Abstract
Background
The S100/calgranulin gene appears to modulate neuroinflammation following cerebral ischemia and could be a valuable biomarker for stroke prognosis, according to growing research. This study aimed at evaluating the correlation between calgranulin gene variants and susceptibility to ischemic stroke (IS) in the Southern Chinese population.
Methods
Using an enhanced multi-temperature ligase detection reaction genotyping, 310 IS patients and 324 age-matched healthy controls were genotyped to identify five calgranulin gene variants.
Results
According to the obtained results, the S100A8 rs3795391, rs3806232, and S100A12 rs2916191 variants were linked to a higher risk of IS, while the S100A9 rs3014866 variant was associated with a lower risk of IS. Moreover, the T-T-C-A-T, T-T-C-G-T, or C-C-C-G-C haplotypes have been linked to a greater risk of developing IS, according to haplotype analysis. The occurrence of the variant C allele there in S100A8 rs3795391, rs3806232, and S100A12 rs2916191 variants may impart a greater risk of stroke in the LAA subtype, according to further stratification by IS subtypes, while the T allele of the S100A9 rs3014866 variant may be linked to a reduced risk of stroke of all subtypes. Furthermore, patients with the variant C allele of the S100A8 rs3795391, rs3806232, and S100A12 rs2916191 variants presented with increased circulating S100A8 and S100A12 levels and larger infarct volumes relative to those with the major TT genotype.
Conclusion
Our findings suggest that calgranulin gene variants are linked to IS susceptibility, implying that the calgranulin gene may be a potential biomarker for IS prevention and personalized treatment.
Introduction
Stroke is a complex disorder that is one of the most common causes of adult disability across the globe. In China, the rate of stroke has risen considerably in recent decades, and around 80% of strokes are ischemic. Ischemic stroke (IS) occurs when blood flow within the area of an occluded blood vessel is disrupted, depriving local brain tissue of oxygen and resulting in malacia and necrosis.Citation1 A growing body of evidence supports that both genetic and environmental factors play pivotal roles in the pathophysiology of IS.Citation2 Atherosclerosis, chronic inflammation of blood vessel walls, has been recognized as an important pathogenic factor for IS. Furthermore, S100, a calcium-binding protein belonging to the EF-Hand family, has been considerably expressed in the human brain, particularly in astrocytes. The underlined protein family has been shown to play a significant role in various pathological activities, including proliferation, differentiation, cytoskeletal assembly, protein phosphorylation, and intracellular calcium homeostasis.Citation3,Citation4 Some S100 proteins regulate cellular function in an autocrine or paracrine manner by activating surface receptors such as RAGE receptors to promote NF-κB signaling, recruit and activate cellular pro-inflammatory effectors, thereby facilitating inflammation.Citation5 Up to now, more than 25 S100 proteins have been discovered, among which S100A1, S100B, S100A6, S100A7, S100A8, S100A9 and S100A12 been reported to be involved in the pathogenesis of multiple neurological disorders including Alzheimer’s disease,Citation6 Parkinson’s diseaseCitation7 and stroke.Citation8,Citation9,Citation10
The S100/calgranulins family comprising S100A8, S100A9, and S100A12 proteins, are primarily expressed in myeloid-derived cells including monocytes, neutrophils, and dendritic cells.Citation11,Citation12 Numerous investigations have shown that calgranulins play a role in a variety of inflammatory disorders including inflammatory bowel disease,Citation13 rheumatoid arthritis,Citation14 myocardial infarctionCitation15 and IS.Citation16 Furthermore, S100A8 and S100A9 typically exist as S100A8/A9 heterodimers, and they usually modulate inflammatory processes through both intracellular and extracellular pathways. S100A8/A9, an innate immune mediator with an inflammatory component, is elevated in the plasma of patients with acute IS that led to accelerating atherosclerotic plaque rupture and, as a result, stroke progression.Citation17 In recent decades, it has been revealed that elevated S100A8/A9 levels are also linked to a higher probability of bad prognosis in IS.Citation16 When compared to patients with TIA or healthy controls, S100A12 was considerably higher in patients with acute IS.Citation18 According to prospective research, S100A12 levels in the blood have been linked to poor functional outcomes in patients with acute IS.Citation19 In vitro evidence also suggests that S100A12 promotes inflammation, oxidative stress, and apoptosis induced by oxygen-glucose deprivation/reperfusion through activating ERK signaling.Citation20 These findings have led us to hypothesize that the calgranulin genes may have a role in IS pathogenesis.
