Abstract
Background
Nonalcoholic steatohepatitis (NASH) is considered to be part of the nonalcoholic fatty liver disorders and its incidence is increasing. Imm124-E (Immuron Ltd, Melbourne, Australia), containing hyperimmune bovine colostrum, has been shown to exert an immunomodulatory effect and to alleviate target organ damage in animal models of NASH. The aim of our study was to determine the safety and efficacy of oral administration of Imm124-E to patients with insulin resistance and NASH.
Methods
In an open-label trial, ten patients with biopsy-proven NASH and insulin resistance were orally treated with Imm124-E for 30 days.
Results
Oral administration of Imm124-E was safe, and no side effects were noted. Alleviation of insulin resistance was reflected by significantly improved hemoglobin A1c (HbA1c) values in all ten treated patients. For between five and eight responders, the following effects were noted: a decrease in fasting glucose levels; improved oral glucose tolerance test (OGGT) and homeostatic model assessment insulin resistance (HOMA) scores; and alleviation in lipid profile. These effects were accompanied by increased serum levels of glucagon-like peptide 1 (GLP-1), adiponectin and T regulatory cells.
Conclusion
Hyperimmune colostrum alleviates NASH.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common of all liver disorders. Citation1–Citation5 The spectrum of histological abnormalities defined by NAFLD includes simple steatosis and nonalcoholic steatohepatitis (NASH), as its more extreme form.Citation6 NASH has emerged as the major cause of chronic liver disease in developed countries.Citation7 The high prevalence of these conditions is expected to increase concurrent with the epidemics of obesity and type 2 diabetes mellitus, which are the major risk factors for NAFLD and NASH.Citation8 The pathogenesis of NASH is unknown; however, the hypothesis that NASH is associated with peripheral insulin resistance has been intensively explored.Citation9
Indeed, patients with NASH and fatty liver were found to have higher serum insulin levels than normal individuals.Citation9,Citation10 No therapy has been proven effective for patients with NASH.
Bovine colostrum (BC) is the milk of lactating mammals that is secreted during the first 72 hours following birth. BC differs from regular milk and contains abundant bioactive components – including growth factors, immunoglobulins, lactoperoxidase, lysozyme, lactoferrin, nucleosides, vitamins, peptides, and oligosaccharides – that are of increasing relevance to human health.Citation11,Citation12 The value of BC as a biological medicine was well documented in several clinical trials.Citation13 Colostrum has been shown to balance blood sugar levels, and a suggested mechanism for this is associated with its high content of insulin-like growth factor (IGF-1), which can stimulate glucose utilization and may be beneficial for the treatment of diabetic patients. But the main actions of BC include antibacterial effects and a modulation of the immune response. The ability of BC to neutralize lipopolysaccharides (LPS) and to inhibit enteropathogenic endotoxemia in animal models was investigated.Citation13 Oral administration of BC was shown to reduce the influx of LPS from the gut and this appears to be a major mechanism underlying BC’s therapeutic effect.Citation13
Immunoglobulins are the main immune components of the acquired immune system presented in colostrum. IgG is the major immunoglobulin class present in ruminant milk, in contrast to IgA being the major immunoglobulin present in human milk.Citation13,Citation14 The immunological activity of bovine IgG in milk from cows immunized against human pathogens is similar to that of IgG in human milk, demonstrating the benefit of hyperimmune bovine milk in the human diet.Citation15,Citation16 Skim milk from cows immunized with human enteropathogenic microorganisms provides protection on cholesterol metabolism that has been demonstrated in rodents and in clinical trials.Citation17–Citation19
Regulatory T cells (Tregs) are essential in controlling a variety of immune responses. These responses involve some inflammation pathways that are well accepted as key elements in obesity-associated insulin resistance. Recently, the impact of Tregs on the glucose homeostasis in mice with diet-induced obesity was explored. CD4+ T lymphocytes resident in visceral adipose tissue were shown to control insulin resistance in mice with diet-induced obesity, and analyses of human tissue suggested that a similar process may also occur in humans.Citation20 Another study reported that CD4+ Foxp3+ cells with a unique phenotype were highly enriched in the abdominal fat of normal mice, but their numbers were strikingly reduced in insulin-resistant models of obesity.Citation21
A study that was conducted in our laboratory showed that induction of NKT cells by hyperimmune BC (enriched with anti-LPS antibodies) had an ameliorating effect in ob/ob mice.Citation22
The aim of the present pilot study was to determine the safety of oral administration of Imm124-E, an anti-LPS hyperimmune BC, in humans; and to assess its immunomodulatory effects on Tregs, glucose homeostasis, various serum cytokines, and lipid profile, in NASH patients.
