chen J, Chen YQ, Shi YJ, et al. VX-765 reduces neuroinflammation after spinal cord injury in mice. Neural Regen Res. 2021;16(9):1836–1847. doi:10.4103/1673-5374.306096
- Zhou Z, Tian X, Mo B, et al. Adipose mesenchymal stem cell transplantation alleviates spinal cord injury-induced neuroinflammation partly by suppressing the Jagged1/Notch pathway. Stem Cell Res Ther. 2020;11(1):212. doi:10.1186/s13287-020-01724-5
- Urdzíková L, Růžička J, LaBagnara M, et al. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int J Mol Sci. 2014;15(7):11275–11293. doi:10.3390/ijms150711275
- Tsumuraya T, Ohtaki H, Song D, et al. Human mesenchymal stem/stromal cells suppress spinal inflammation in mice with contribution of pituitary adenylate cyclase-activating polypeptide (PACAP). J Neuroinflammation. 2015;12:35. doi:10.1186/s12974-015-0252-5
- Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29(8):1614–1625. doi:10.1089/neu.2011.2109
- Maeda Y, Otsuka T, Takeda M, et al. Transplantation of rat cranial bone-derived mesenchymal stem cells promotes functional recovery in rats with spinal cord injury. Sci Rep. 2021;11(1):21907. doi:10.1038/s41598-021-01490-1
- Bao CS, Li XL, Liu L, Wang B, Yang FB, Chen LG. Transplantation of Human umbilical cord mesenchymal stem cells promotes functional recovery after spinal cord injury by blocking the expression of IL-7. Eur Rev Med Pharmacol Sci. 2018;22(19):6436–6447. doi:10.26355/eurrev_201810_16056
- Papa S, Vismara I, Mariani A, et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J Control Release. 2018;278:49–56. doi:10.1016/j.jconrel.2018.03.034
- Matsubara K, Matsushita Y, Sakai K, et al. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity. J Neurosci. 2015;35(6):2452–2464. doi:10.1523/JNEUROSCI.4088-14.2015
- Fu Q, Liu Y, Liu X, et al. Engrafted peripheral blood-derived mesenchymal stem cells promote locomotive recovery in adult rats after spinal cord injury. Am J Transl Res. 2017;9(9):3950–3966.
- Nitzsche F, Muller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35(6):1446–1460. doi:10.1002/stem.2614
- Ferrini E, Stellari FF, Franceschi V, et al. Persistency of mesenchymal stromal/stem cells in lungs. Front Cell Dev Biol. 2021;9:709225. doi:10.3389/fcell.2021.709225
- Markov A, Thangavelu L, Aravindhan S, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021;12(1):192. doi:10.1186/s13287-021-02265-1
- Hassanshahi G, Roohi MA, Esmaeili SA, Pourghadamyari H, Nosratabadi R. Involvement of various chemokine/chemokine receptor axes in trafficking and oriented locomotion of mesenchymal stem cells in multiple sclerosis patients. Cytokine. 2021;148:155706. doi:10.1016/j.cyto.2021.155706
- Li Q, Lian Y, Deng Y, et al. mRNA-engineered mesenchymal stromal cells expressing CXCR2 enhances cell migration and improves recovery in IBD. Mol Ther Nucleic Acids. 2021;26:222–236. doi:10.1016/j.omtn.2021.07.009
- Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal stem cells for neurological disorders. Adv Sci. 2021;8(7):2002944. doi:10.1002/advs.202002944
- Johnson LDV, Pickard MR, Johnson WEB. The comparative effects of mesenchymal stem cell transplantation therapy for spinal cord injury in humans and animal models: a systematic review and meta-analysis. Biology. 2021;10(3):230. doi:10.3390/biology10030230
- Lindsay SL, Barnett SC. Therapeutic potential of niche-specific mesenchymal stromal cells for spinal cord injury repair. Cells. 2021;10(4):901. doi:10.3390/cells10040901
- Silva-Carvalho AE, Cardoso MH, Alencar-Silva T, et al. Dissecting the relationship between antimicrobial peptides and mesenchymal stem cells. Pharmacol Ther. 2021:108021. doi:10.1016/j.pharmthera.2021.108021
- Miceli V, Bulati M, Iannolo G, Zito G, Gallo A, Conaldi PG. Therapeutic properties of mesenchymal stromal/stem cells: the need of cell priming for cell-free therapies in regenerative medicine. Int J Mol Sci. 2021;22(2):763. doi:10.3390/ijms22020763
- Lavoie JR, Rosu-Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie. 2013;95(12):2212–2221. doi:10.1016/j.biochi.2013.06.017
- Sykova E, Cizkova D, Kubinova S. Mesenchymal stem cells in treatment of spinal cord injury and amyotrophic lateral sclerosis. Front Cell Dev Biol. 2021;9:695900. doi:10.3389/fcell.2021.695900
- Lv B, Zhang X, Yuan J, et al. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res Ther. 2021;12(1):36. doi:10.1186/s13287-020-02090-y
- Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–2185. doi:10.1002/sctm.17-0129
- Antonios JP, Farah GJ, Cleary DR, Martin JR, Ciacci JD, Pham MH. Immunosuppressive mechanisms for stem cell transplant survival in spinal cord injury. Neurosurg Focus. 2019;46(3):E9. doi:10.3171/2018.12.FOCUS18589
- Chen D, Zeng W, Fu Y, Gao M, Lv G. Bone marrow mesenchymal stem cells combined with minocycline improve spinal cord injury in a rat model. Int J Clin Exp Pathol. 2015;8(10):11957–11969.