The calgranulin genes encoding S100A8, S100A9, and S100A12 are located on chromosome 1q21. Previous studies have reported the associations between calgranulin genes polymorphisms and the risk of numerous diseases. Sun et al indicated that the S100A8 gene polymorphisms may influence the susceptibility and severity of aggressive periodontitis.Citation21 Blanco-Rojo et al found that decreased T2D risk is linked with minor allele (T) of the S100A9 variant rs3014866 in three populations of varied ancestry including CORDIOPREV, GOLDN, and BPRHS.Citation22 Salminen et al revealed that genetic polymorphism in the S100A9-S100A12-S100A8 locus influences serum and plasma MMP-8 levels and may be linked to the risk of cardiovascular disease in a genome-wide association study.Citation23 The calgranulin family, as a key pro-inflammatory mediator, has a variety of regulatory activities in IS and other inflammatory disorders. Hence, the calgranulin genes appear to be good candidate genes for transmitting hereditary susceptibility to IS. The purpose of the present study was to investigate the association of S100A8, S100A9, and S100A12 polymorphisms with IS in a case-control study of the southern Chinese population.
Materials and Methods
Participant’s Recruitment
Between 2018 and 2020, we recruited 310 patients with IS (209 males, 101 females) from the Department of Neurology at Guangdong Medical University’s Affiliated Hospital. The clinical signs and symptoms were recorded to diagnose IS. All of the patients had regular blood tests as well as computed tomography (CT) scans and/or magnetic resonance imaging (MRI) studies. Two neurologists used the TOASTCitation18 criteria to categorize IS into five subgroups based on clinical signs and neuroimaging findings. The study excluded patients with a history of transient IS, cerebral hemorrhage, coronary artery disease, atrial fibrillation, haematologic illnesses, subarachnoid hemorrhage, systemic inflammatory diseases, autoimmune diseases, malignant tumors, and chronic infectious diseases. The control subjects were 324 participants (153 male and 171 female) who were selected during the same time from the Health Examination Center of Guangdong Medical University’s Affiliated Hospital and were found similar in age and race to the IS subjects. Subjects with a recent history of the cerebrovascular disorder or myocardial infarction were excluded from the study. All of the registered participants were Han Chinese adults from Guangdong Province who were genetically unrelated. Each participant gave their informed consent in the written form. The present study was conducted in compliance with the Declaration of Helsinki and its approval was provided by the Guangdong Medical University Affiliated Hospital’s Ethics Committee (No: YJYS2018053).
SNP Selection and Genotyping
Based on prior published results, five SNPs were chosen: S100A8 rs3795391 and rs3806232, S100A9 rs3014866 and rs1560833, and S100A12 rs2916191.Citation21–24 The TIANamp Blood DNA kit (Tiangen Biotech, Beijing, China) was employed to extract genomic DNA from each person’s peripheral leukocytes based on guidelines provided by the manufacturer. Following that, a DNA spectrophotometer (ND-1000, NanoDrop, Wilmington USA) was employed to evaluate the purity and concentration of DNA. The primers were utilized to genotype the chosen calgranulin SNPs using an iMLDR-TM method. The primers are indicated in Table S1. The iMLDR-TM reactions and PCR methods were carried out as stated previously.Citation25
Enzyme-Linked Immunosorbent Assay
As the diagnosis was confirmed, participants’ peripheral venous blood samples were taken. S100A9, S100A8, and S100A12 levels in serum were measured (in duplicate) employing ELISA kits (R&D Systems, Minneapolis, MN, USA), as suggested by the manufacturer.
Quantification of Infarct Volume
MIPAV software (Medical Image Processing, Analysis, and Visualization, version 3.0; NIH, Bethesda, MD) was used to calculate infarct volumes based on DWI.Citation26 To discriminate acute from non-acute diffusion variation, a semiautomatic segmentation approach was used to characterize acute diffusion lesions on a slice-by-slice basis, relying on apparent diffusion coefficient and fluid-attenuated inversion recovery imaging sequences. On multiplying the slice thickness by the entire lesion area, DWI infarct volumes may be determined.