Although the medical evidence of a benefit in this small study is limited, our data show that Imm124-E is safe and was able to improve glycemic control. This beneficial effect was accompanied by improvements of insulin resistance and hyperlipidemia, and changes in the level of cytokines and several subsets of Tregs.
These data suggest a commercially useful procedure for preparing hyperimmune BC containing high levels of anti-LPS antibodies, that could allow production of a safe treatment for NASH.
Methods
Patient population
This was an open-label and single-center trial that was conducted in patients with type 2 diabetes and NASH. The study was performed in accordance with the guidelines of the Hebrew University-Hadassah Institutional Committee for Human Clinical Trials and with the approval of the Israel Ministry of Health Committee for Human Trials. All patients provided written informed consent before the study.
Inclusion criteria
Ten patients were enrolled in the open-label trial. Participants (men and women between 18 and 60 years old) were evaluated for eligibility after they signed a written informed-consent form. The diagnosis of NASH was based on a liver biopsy score of 4 or above and altered glucose metabolism, including diabetes (nontreated or treated with up to two drugs without any change in medication during the two months prior to enrollment), impaired fasting glucose or impaired glucose tolerance, and hemoglobin A1c (HbA1c) between 5.5% and 14%. Impaired fasting glucose was defined as >100 mg/dL. Impaired glucose tolerance was defined as a blood sugar level > 140 mg/dL 2 hours post–glucose load and an HbA1c between 5.5% and 8%. No evidence of other viral or immunemediated liver disease was present.
Exclusion criteria
Patients meeting any of the following criteria were excluded: active coinfection with hepatitis A, B, or C viruses; the presence of human immunodeficiency virus (HIV) infection, hepatocellular carcinoma, fulminant liver failure, severe deteriorating synthetic liver functions, or a clinically significant infectious, immune-mediated or malignant disease; any history of treatment with immunomodulatory drugs, including steroids and nonsteroidal anti-inflammatory drugs (NSAID), at any time within the previous four weeks; a history of coagulopathy; women with childbearing potential unless surgically sterile or using adequate contraception (ie, IUD, oral or Depo-Provera contraceptive or barrier plus spermicidal); anemia (Hb < 10.5 gm/dL), thrombocytopenia (platelets < 100 K/μL) or lymphopenia (absolute lymphocyte count < 0.7 ×109/L); or allergy to cow milk or lactose intolerant.
Therapy and laboratory follow up
After complete medical evaluation and liver biopsy, patients who qualified for therapy were given Imm124-E (Immuron Ltd, Melbourne, Australia), an anti-LPS hyperimmune BC, in a dose of 600 mg three times daily (total of 1800 mg/day) for 30 days. Patients were followed for 60 days through regular weekly scheduled visits that included physical examination, ongoing medical history review, and laboratory tests. Safety was assessed by monitoring the patients for adverse events. Blood was drawn at each visit for determination of complete blood counts (CBC), sedimentation rate (ESR), and standard chemistries, including liver enzymes, international normalized ratio (INR), lipid profile, C-reactive protein (CRP), HbA1c, and serum insulin level. All patients underwent a repeat oral glucose tolerance test (OGTT) and a homeostatic model of assessment insulin resistance (HOMA) score evaluation at the end of the study.
Primary endpoint
The safety of oral administration of Imm124-E was the primary endpoint.
Secondary endpoints
The secondary endpoints included reduction of the liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transpeptidase (γ-GT) by 10%, improvement in insulin sensitivity (Matsuda index from oral glucose tolerance assessment), and improved lipid profile.
The levels of liver enzymes (AST and ALT, alkaline phosphatase [AP] and γ-GT), serum fasting glucose, insulin, and plasma lipids (cholesterol and triglycerides) were determined using standard methods.