- An N, Yang J, Wang H, et al. Mechanism of mesenchymal stem cells in spinal cord injury repair through macrophage polarization. Cell Biosci. 2021;11(1):41. doi:10.1186/s13578-021-00554-z
- Yang R, Gao H, Chen L, et al. Effect of peripheral blood-derived mesenchymal stem cells on macrophage polarization and Th17/Treg balance in vitro. Regen Ther. 2020;14:275–283. doi:10.1016/j.reth.2020.03.008
- Eggenhofer E, Hoogduijn MJ. Mesenchymal stem cell-educated macrophages. Transplant Res. 2012;1(1):12. doi:10.1186/2047-1440-1-12
- Cho DI, Kim MR, Jeong HY, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi:10.1038/emm.2013.135
- Selleri S, Bifsha P, Civini S, et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7(21):30193–30210. doi:10.18632/oncotarget.8623
- Takafuji Y, Hori M, Mizuno T, Harada-Shiba M. Humoral factors secreted from adipose tissue-derived mesenchymal stem cells ameliorate atherosclerosis in Ldlr-/- mice. Cardiovasc Res. 2019;115(6):1041–1051. doi:10.1093/cvr/cvy271
- Sun G, Li G, Li D, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. doi:10.1016/j.msec.2018.04.006
- Zhai X, Chen K, Yang H, et al. Extracellular vesicles derived from CD73 modified human umbilical cord mesenchymal stem cells ameliorate inflammation after spinal cord injury. J Nanobiotechnology. 2021;19(1):274. doi:10.1186/s12951-021-01022-z
- Lee JR, Kyung JW, Kumar H, et al. Targeted delivery of mesenchymal stem cell-derived nanovesicles for spinal cord injury treatment. Int J Mol Sci. 2020;21(11):4185. doi:10.3390/ijms21114185
- Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31(10):2042–2046. doi:10.1002/stem.1400
- Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi:10.1038/nm.1905
- Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–195. doi:10.1038/mt.2011.189
- Song X, Xie S, Lu K, Wang C. Mesenchymal stem cells alleviate experimental asthma by inducing polarization of alveolar macrophages. Inflammation. 2015;38(2):485–492. doi:10.1007/s10753-014-9954-6
- Braza F, Dirou S, Forest V, et al. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016;34(7):1836–1845. doi:10.1002/stem.2344
- Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica. 2013;98(6):888–895. doi:10.3324/haematol.2012.078055
- Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114(1):220–229. doi:10.1002/jcb.24357
- Maldonado-Lasuncion I, Haggerty AE, Okuda A, et al. The effect of inflammatory priming on the therapeutic potential of mesenchymal stromal cells for spinal cord repair. Cells. 2021;10(6):1316. doi:10.3390/cells10061316
- Chen L, Zhang Q, Chen QH, et al. Combination of G-CSF and AMD3100 improves the anti-inflammatory effect of mesenchymal stem cells on inducing M2 polarization of macrophages through NF-kappaB-IL1RA signaling pathway. Front Pharmacol. 2019;10:579. doi:10.3389/fphar.2019.00579
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118(2):330–338. doi:10.1182/blood-2010-12-327353
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi:10.1172/JCI59643
- Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356(6337):513–519. doi:10.1126/science.aal3535
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi:10.1146/annurev-immunol-031210-101312
- Qian D, Li L, Rong Y, et al. Blocking Notch signal pathway suppresses the activation of neurotoxic A1 astrocytes after spinal cord injury. Cell Cycle. 2019;18(21):3010–3029. doi:10.1080/15384101.2019.1667189
- Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi:10.1038/nature21029
- Jiang D, Gong F, Ge X, et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology. 2020;18(1):105. doi:10.1186/s12951-020-00665-8
- Schäfer S, Calas A-G, Vergouts M, Hermans E. Immunomodulatory influence of bone marrow-derived mesenchymal stem cells on neuroinflammation in astrocyte cultures. J Neuroimmunol. 2012;249(1–2):40–48. doi:10.1016/j.jneuroim.2012.04.018