Statistical Evaluation
SPSS version 19.0 (IBM, NY, USA) and GraphPad Prism 4.0 (GraphPad Software, Inc., San Diego, CA, USA) were used to perform statistical evaluations. The Chi-squared test or Fisher’s exact test was used to evaluate allele frequencies and genotype distribution among IS patients and controls. The link between the S100A8, S100A9, and S100A12 genotypes and IS were assessed using the OR and 95% CI. HWE software was used to assess the Hardy-Weinberg equilibrium (HWE). Haploview was used to examine linkage disequilibrium (LD) and haplotypes. Student’s t-test for normally distributed data or the Mann–Whitney U-test for nonparametric data were used to compare levels of S100A8, S100A9, and S100A12 with different genotypes in the patient and control groups. A multiple linear regression model was used to examine the correlations between the genotypes of the S100A8, S100A9, and S100A12 polymorphisms with the DWI infarct volume. Linear regressions were adjusted for age, gender, smoking, hypertension, diabetes mellitus and hyperlipidaemia. Bonferroni correction was applied for multiple comparisons with control type 1 error. For all the evaluations, two-tailed P value under 0.05 was regarded as statistically considerable.
Results
Demographic Characteristics
In total, 634 participants (310 IS patients and 324 healthy controls) were enrolled in the study, and their demographics and medical conditions were detailed in . In terms of age (P = 0.93), no considerable differences were observed in the levels of serum uric acid (P = 0.59), triglyceride (P = 0.61), total cholesterol (P = 0.22), low-density lipoprotein (LDL) (P = 0.71), high-density lipoprotein (HDL) (P = 0.26), and homocysteine (P = 0.78) between patients and controls. However, substantial variations in sex, smoking status, hypertension, and diabetes were recorded between the control and SI groups (P < 0.001).
Table 1 Characteristics of Ischemic Stroke Cases and Controls
Association Between the Calgranulin Gene Variants and the Risk of IS
lists the allele and genotype frequencies for the calgranulin gene variants in the healthy (control) as well as IS patients. The HWE test revealed no selection bias in either group (P > 0.05). Figure S1 depicts the linkage pattern of polymorphisms within the calgranulin gene. The X2 test was used to compare the genotype distributions of IS patients and healthy individuals. The obtained results revealed a significant statistical relationship between the S100A8 rs3795391 (P = 0.0040) and rs3806232 (P = 0.0040), S100A9 rs3014866 (P < 0.001), and S100A12 rs2916191 (P < 0.0010) variants and the risk of IS. In a dominant model (TT vs TC/CC, TT vs TC/CC, CC vs CT/TT and TT vs TC/CC, respectively), considerable differences in the frequencies were seen between the IS group and the controls for rs3795391 (OR = 1.98, 95% CI: 1.27–3.07), rs3806232 (OR = 2.0, 95% CI: 1.28–3.12), rs3014866 (OR = 0.65, 95% CI: 0.47–0.90), and rs2916191 (OR = 1.80, 95% CI: 1.17–2.75,) with P values of 0.0040, 0.0040, <0.001, and 0.0090, respectively. The recessive model’s rs3795391, rs3806232, and rs2916191 variants showed no considerable differences between the IS group and controls (P > 0.05). The recessive model for S100A9 rs3014866 frequency (CC+CT vs TT) in the IS group was significantly different from that in the controls (OR = 0.45, 95% CI: 0.29–0.69, P < 0.0010). The IS group had a considerably higher frequency of the variant C allele at rs3795391 (OR = 2.01, 95% CI: 1.33–3.04, P = 0.0040), rs3806232 (OR = 2.03, 95% CI: 1.34–3.09, P = 0.0040), and rs2916191 (OR = 1.84, 95% CI: 1.23–2.74, P = 0.0060), as well as the variant T allele at rs3014866 (OR = 0.65, 95% CI: 0.52–0.81, P < 0.001). On the other hand, the genotype and allele distributions for the S100A9 rs1560833 variant were found to be similar between IS patients and controls (P > 0.05).
Table 2 Genotype and Allele Frequencies of S100A8/A9 and S100A12 Polymorphisms Between IS Patients and Controls, and Corresponding ORs for IS
Haplotype Analysis
The frequencies of the T-T-C-A-T (OR = 1.38, 95% CI: 1.03–1.84, P = 0.040), T-T-C-G-T (OR = 1.54, 95% CI: 1.14–2.07, P = 0.010), and C-C-C-G-C (OR = 2.82, 95% CI: 1.63–4.86, P < 0.001) haplotypes (according to the rs3795391-rs3806232-rs3014866-rs1560833-rs2916191 variants) were found to be considerably elevated in IS patients relative to the control group. Following adjustment for sex, age, smoking, diabetes mellitus, hyperlipidemia, and hypertension, these haplotypes were related to an elevated risk of IS ().