Colostrum collection, processing and preparation
Imm124-E was prepared from cows immunized with a vaccine containing an extract of the surface antigens from the most common varieties of enterotoxigenic E. coli. To prepare each batch of Imm124-E powder, colostrum was collected appropriately from immunized cows and was frozen in individual bags for testing. For processing, colostrum was thawed and pooled, and the fat was removed. Each batch was pasteurized. Colostrum was then concentrated by ultrafiltration to reduce the volume before freeze-drying. The ultrafiltration step reduced lactose in the final powder to less than 7%, compared with about 50% in skim milk powder. Colostrum was manufactured and then tested by an accredited testing lab (Dairy Technical Services, Kensingtone, VIC, Australia) against specifications for protein, moisture, lactose, fat, antibiotics, and microbiology parameters.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) from blood samples collected at day 0 and day 30 were isolated using a Ficoll-Hypaque gradient. Cells were resuspended in Phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA). For surface staining, PBMCs were incubated with either fluorochrome-conjugated antibodies against the indicated cell surface markers at the recommended dilution or with isotype control antibodies, for 30 minutes at 4°C. We used the following cell surface antibodies: CD4-FITC, CD25-PE, CD8-FITC, CD56-FITC, CD69-PE, CD3-APC, CD62-PE, and HLA-DR-APC (eBioscience Inc, San Diego, CA). Cells were then washed in PBS containing 1% BSA and fixed with fixation buffer (eBiosciences) for another 50 minutes. For intracellular staining of Foxp3, cells were permeabilized with Foxp3 staining buffer (eBioscience) after fixation and stained with APC-conjugated antibodies against Foxp3 (eBioscience). The stained cells were then washed twice and resuspended in 250 μL of PBS containing 1% BSA and kept at 4°C. A total of 106 stained cells in 250 μL of PBS containing 1% BSA were subsequently analyzed using a FACS LSR II instrument (BD Biosciences, Franklin Lakes, NJ) with the FCS express V.3 software (DeNovo Software, Los Angeles, CA). Only live cells were counted, and the background fluorescence from non-antibody-treated lymphocytes was subtracted.
Measurements of circulating cytokines and adiponectin
Blood was drawn from all patients on day 1 and on day 30 of the study. Serum levels of interleukin-6 (IL-6) were determined using a “sandwich” enzyme-linked immunosorbent assay (ELISA) using commercial kits (Quantikine®; R&D Systems Inc, Minneapolis, MN), according to the manufacturer’s instructions. Glucagon-like peptide-1 (GLP-1) was tested by the following method: Blood was collected from all patients after a 12-hour overnight fasting at 180 minutes time point of OGTT. The blood was collected in ice-cooled EDTA tubes and immediately (<30 seconds) after collection, 20 μL of dipeptidyl peptidase 4 (DPP-4) inhibitor was added to 2 mL of plasma, according to manufacturer’s instructions. The tubes were inverted and were then centrifuged immediately at 1000 × g for 10 minutes in a refrigerated centrifuge. After plasma separation, the specimens were stored at −70°C until the ELISA assay was run. The circulating level of human GLP-1 was quantified using a commercial ELISA kit from Millipore Corp (Billerica, MA) according to the manufacturer’s instructions. Serum levels of human adiponectin were determined using a commercial ELISA kit from Linco Research Inc (St Charles, MO). Serum was diluted 500 fold before the assay, and 20 μL of diluted serum, standard samples and controls, were plated in duplicate on a mouse anti-human adiponectin-coated plate and examined using an ELISA reader at 450 nm, according to the manufacturer’s instructions.
HOMA score
HOMA is designed to predict the homeostatic concentrations of fasting insulin and glucose, which arise from varying degrees of beta-cell deficiency and insulin resistance. The model is nonlinear, but can be simply approximated. Two types of HOMA scores are currently being evaluated in clinical practice for determining fasting glucose and insulin levels. HOMA IR = insulin resistance = (fasting insulin in mU/L) × (fasting plasma glucose in mmol/L)/22.5. HOMA B = beta-cell function [%] = 20 × (fasting insulin in mU/L)/([fasting glucose in mmol/L] − 3.5).
In our study, we have used the HOMA IR levels.
Sample size and power
This study was not geared to detect rarely occurring treatment- associated adverse events. Summary statistics of all clinical and laboratory variables were calculated by time point; Paired t-tests were performed to test the statistical significance of all the before and after treatment measures. A two-tailed P-value of 0.05 was considered statistically significant.
Results
Determination of anti-LPS antibodies in Imm124-E
Details about the IgG antibodies in Imm124-E (an anti-LPS hyperimmune BC) obtained post–cow’s immunization with several strains of enteropathogenic E. coli were recently published.Citation22 The specific antibody titers in Imm124-E were analyzed by a validated in-house ELISA against a pool of antigens from these strains of E. coli.Citation22
Safety parameters
All patients completed the study according to the protocol. No treatment-related adverse events were noted in any of the clinical and laboratory parameters tested during treatment or follow up.