- Wang S, Jia Y, Cao X, et al. HUCMSCs transplantation combined with ultrashort wave therapy attenuates neuroinflammation in spinal cord injury through NUR77/ NF-kappaB pathway. Life Sci. 2021;267:118958. doi:10.1016/j.lfs.2020.118958
- Wang L, Pei S, Han L, et al. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFkappaB P65 subunit in spinal cord injury. Cell Physiol Biochem. 2018;50(4):1535–1559. doi:10.1159/000494652
- Ceyzeriat K, Abjean L, Carrillo-de Sauvage MA, Ben Haim L, Escartin C. The complex STATes of astrocyte reactivity: how are they controlled by the JAK-STAT3 pathway? Neuroscience. 2016;330:205–218. doi:10.1016/j.neuroscience.2016.05.043
- Gu Y, He M, Zhou X, et al. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by suppressing apoptosis in astrocyte. Sci Rep. 2016;6:18587. doi:10.1038/srep18587
- Norden DM, Fenn AM, Dugan A, Godbout JP. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62(6):881–895. doi:10.1002/glia.22647
- Luo K. Signaling cross talk between TGF-beta/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9(1):a022137. doi:10.1101/cshperspect.a022137
- Liu R, Wang W, Wang S, Xie W, Li H, Ning B. microRNA-21 regulates astrocytic reaction post-acute phase of spinal cord injury through modulating TGF-beta signaling. Aging. 2018;10(6):1474–1488. doi:10.18632/aging.101484
- Li T, Liu T, Chen X, et al. Microglia induce the transformation of A1/A2 reactive astrocytes via the CXCR7/PI3K/Akt pathway in chronic post-surgical pain. J Neuroinflammation. 2020;17(1):211. doi:10.1186/s12974-020-01891-5
- Liu F, Qiu H, Xue M, et al. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019;10(1):345. doi:10.1186/s13287-019-1447-y
- Li X, Dong Y, Yin H, Qi Z, Wang D, Ren S. Mesenchymal stem cells induced regulatory dendritic cells from hemopoietic progenitor cells through Notch pathway and TGF-beta synergistically. Immunol Lett. 2020;222:49–57. doi:10.1016/j.imlet.2020.03.005
- Luz-Crawford P, Kurte M, Bravo-Alegria J, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4(3):65. doi:10.1186/scrt216
- Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019;10:1191. doi:10.3389/fimmu.2019.01191
- Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1–2):47–54. doi:10.1016/j.imlet.2012.06.001
- Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–1244. doi:10.1089/scd.2013.0479
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi:10.1182/blood-2003-11-3909
- Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. doi:10.1038/ni.3002
- Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi:10.1182/blood-2006-02-002246
- Bouffi C, Bony C, Courties G, Jorgensen C, Noel D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5(12):e14247. doi:10.1371/journal.pone.0014247
- Matysiak M, Orlowski W, Fortak-Michalska M, Jurewicz A, Selmaj K. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J Neuroimmunol. 2011;233(1–2):106–111. doi:10.1016/j.jneuroim.2010.12.004
- Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. doi:10.1634/stemcells.2007-0554
- Wang K, Shi YJ, Song ZL, et al. Regulatory effect of rat bone marrow mesenchymal stem cells on Treg/Th17 immune balance in vitro. Mol Med Rep. 2020;21(5):2123–2130. doi:10.3892/mmr.2020.11019
- Melief SM, Schrama E, Brugman MH, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31(9):1980–1991. doi:10.1002/stem.1432
- Lee HJ, Kim SN, Jeon MS, Yi T, Song SU. ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Sci Rep. 2017;7:44486. doi:10.1038/srep44486
- Martinez AM, Goulart CO, Ramalho Bdos S, Oliveira JT, Almeida FM. Neurotrauma and mesenchymal stem cells treatment: from experimental studies to clinical trials. World J Stem Cells. 2014;6(2):179–194. doi:10.4252/wjsc.v6.i2.179