Table 3 The Frequencies of Haplotypes of S100A8/A9 and S100A12 Gene in Patients and Controls
Associations Between the Calgranulin Gene Variants and Demographic Characteristics
– shows the correlations of S100A8, S100A9, and S100A12 variants with demographic factors. After stratifying participants by age, sex, hypertension, diabetes, and smoking status, we discovered that the S100A8 rs3795391 C allele was linked to an elevated risk of IS in individuals over 70 (P = 0.010), men (P = 0.013), and non-diabetic patients (P = 0.010) (). The S100A8 rs3806232 C allele was found to be correlated with an elevated risk of IS in non-diabetic patients (P = 0.010) (). The S100A12 rs2916191 C allele was associated with a higher risk of IS in male (P = 0.030), non-diabetic (P = 0.020) and non-hypertensive patients (P < 0.01) (). In contrast, a decreased risk of IS was correlated with the variant T allele at S100A9 rs3014866 in male (P = 0.0050) and nondiabetic patients (P = 0.010) (). When stratified by sex, age, hypertension, diabetes, and status of smoking, no considerable variations in S100A9 rs1560833 variant distributions and IS risk were found (P > 0.05) ().
Table 4 A Comparison Between the Baseline Characteristics of the S100A8 rs3795391 Genotypes and Alleles in the IS Patient and Control Groups
Table 5 A Comparison Between the Baseline Characteristics of the S100A8 rs3806232 Genotypes and Alleles in the IS Patient and Control Groups
Table 6 A Comparison Between the Baseline Characteristics of the S100A9 rs3014866 Genotypes and Alleles in the IS Patient and Control Groups
Table 7 A Comparison Between the Baseline Characteristics of the S100A9 rs1560833 Genotypes and Alleles in the IS Patient and Control Groups
Table 8 A Comparison Between the Baseline Characteristics of the S100A12 rs2916191 Genotypes and Alleles in the IS Patient and Control Groups
Associations Between the Calgranulin Gene Variants and Stroke Subtypes
We divided IS patients into stroke subgroups based on TOAST categorization to see if the S100A8, S100A9, and S100A12 variants were localized to a certain subset. Carriers with the C allele of the S100A8 rs3795391 and rs3806232 and S100A12 rs2916191 variants (P < 0.05) appeared to have an elevated risk of stroke of the LAA or SAO (small-artery occlusion) subtype relative to the controls, as illustrated in , and . In contrast, the S100A9 rs3014866 T allele appeared to run a lower risk of stroke with all subtypes (P < 0.05) (). However, no statistical correlation was found between the S100A9 rs1560833 variant and all stroke subtypes when compared with the controls (P > 0.05) ().
Table 9 The Relationship Between S100A8 rs3795391 Genotypes and is Stratified by TOAST Classification in IS Patients
Table 10 The Relationship Between S100A8 rs3806232 Genotypes and is Stratified by TOAST Classification in IS Patients
Table 11 The Relationship Between S100A9 rs3014866 Genotypes and is Stratified by TOAST Classification in IS Patients
Table 12 The Relationship Between S100A9 rs1560833 Genotypes and is Stratified by TOAST Classification in IS Patients
Table 13 The Relationship Between S100A12 rs2916191 Genotypes and is Stratified by TOAST Classification in IS Patients
The Serum Levels of the Calgranulins Based on Different Genotypes
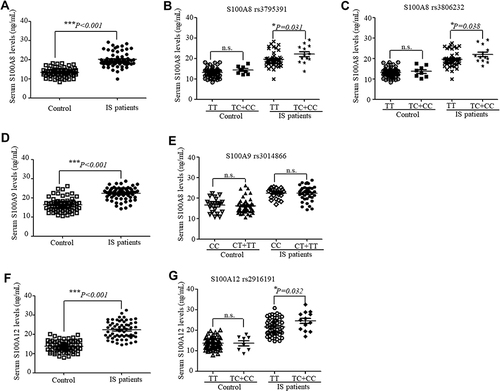
The serum levels of S100A8, S100A9, and S100A12 were detected in 62 IS patients and 66 controls. As indicated in , the serum levels of S100A8 (), S100A9 () and S100A12 () (with P-values of < 0.001, < 0.001, and < 0.001, accordingly) were considerably elevated in the IS patients when compared with the control. Moreover, we assessed whether there was an association between the serum levels of S100A8, S100A9, and S100A12 in IS patients and the calgranulin gene genotypes. When the samples were stratified based on S100A8 genotypes, the serum S100A8 levels were significantly higher in patients with the TC+CC genotypes in the rs3795391 (P = 0.031) () or rs3806232 (P = 0.038) () variant than those with the TT genotypes. Serum S100A9 levels in IS patients of various genotypes did not differ significantly when patients were divided according to their S100A9 genotype (). In addition, a considerable elevation in serum S100A12 levels was observed in IS patients carrying the mutated rs2916191 TC+CC genotypes (P = 0.032) (). However, no considerable correlations were detected in the healthy controls carrying the major common alleles compared with the mutated alleles of S100A8, S100A9, and S100A12 variants (P > 0.05) ( and ).