Imm124-E ameliorates insulin resistance
To determine the effect of Imm124-E on the glycemic control in NASH patients, especially in the diabetic patients with impaired glucose tolerance, we tested several parameters. A recommended target of HbA1c in clinical trials is a 6.5% to 7.0% decrease.Citation23 shows the significant improvement in HbA1c values in all ten treated patients (7.49% vs 6.38% for day 1 vs day 30, respectively). The treatment with Imm124-E for 30 days caused a 14.8% decrease in HbA1c values in all ten patients. shows that Imm124-E also exerted a beneficial effect on insulin resistance, as assessed by measuring the early peak of insulin secretion at 30 minutes after glucose administration (OGTT of 75 g of glucose); after 30 minutes, insulin secretion increased from 310 to 538.4 pmol/L for day 1 vs day 30, in five of the treated patients.
Figure 1 Effect of Imm124-E on insulin resistance, as measured on days 1 and 30. Graphs show (A) The mean percentage of HbA1c in all ten treated patients; (B) Serum insulin 30 minutes after starting the OGTT, in five treated patients. The serum insulin level was measured after overnight fasting and oral administration of 75 g of glucose; (C) Fasting plasma glucose levels in five treated patients. The fasting plasma glucose levels were measured after overnight fasting; (D) The mean percentage of OGTT in five treated patients. The assay was performed every 30 minutes for 3 hours; (E) HOMA score calculation was applied as HOMA IR = (fasting insulin in mU/L) × (fasting plasma glucose in mmol/L)/22.5. Graphs (A–C) indicate means ± SD; Graph (D) indicates means ± SD of AUC.
Abbreviations: HbA1c, hemoglobin A1c; OGTT, oral glucose tolerance test; AUC, area under the curve; HOMA, homeostasis model assessment; HOMA IR, insulin resistance; SD, standard deviation.
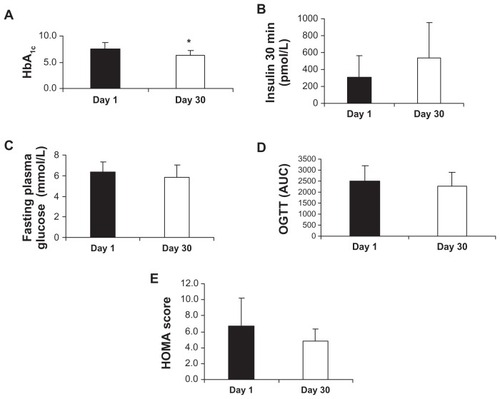
shows a reduction in the fasting plasma glucose levels of treated patients (6.3 vs 5.8 mmol/L for day 1 vs day 30, for five patients). shows an improvement (P = 0.08) in the results of the OGTT, as indicated by the area under the curve (2492 vs 2252 for day 1 vs day 30, respectively, for five patients), and shows the improved HOMA scores (6.7 vs 4.8 for day 1 vs day 30, respectively, for six patients).
Taken together, these results show an improvement in insulin resistance in patients treated with Imm124-E.
Oral administration of Imm124-E increases regulatory T cells
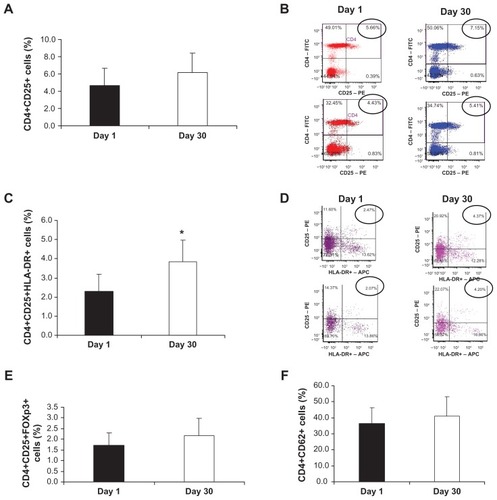
In obesity-related disorders, such as NASH, chronic local inflammation is present in adipose tissue, and cells of the innate immune system are crucially involved in adipose inflammation and systemic metabolic abnormalities. Citation24 We characterized several subsets of peripheral T cells from PBMCs using flow cytometry. shows that CD4+CD25+ cells were elevated 30 days after oral treatment with Imm124-E in 7 out of the 10 patients (4.63% vs 6.28% for day 1 vs day 30, respectively). This elevation is demonstrated in with representative fluorescence-activated cell sorting (FACS) plots taken from the analysis of two patients. A significant (P = 0.002) increase in CD4+CD25+ HLA-DR cells (2.3% vs 3.8%, gated on CD4, for day 1 vs day 30) was noted in the PBMCs of seven treated patients, as presented in . A representative dot plot of CD4+CD25+ HLA-DR cells is shown in . An increase in CD4+CD25+Foxp3+ cells was noted in seven out of ten treated patients (, 1.7% vs 2.2%, in responders). shows an increase in CD4+CD62+ cells that was noted in six patients (36.5% vs 41%, in responders, gated for CD4 cells). Taken together, the data presented here show that oral administration of Imm124-E was associated with alterations of regulatory T lymphocytes, which may contribute to some of its anti-inflammatory effects.