- Zhilai Z, Biling M, Sujun Q, et al. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016;1642:426–435. doi:10.1016/j.brainres.2016.04.025
- Mammana S, Gugliandolo A, Cavalli E, et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J Tissue Eng Regen Med. 2019;13(7):1109–1121. doi:10.1002/term.2857
- Wang W, Huang X, Lin W, et al. Hypoxic preconditioned bone mesenchymal stem cells ameliorate spinal cord injury in rats via improved survival and migration. Int J Mol Med. 2018;42(5):2538–2550. doi:10.3892/ijmm.2018.3810
- Noori L, Arabzadeh S, Mohamadi Y, et al. Intrathecal administration of the extracellular vesicles derived from human Wharton’s jelly stem cells inhibit inflammation and attenuate the activity of inflammasome complexes after spinal cord injury in rats. Neurosci Res. 2021;170:87–98. doi:10.1016/j.neures.2020.07.011
- Liu W, Rong Y, Wang J, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17(1):47. doi:10.1186/s12974-020-1726-7
- Lankford KL, Arroyo EJ, Nazimek K, Bryniarski K, Askenase PW, Kocsis JD. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018;13(1):e0190358. doi:10.1371/journal.pone.0190358
- Fan L, Dong J, He X, Zhang C, Zhang T. Bone marrow mesenchymal stem cells-derived exosomes reduce apoptosis and inflammatory response during spinal cord injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway. Hum Exp Toxicol. 2021;40(10):1612–1623. doi:10.1177/09603271211003311
- Shang AJ, Hong SQ, Xu Q, et al. NT-3-secreting human umbilical cord mesenchymal stromal cell transplantation for the treatment of acute spinal cord injury in rats. Brain Res. 2011;1391:102–113. doi:10.1016/j.brainres.2011.03.019
- Li G, Che MT, Zeng X, et al. Neurotrophin-3 released from implant of tissue-engineered fibroin scaffolds inhibits inflammation, enhances nerve fiber regeneration, and improves motor function in canine spinal cord injury. J Biomed Mater Res A. 2018;106(8):2158–2170. doi:10.1002/jbm.a.36414
- Allahdadi KJ, De santana TA, Santos GC, et al. IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res Ther. 2019;10(1):146. doi:10.1186/s13287-019-1223-z
- Liu W, Wang Y, Gong F, et al. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36(3):469–484. doi:10.1089/neu.2018.5835
- Li Z, Wang Q, Hu H, Zheng W, Gao C. Research advances of biomaterials-based microenvironment-regulation therapies for repair and regeneration of spinal cord injury. Biomed Mater. 2021;16(5):052002. doi:10.1088/1748-605X/ac1d3c
- Ha XQ, Yang B, Hou HJ, Cai XL, Xiong WY, Wei XP. Protective effect of rhodioloside and bone marrow mesenchymal stem cells infected with HIF-1-expressing adenovirus on acute spinal cord injury. Neural Regen Res. 2020;15(4):690–696. doi:10.4103/1673-5374.266920
- Amiri F, Jahanian-Najafabadi A, Roudkenar MH. In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments: in vitro augmentation of mesenchymal stem cells viability. Cell Stress Chaperones. 2015;20(2):237–251. doi:10.1007/s12192-014-0560-1
- Yang Y, Lee EH, Yang Z. Hypoxia conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng Part B Rev. 2021. doi:10.1089/ten.TEB.2021.0145
- Philipp D, Suhr L, Wahlers T, Choi YH, Paunel-Gorgulu A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 2018;9(1):286. doi:10.1186/s13287-018-1039-2
- Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–569. doi:10.1038/nature06306
- Guo H, Zhao N, Gao H, He X. Mesenchymal stem cells overexpressing interleukin-35 propagate immunosuppressive effects in mice. Scand J Immunol. 2017;86(5):389–395. doi:10.1111/sji.12613
- Dooley D, Lemmens E, Vangansewinkel T, et al. Cell-based delivery of interleukin-13 directs alternative activation of macrophages resulting in improved functional outcome after spinal cord injury. Stem Cell Reports. 2016;7(6):1099–1115. doi:10.1016/j.stemcr.2016.11.005