Figure 1 The serum calgranulin levels in IS patients (n=62) and controls (n=66) stratified based on corresponding genotypes of S100A8, S100A9 and S100A12. The serum S100A8 (A), S100A9 (D), and S100A12 (F) levels in IS patients and controls were measured using ELISA. The serum S100A8 levels in IS patients and controls were stratified according to different genotypes of S100A8 rs3795391 (B) and rs3806232 (C). The serum S100A9 levels in IS patients and controls were stratified according to S100A9 rs3014866 genotypes (E). The serum S100A12 levels in IS patients and controls were stratified according to S100A12 rs2916191 genotypes (G). The data are shown as medians with interquartile ranges (*P < 0.05, ***P < 0.001).
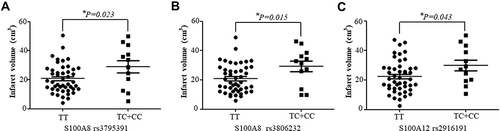
Association of the Calgranulin Gene Variants with the Infarct Volume
Associations of the S100A8, S100A9 and S100A12 variants with the actual volumetric measurements of infarct volume by diffusion-weighted magnetic resonance imaging (DWI) in 58 individuals suffering from IS were evaluated. Their obtained results are presented in . The mean infarct volumes in the IS patients with the S100A8 (rs3795391 and rs3806232) ( ) and S100A12 (rs2916191) () variant genotypes (TC+CC) were found to be larger when compared to those patients associated with the major TT genotype (P = 0.023, P = 0.015 and P = 0.043, respectively).
Discussion
In this hospital-based case-control study, we demonstrate for the first time that S100A8 rs3795391, rs3806232, S100A9 rs3014866, and S100A12 rs2916191 variants are associated with susceptibility to IS in a Southern Chinese population. Haplotype analysis indicated that individuals with the T-T-C-A-T, T-T-C-G-T, or C-C-C-G-C haplotype may have an increased risk of IS. Further stratification revealed that the S100A8 rs3795391 and rs3806232 and S100A12 rs2916191 variants may confer an increased risk of LAA or SAO subtype stroke, while the S100A9 rs3014866 variant may associate with a lower risk of stroke of all subtypes. The circulating calgranulins levels were considerably elevated in the IS patients with the variant C alleles of the S100A8 rs3795391, rs3806232, and S100A12 rs2916191 variants than in those carrying the major TT genotypes. Moreover, patients with the S100A8 rs3795391, rs3806232, and S100A12 rs2916191 variant C alleles have greater infarct volumes than those with the main TT genotypes.
The most important pathologic underpinning of IS is atherosclerosis, which is a chronic inflammation of the vascular wall driven by activated T- and B-lymphocytes, macrophages, and smooth muscle cells with high calgranulin expression. Guo et al indicated that higher serum S100A8/9 levels were linked with an increased risk of poor prognosis for IS.Citation16 According to Wakisaka et al, an elevated plasma S100A12 level at admission has been linked to poor functional outcomes in individuals with acute IS.Citation19 S100A8/A9 was reported to induce neuroinflammation and enhance the pathophysiology of IS,Citation27,Citation28 and inhibiting S100A8/A9 reduced the size, inflammation, and vulnerability of atherosclerotic plaques in mice.Citation29 Moreover, APOE-/- mice overexpressing S100A12 showed a significant increase in atherosclerotic plaque size, accompanied by increased oxidative stress and vascular calcification. In addition, inhibitor ABR-215757 could competitively bind with S100A12 in vitro and reduce atherosclerosis.Citation30 Given these facts, the calgranulin genes may serve a considerable role in the occurrence and development of IS. Calgranulin may be involved in the pathogenesis and prognosis of IS through several possible pathophysiological pathways. First, calgranulins are involved in the accumulation of neutrophils and macrophages, macrophage cytokine production, and smooth muscle cell proliferation in response to vascular inflammatory injury.Citation31 Second, calgranulins can enhance activation of the RAGE or TLR4 receptors and promote atherosclerosis.Citation30,Citation32 Third, calgranulins stimulate the secretion of proinflammatory cytokines and are associated with inflammatory damage during cerebral ischemia-reperfusion.Citation27,Citation30 Despite these developments, the calgranulin alleles that cause IS remain elusive.