Figure 2 Effect of Imm124-E on Tregs in PBMCs. PBMCs were isolated from patients treated with Imm124-E at day 1 and at day 30. The cells were stained for various cell markers and then analyzed using flow cytometry. Graphs show (A) The mean percentage of CD4+CD25+ cells from seven patients; (B) Representative FACS plots taken from two patients showing the increase in the distribution of CD4+CD25+ cells (circled). The numbers in the top right quadrate indicate the frequency of CD4+CD25+ cells, gated on CD4; (C) The mean percentage of CD4+CD25+HLA-DR+ cells from seven patients; (D) Representative FACS plots taken from two patients showing the increase in CD4+CD25+HLA-DR+ cells (circled). The numbers in the top right quadrate indicate the frequency of CD4+CD25+HLA-DR+ cells, gated on CD4; (E) The mean percentage of CD4+CD25+FOXP3+ cells from five patients; (F) The mean percentage of CD4+CD62L+ cells from six patients. Graphs (A, C, E and F) indicate means ± SD.
Abbreviations: Tregs, regulatory T cells; PBMC, peripheral blood mononuclear cells; FACS, fluorescence-activated cell sorting; SD, standard deviation.
Effect of Imm124-E on serum levels IL-6
shows the net increase in serum IL-6 levels in six treated patients (4.6 vs 5.5 pg/mL, in responders) in response to oral administration of Imm124-E, as measured by ELISA.
Figure 3 Effect of Imm124-E on serum levels of IL-6, GLP-1, and adiponectin. All sera were measured using ELISA kits at days 1 and 30 of the trial in all treated patients. Graphs show (A) Serum levels of IL-6 from six patients; (B) Serum levels of GLP-1 post–glucose tolerance test from five patients; (C) Serum levels of adiponectin from eight patients.
Abbreviations: IL-6, interleukin-6; GLP-1, glucagon-like peptide 1; ELISA, enzyme-linked immunosorbent assay; SD, standard deviation.
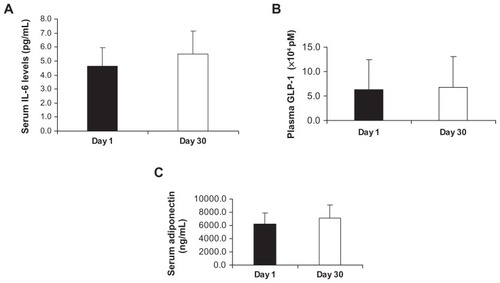
Effect of oral administration of Imm124-E on serum levels of GLP-1 and adiponectin
The central role of GLP-1 in glucose tolerance has raised questions about the possible involvement of this peptide in the pathogenesis of diabetes. A recent study found that the sensitivity of diabetic patients to GLP-1 was significantly reduced relative to nondiabetic individuals.Citation25 We compared the circulating levels of serum GLP-1 before and after carrying out OGTT on day 1 and on day 30. shows that oral treatment with Imm124-E for 30 days increased serum levels of GLP-1 post-OGTT in five of the treated patients (6.31 vs 6.78 × 104 pM, in responders). shows the serum levels of adiponectin, which were increased in eight patients (6181 vs 7069 ng/mL).
Effect of Imm124-E on lipid profile
shows a beneficial effect of the Imm124-E on serum levels of total cholesterol in five treated patients (5.8 vs 4.5 μmol/L, for day 1 vs day 30, respectively). Similar results were obtained with serum low-density lipoproteins (LDL) measurements (3.8 vs 2.7 μmol/L, for day 1 vs day 30, respectively, ). A slight improvement in serum triglycerides levels was noted in five patients (data not shown).
Figure 4 Effect of Imm124-E on lipid profile. All sera were measured at days 1 and 30 of the trial in all treated patients. Graphs show (A) Total cholesterol levels from five patients; (B) LDL levels from seven patients.