S100 calgranulin levels have been linked to a positive relationship between higher IS risk and a bad prognosis. In a previous investigation, elevated plasma S100A8/A9 concentrations were found to be considerably linked to an increased risk of poor clinical outcomes three months following the onset of IS.Citation16 Another study showed that plasma S100A8/A9 is an independent predictor of 3-month mortality and helps identify patients with poor AIS prognosis.Citation33 Furthermore, circulating S100A12 levels have been linked to functional outcomes following IS, and short-term mortality, severity, and inflammatory process following spontaneous intracerebral hemorrhage.Citation34 It is highly speculated that the calgranulin gene variants might affect the progression and prognosis of IS by influencing the circulating calgranulins. Hence, we investigated the relationship between calgranulin gene variants and S100A8, S100A9, and S100A12 levels. Our study showed that patients with the variant C allele of the S100A8 rs3795391, rs3806232, and S100A12 rs2916191 variants exhibited higher circulating S100A8 and S100A12 levels in IS, relative to those having the common T allele. Hence, it is believed that individuals with the mutant C allele at the rs3795391, rs3806232, and rs2916191 loci expressing elevated S100A8 or S100A12 levels may have enhanced pro-inflammatory signaling, which could exacerbate the consequent cellular responses to cerebral ischemia injury.
The damage to brain tissue induced by cerebral ischemia activates several pro-inflammatory and apoptotic cascades, resulting in the accumulation of many triggered microglia, infiltrating macrophages, and lymphocytes in the infarct area.Citation35 It is reported that S100A9 downregulation may reduce the infarct volume and brain swelling in a murine MCAO model.Citation36 Additional studies indicated that blocking calprotectin could reduce infarct volume, vascular inflammation, and vulnerability of atherosclerotic plaques.Citation29,Citation37,Citation38 Danger-associated molecular patterns, including S100A8/A9 and HMGB1 are warning signals released by apoptotic cells in a variety of situations, including ischemia and hypoxia.Citation39 In the post-ischemic brain, inhibiting HMGB1 signaling with short hairpin RNA reduced infarct size, microglial stimulation, and the production of pro-inflammatory mediators.Citation40 Our previous study reported that the HMGB1 rs2249825 variant was associated with low infarct volume and was a protective factor for IS in the Han Chinese population.Citation41 Because of the involvement of S100 calgranulins in vascular inflammation, we hypothesized that they may have a role in stroke infarct size. Patients with the variant C alleles of the S100A8 rs3795391, rs3806232, or S100A12 rs2916191 variant have greater infarct sizes than those with the major T alleles, according to the findings. This is also supported by the evidence that the variant C alleles of the S100A8 rs3795391, rs3806232, or S100A12 rs2916191 variant were associated with increased circulating S100A8 and S100A12 levels, suggesting the calgranulin gene variants may induce the different expression of S100A8 and S100A12, which is associated with the infarct size.
There are several limitations to our case-control study. First, the study sample was not sufficiently large, which could have resulted in non-representative results. Second, potential biases such as information, selection, and confounding bias cannot be eliminated. Third, this study only investigated five calgranulin gene polymorphisms, although other variants may have had a role in IS susceptibility. Fourth, numerous environmental risk factors, such as smoking, diabetes, hypertension, hyperlipidemia, and hypercholesterolemia could have obscured the relationship between calgranulin gene variants and IS. Finally, the particular methods by which these calgranulin gene variants affect the expression of S100A8, S100A9, and S100A12 are unknown. Before the conclusions are evaluated for predicting individuals’ risk of having IS, more independent validation of these outcomes in larger groups with varied ethnic backgrounds should be undertaken.
Finally, our research is the first to show a correlation between calgranulin gene variants and susceptibility to IS in a Southern Chinese population. The S100A8 rs3795391, rs3806232, and S100A12 rs2916191 variant C allele is linked to elevated circulating S100A8 and S100A12 levels as well as larger infarct volumes in IS patients. Our research could lead to a better understanding of individual susceptibility to IS and the development of effective IS control and preventive strategies. Future research is needed to confirm our findings and give an understanding of how calgranulin variants affect IS pathogenesis.
Author Contributions
All authors have made significant contributions to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors have no actual or potential conflicts of interest related to this manuscript.
Acknowledgments
This work was supported by funding from the National Nature Science Foundation of China (grant numbers 81571157, 81300929 and 81670252), the Natural Science Foundation of Guangdong Province (grant numbers 2019A1515011424 and 2019A1515011306), the Youth Cultivation Foundation of Guangdong Medical University (grant number GDMUQ2021006), the Medical Scientific Research Foundation of Guangdong Province, China (grant number:A2022139) and the Non-funded Science and Technology Research Foundation of Zhanjiang City (grant number:2021B01370). We also thank MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
References
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1). e2–e220.
- Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–495. doi:10.1161/CIRCRESAHA.116.308398
- Donato R, Sorci G, Riuzzi F, et al. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793(6):1008–1022. doi:10.1016/j.bbamcr.2008.11.009
- Donato R, Cannon BR, Sorci G, et al. Functions of S100 proteins. Curr Mol Med. 2013;13(1):24–57. doi:10.2174/156652413804486214
- Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793(6):993–1007. doi:10.1016/j.bbamcr.2008.11.016
- Cristovao JS, Gomes CM. S100 proteins in alzheimer’s disease. Front Neurosci. 2019;13:463. doi:10.3389/fnins.2019.00463
- Angelopoulou E, Paudel YN, Piperi C. Emerging role of S100B protein implication in Parkinson’s disease pathogenesis. Cell Mol Life Sci. 2021;78(4):1445–1453. doi:10.1007/s00018-020-03673-x
- He Y, Cai Z, Chen Y. Role of S-100beta in stroke. Int J Neurosci. 2018;128(12):1180–1187. doi:10.1080/00207454.2018.1481065
- Michetti F, D’Ambrosi N, Toesca A, et al. The S100B story: from biomarker to active factor in neural injury. J Neurochem. 2019;148(2):168–187. doi:10.1111/jnc.14574
- Nash DL, Bellolio MF, Stead LG. S100 as a marker of acute brain ischemia: a systematic review. Neurocrit Care. 2008;8(2):301–307. doi:10.1007/s12028-007-9019-x
- Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81(1):28–37. doi:10.1189/jlb.0306170
- Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41(4):821–842. doi:10.1007/s00726-010-0528-0
- Foell D, Wittkowski H, Ren Z, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216(2):183–192. doi:10.1002/path.2394
- Jonsson MK, Sundlisaeter NP, Nordal HH, et al. Calprotectin as a marker of inflammation in patients with early rheumatoid arthritis. Ann Rheum Dis. 2017;76(12):2031–2037. doi:10.1136/annrheumdis-2017-211695
- Frangogiannis NG. S100A8/A9 as a therapeutic target in myocardial infarction: cellular mechanisms, molecular interactions, and translational challenges. Eur Heart J. 2019;40(32):2724–2726. doi:10.1093/eurheartj/ehz524
- Guo D, Zhu Z, Xu T, et al. Plasma S100A8/A9 concentrations and clinical outcomes of ischemic stroke in 2 independent multicenter cohorts. Clin Chem. 2020;66(5):706–717. doi:10.1093/clinchem/hvaa069
- Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003;60(6):569–580. doi:10.1002/jemt.10299
- Armstrong CW, Bosio E, Neil C, Brown SG, Hankey GJ, Fatovich DM. Distinct inflammatory responses differentiate cerebral infarct from transient ischaemic attack. J Clin Neurosci. 2017;35:97–103. doi:10.1016/j.jocn.2016.09.011
- Wakisaka Y, Ago T, Kamouchi M, et al. Plasma S100A12 is associated with functional outcome after ischemic stroke: research for Biomarkers in Ischemic Stroke. J Neurol Sci. 2014;340(1–2):75–79. doi:10.1016/j.jns.2014.02.031
- Zhang X, Shen R, Shu Z, Zhang Q, Chen Z. S100A12 promotes inflammation and apoptosis in ischemia/reperfusion injury via ERK signaling in vitro study using PC12 cells. Pathol Int. 2020;70(7):403–412. doi:10.1111/pin.12924
- Sun X, Meng H, Shi D, et al. Analysis of plasma calprotectin and polymorphisms of S100A8 in patients with aggressive periodontitis. J Periodontal Res. 2011;46(3):354–360. doi:10.1111/j.1600-0765.2011.01350.x
- Blanco-Rojo R, Delgado-Lista J, Lee Y-C, et al. Interaction of an S100A9 gene variant with saturated fat and carbohydrates to modulate insulin resistance in 3 populations of different ancestries. Am J Clin Nutr. 2016;104(2):508–517. doi:10.3945/ajcn.116.130898
- Salminen A, Vlachopoulou E, Havulinna AS, et al. Genetic variants contributing to circulating matrix metalloproteinase 8 levels and their association with cardiovascular diseases: a genome-wide analysis. Circ Cardiovasc Genet. 2017;10:6. doi:10.1161/CIRCGENETICS.117.001731
- Farkas G Jr, Tiszlavicz Z, Takacs T, et al. Analysis of plasma levels and polymorphisms of S100A8/9 and S100A12 in patients with acute pancreatitis. Pancreas. 2014;43(3):485–487. doi:10.1097/MPA.0000000000000046
- Li Y, Liao F, Yin XJ, et al. An association study on ADAM10 promoter polymorphisms and atherosclerotic cerebral infarction in a Chinese population. CNS Neurosci Ther. 2013;19(10):785–794.