Abbreviations: LDL, low-density lipoprotein; SD, standard deviation.
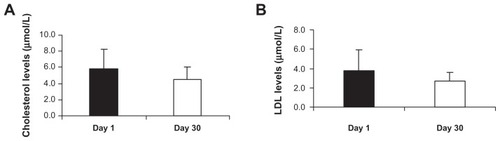
The data presented here suggest that oral administration of Imm124-E has an ameliorating effect on the lipid profile in treated NASH patients.
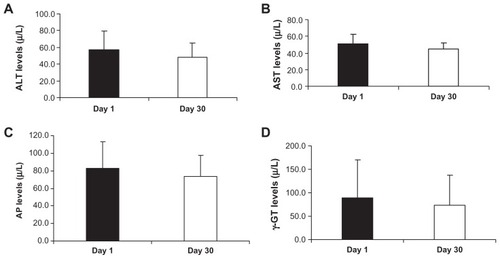
Effect of oral administration of Imm124-E on liver injury
show the effect of oral administration of Imm124-E on serum levels of ALT, AST, AP and γ-GT, respectively. ALT levels were decreased in five patients (57.4 vs 48.6 μ/L, for day 1 vs day 30). AST levels were decreased in five of the treated subjects (51.2 vs 44.6 μ/L, for day 1 vs day 30). AP levels were improved in eight of the treated patients (83.1 vs 73.9 μ/L, for day 1 vs day 30), and γ-GT levels were decreased in five treated patients (88.2 vs 73.2 μ/L, for day 1 vs day 30).
Figure 5 Effect of Imm124-E on liver enzymes. The serum liver enzymes were measured in treated patients at days 1 and 30 of the study. Graphs show (A) ALT levels from five patients; (B) AST levels from five patients; (C) AP levels from eight patients; (D) γ-GT levels from five patients.
Abbreviations: ALT, alanine aminotransferase; SD, standard deviation; AST, aspartate aminotransferase; AP, alkaline phosphatase; γ-GT, gamma-glutamyl transpeptidase.
Effect of oral administration of Imm124-E on body weight
Oral administration of Imm124-E led to no change in body weight.
Discussion
The data presented in our study show that Imm124-E is a safe oral treatment. No side effects were noted in any of the treated patients. Imm124-E led to a clear improvement in insulin resistance and to alleviation of related liver injury in NASH patients. These effects of Imm124-E were associated with an increased distribution of T regulatory lymphocytes and an improved lipid profile in treated patients.
Immediately postpartum, high concentrations of various immune components can be found in colostrum, with immunoglobulins making up approximately 5% of the colostrum content.Citation26 Colostrum is also rich in cytokines and other immune agents that provide bacteriostatic, bactericidal, antiviral, anti-inflammatory, and immunoprotection against infection.Citation27,Citation28
The value of BC as a biologic in medicine is well documented.Citation13 BC has been already shown to balance blood sugar levels. The high content of IGF-I in BC, which has been shown to stimulate glucose transport in type 2 diabetes,Citation29 may provide a possible explanation for such an effect. An efficient treatment to achieve and sustain glycemic control is measured by improvement in HbA1c.Citation30 We show a significant decrease of more than 14% in all ten treated patients, in terms of HbA1c values. Reduced fasting glucose levels, and improved HOMA score and OGTT strengthened the enhancement of glycemic control exerted by Imm124-E. Furthermore, oral administration of Imm124-E induced an increase in the levels of circulating GLP-1, a gut hormone that plays an important role in regulating glucose homeostasis by both pancreatic and extrapancreatic mechanisms (defects of GLP-1 in type 2 diabetes result in inappropriately low insulin secretion after oral ingestion of nutrients).Citation25
Passive immunization based on bovine colostral preparations is an area of active research. BC-based immune milk products have proven efficacy in prophylaxis and treatment against various infectious diseases in humans, such as diarrheal diseases caused by E. coli, rotavirus, and other pathogens.Citation31,Citation32 However, the treatment of NASH with colostrum enriched with anti-LPS antibodies has not been previously published. BC contains immunoglobulins that provide the newborn calf with protection against microbial infections until its own immune system matures. The concentration of antibodies against pathogens in colostrum can be raised by immunizing cows with pathogens or their antigens. While normal BC contains active IgG against specific enteric pathogens, their specificity is dictated by previous systemic challenge, and often the concentration is too low to afford optimal protection.Citation33 Therefore, treatment with hyperimmune colostrum is more effective.