- Buck BH, Liebeskind DS, Saver JL, et al. Association of higher serum calcium levels with smaller infarct volumes in acute ischemic stroke. Arch Neurol. 2007;64(9):1287–1291. doi:10.1001/archneur.64.9.1287
- Sun P, Li Q, Zhang Q, Xu L, Han JY. Upregulated expression of S100A8 in mice brain after focal cerebral ischemia reperfusion. World J Emerg Med. 2013;4(3):210–214. doi:10.5847/wjem.j.issn.1920-8642.2013.03.010
- Shichita T, Ito M, Morita R, et al. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat Med. 2017;23(6):723–732. doi:10.1038/nm.4312
- Yan L, Bjork P, Butuc R, et al. Beneficial effects of quinoline-3-carboxamide (ABR-215757) on atherosclerotic plaque morphology in S100A12 transgenic ApoE null mice. Atherosclerosis. 2013;228(1):69–79. doi:10.1016/j.atherosclerosis.2013.02.023
- Oesterle A, Bowman MA. S100A12 and the S100/calgranulins: emerging biomarkers for atherosclerosis and possibly therapeutic targets. Arterioscler Thromb Vasc Biol. 2015;35(12):2496–2507. doi:10.1161/ATVBAHA.115.302072
- Croce K, Gao H, Wang Y, et al. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120(5):427–436. doi:10.1161/CIRCULATIONAHA.108.814582
- Ehlermann P, Eggers K, Bierhaus A, et al. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi:10.1186/1475-2840-5-6
- Marta-Enguita J, Navarro-Oviedo M, Rubio-Baines I, et al. Association of calprotectin with other inflammatory parameters in the prediction of mortality for ischemic stroke. J Neuroinflammation. 2021;18(1):3. doi:10.1186/s12974-020-02047-1
- Qian SQ, He SR, Li BB, Qian J, Zheng XD. Serum S100A12 and 30-day mortality after acute intracerebral hemorrhage. Clin Chim Acta. 2018;477:1–6. doi:10.1016/j.cca.2017.11.032
- Singh V, Roth S, Veltkamp R, Liesz A. HMGB1 as a key mediator of immune mechanisms in ischemic stroke. Antioxid Redox Signal. 2016;24(12):635–651. doi:10.1089/ars.2015.6397
- Ziegler G, Prinz V, Albrecht MW, et al. Mrp-8 and −14 mediate CNS injury in focal cerebral ischemia. Biochim Biophys Acta. 2009;1792(12):1198–1204. doi:10.1016/j.bbadis.2009.10.003
- Kawano T, Shimamura M, Nakagami H, et al. Therapeutic vaccine against S100A9 (S100 calcium-binding protein A9) inhibits thrombosis without increasing the risk of bleeding in ischemic stroke in mice. Hypertension. 2018;72(6):1355–1364. doi:10.1161/HYPERTENSIONAHA.118.11316
- Bjork P, Bjork A, Vogl T, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7(4):e97. doi:10.1371/journal.pbio.1000097
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–289. doi:10.1038/nri2215
- Kim JB, Sig Choi J, Yu YM, et al. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26(24):6413–6421. doi:10.1523/JNEUROSCI.3815-05.2006
- Li Y, Zhu J, Chen L, et al. Genetic predisposition to ischaemic stroke by RAGE and HMGB1 gene variants in Chinese Han population. Oncotarget. 2017;8(59):100150–100164. doi:10.18632/oncotarget.22112