LPS and endogenous gut-derived bacterial endotoxins have been suggested to play a role in NASH. The increased permeability in liver disease related to steatohepatitis appears to be caused by the disruption of intracellular tight junctions in the intestine. This has been shown to correlate with a high prevalence of small intestine bacterial overgrowth.Citation34 It has also been shown that a lifestyle that included high-intensity and high-volume exercise induced favorable changes in inflammatory markers in relation to endotoxin treatment.Citation35 Another study showed that probiotic bacteria prevented hepatic damage and maintained colonic barrier function in a mouse model of sepsis; in this report, orally administered probiotics prevented liver and intestinal damage through a peroxisome proliferator-activated receptor (PPAR)-dependent mechanism.Citation36 In our study, the association between gut-derived bacterial endotoxins and the efficacy of Imm124-E in treating NASH was not established and has to be further studied.
The link between inflammatory and metabolic signaling is a delicate balance. An important feature of inflammation is the infiltration of inflamed tissues by immune cells.Citation37 In the last few years several reports have described the involvement of regulatory T cells in the cross junction of inflammation, liver disease, and insulin resistance.Citation20,Citation21,Citation24 For example, CD4+ T lymphocytes resident in the visceral adipose tissue were shown to control insulin resistance in mice with diet-induced obesity.Citation20 Also, treatment of humans with anti CD3 was shown to affect Tregs along with alteration in cytokine secretion and PBMC proliferation.Citation38 In our study, after one month of oral treatment of Imm124-E, clear changes were found in various subtypes of Tregs. The effect on Tregs was accompanied by an increase in serum IL-6 and a small increase in adiponectin levels.
A bulk of data has accumulated during the last two decades that supports the notion that insulin resistance in diabetes is primarily a lipid disease. Treatment with Imm124- E had a beneficial effect on the lipid profile and liver injury of treated patients. Also, skim milk from cows immunized with a polyvalent human gut bacterial vaccine was claimed to reduce significantly elevated blood cholesterol and triglyceride concentrations.Citation19
This hyperimmune BC may serve as an easy and safe method for generating antigen-specific antibodies and as a source of immune adjuvants.
In summary, oral administration of Imm124-E is safe and exerts an immunomodulatory effect in patients with type 2 diabetes, hyperlipidemia, and NASH. The anti-inflammatory effect and the promotion of Tregs are associated with alleviation of insulin resistance, hyperlipidemia, and liver damage in these patients. Further trials will aim at studying a larger cohort to elucidate the immunomodulatory effect at the level of adipose tissue.
Disclosure
Y Ilan is the medical director of Immuron. Victor Wong, Brian Muller, Grant Rawlin are employees of Immuron. This study was supported, in part, by Immuron Ltd. Apart from this, the Authors report no conflicts of interest in this work.
References
- Neuschwander-TetriBAClarkJMBassNMNASH Clinical Research NetworkClinical, laboratory and histological associations in adults with nonalcoholic fatty liver diseaseHepatology201052391392420648476
- DanielSBen-MenachemTVasudevanGMaCKBlumenkehlMProspective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patientsAm J Gastroenterol199994103010301410520861
- ClarkJMBrancatiFLDiehlAMNonalcoholic fatty liver diseaseGastroenterology200212261649165712016429
- SorbiDMcGillDBThistleJLTherneauTMHenryJLindorKDAn assessment of the role of liver biopsies in asymptomatic patients with chronic liver test abnormalitiesAm J Gastroenterol200095113206321011095343
- ByronDMinukGYClinical hepatology: profile of an urban, hospital-based practiceHepatology19962448138158855181
- Neuschwander-TetriBACaldwellSHNonalcoholic steatohepatitis: summary of an AASLD Single Topic ConferenceHepatology20033751202121912717402
- Almeda-ValdésPCuevas-RamosDAguilar-SalinasCAMetabolic syndrome and non-alcoholic fatty liver diseaseAnn Hepatol20098Suppl 1S18S2419381120
- ClarkJMThe epidemiology of nonalcoholic fatty liver disease in adultsJ Clin Gastroenterol200640Suppl 1S5S1016540768
- SanyalAJCampbell-SargentCMirshahiFNonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalitiesGastroenterology200112051183119211266382
- MarchesiniGBriziMBianchiGNonalcoholic fatty liver disease: a feature of the metabolic syndromeDiabetes20015081844185011473047
- MeroAMiikkulainenHRiskiJPakkanenRAaltoJTakalaTEffects of bovine colostrum supplementation on serum IGF-I, IgG, hormone, and saliva IgA during trainingJ Appl Physiol1997834114411519338422
- SéverinSWenshuiXMilk biologically active components as nutraceuticals: reviewCrit Rev Food Sci Nutr2005457–864565616371332
- StruffWGSprotteGBovine colostrum as a biologic in clinical medicine: a review – Part II: clinical studiesInt J Clin Pharmacol Ther200846521122518538107
- StruffWGSprotteGBovine colostrum as a biologic in clinical medicine: a review. Part I: biotechnological standards, pharmacodynamic and pharmacokinetic characteristics and principles of treatmentInt J Clin Pharmacol Ther200745419320217474538
- LoimarantaVTenovuoJVirtanenSGeneration of bovine immune colostrum against Streptococcus mutans and Streptococcus sobrinus and its effect on glucose uptake and extracellular polysaccharide formation by mutans streptococciVaccine19971511126112689286054
- LoimarantaVNuutilaJMarnilaPTenovuoJKorhonenHLiliusEMColostral proteins from cows immunised with Streptococcus mutans/S. sobrinus support the phagocytosis and killing of mutans streptococci by human leucocytesJ Med Microbiol1999481091792610510968
- HowardANMarksJHypocholesterolaemic effect of milkLancet19772803125525669869
- MalinowMRMcLaughlinPThe effect of skim milk on plasma cholesterol in ratsExperientia1975319101210131175732
- GolayAFerraraJMFelberJPSchneiderHCholesterol-lowering effect of skim milk from immunized cows in hypercholesterolemic patientsAm J Clin Nutr1990526101410192239776
- WinerSChanYPaltserGNormalization of obesity-associated insulin resistance through immunotherapyNat Med200915892192919633657
- FeuererMHerreroLCipollettaDLean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parametersNat Med200915893093919633656
- AdarTBen Ya’acovALalazarGOral administration of immunoglobulin G-enhanced colostrum alleviates insulin resistance and liver injury and is associated with alterations in natural killer T cellsClin Exp Immunol2012167225226022236001
- DeFronzoRAStonehouseAHHanJWintleMERelationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trialsDiabet Med201027330931720536494
- IlanYMaronRTukpahAMInduction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob miceProc Natl Acad Sci U S A2010107219765977020445103
- KjemsLLHolstJJVølundAMadsbadSThe influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjectsDiabetes200352238038612540611
- NevilleMCAnatomy and physiology of lactationPediatr Clin North Am2001481133411236721
- BuescherESMcWilliams-KoeppenPSoluble tumor necrosis factor-alpha (TNF-alpha) receptors in human colostrum and milk bind to TNF-alpha and neutralize TNF-alpha bioactivityPediatr Res199844137429667368
- GarofaloRPGoldmanASCytokines, chemokines, and colony-stimulating factors in human milk: the 1997 updateBiol Neonate19987421341429691155
- DohmGLEltonCWRajuMSIGF-I – stimulated glucose transport in human skeletal muscle and IGF-I resistance in obesity and NIDDMDiabetes1990399102810322166697
- NathanDMBuseJBDavidsonMBAmerican Diabetes Association; European Association for Study of DiabetesMedical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of DiabetesDiabetes Care200932119320318945920
- KorhonenHMarnilaPGillHSMilk immunoglobulins and complement factorsBr J Nutr200084Suppl 1S75S8011242450
- HammarströmLWeinerCKTargeted antibodies in dairy-based productsAdv Exp Med Biol200860632134318183936
- HilpertHBrüssowHMietensCSidotiJLernerLWerchauHUse of bovine milk concentrate containing antibody to rotavirus to treat rotavirus gastroenteritis in infantsJ Infect Dis198715611581663110303
- MieleLValenzaVLa TorreGIncreased intestinal permeability and tight junction alterations in nonalcoholic fatty liver diseaseHepatology20094961877188719291785
- LiraFSRosaJCPimentelGDEndotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjectsLipids Health Dis201098220684772
- EwaschukJEndersbyRThielDProbiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsisHepatology200746384185017659579
- HotamisligilGSInflammation and metabolic disordersNature2006444712186086717167474
- IlanYZigmondELalazarGOral administration of OKT3 monoclonal antibody to human subjects induces a dose-dependent immunologic effect in T cells and dendritic cellsJ Clin Immunol201030116717719756989