ABSTRACT
Background
Allergic rhinitis (AR) is a nasal inflammatory disease resulting from a complex interplay between genetic and environmental factors. The association between Toll-like receptor (TLR) signaling pathway and environmental factors in AR pathogenesis remains to be explored. This study aims to assess the genetic association of AR with single nucleotide polymorphisms (SNPs) in TLR signaling pathway, and investigate the roles of gene–gene and gene–environment interactions in AR.
Methods
A total of 452 AR patients and 495 healthy controls from eastern China were enrolled in this hospital-based case–control study. We evaluated putatively functional genetic polymorphisms in TLR2, TLR4 and CD14 genes for their association with susceptibility to AR and related clinical phenotypes. Interactions between environmental factors (such as traffic pollution, residence, pet keeping) and polymorphisms with AR were examined using logistic regression. Models were stratified by genotype and interaction terms, and tested for the significance of gene–gene and gene–environment interactions.
Results
In the single-locus analysis, two SNPs in CD14, rs2563298 (A/C) and rs2569191 (C/T) were associated with a significantly decreased risk of AR. Compared with the GG genotype, the GT and GT/TT genotypes of TLR2 rs7656411 (G/T) were associated with a significantly increased risk of AR. Gene–gene interactions (eg, TLR2 rs7656411, TLR4 rs1927914, and CD14 rs2563298) was associated with AR. Gene–environment interactions (eg, TLR4 or CD14 polymorphisms and certain environmental exposures) were found in AR cases, but they were not significant after Bonferroni correction.
Conclusion
The genetic polymorphisms of TLR2 and CD14 and gene–gene interactions in TLR signaling pathway were associated with susceptibility to AR in this Han Chinese population. However, the present results were limited to support the association between gene–environment interactions and AR.
Introduction
Allergic rhinitis (AR) is a nasal inflammatory disease induced by immunoglobulin E (IgE)-mediated immune response, with main symptoms of itchy nose, rhinorrhea, sneezing and nasal congestion.Citation1 The prevalence of AR keeps increasing worldwide. In China, the AR prevalence in adults increased from 11.1% in 2005 to 17.6% in 2011.Citation2,Citation3 AR and asthma often coexist, both triggered by genetic and environmental factors.Citation4 The development of AR involves not only allergen exposure but also early-life factors, family history, ethnicity, and environmental factors, such as tobacco smoke, cooking fumes, living floors, lifestyle, air pollution and furniture pollution.
Toll-like receptors (TLRs) are type I transmembrane protein natural immune pattern recognition receptors, which can recognize pathogen-associated molecular patterns (PAMP) in nature, initiate intracellular signaling pathways, and activate innate immune response. In addition, TLRs also induce dendritic cell (DC) maturation and T cell activation, thus skewing the adaptive immune response towards Th1,Citation5 and participate in the induction and perpetuation of asthma and atopy.Citation6 Given the mediatory role of TLRs between innate and adaptive immunity, genetic variations of TLR signaling pathway genes may drive the progression of inflammatory and allergic diseases. A number of studies have demonstrated the association between single nucleotide polymorphisms (SNPs) of TLR signaling pathway genes and asthma in diverse populations;Citation7–14 however, little attention has been given to rhinitis.Citation15–18
According to the hygiene hypothesis,Citation19 the allergic diseases may arise from bacterial or viral infections and exposure to non-infectious microbial agents (such as endotoxin) in the environment.Citation20 TLR signaling pathway genes may participate in the protective effect of microbial agents on allergy. Many studies that have examined the interplay between genetic susceptibility and environmental factors in allergic conditions only focus on asthma. A gene–environment interaction has been observed between CD14 rs2569190 and asthma, depending on the endotoxin exposure level in house dust.Citation21 Moreover, the associations between polymorphisms of TLR2, TLR4, TLR6, TLR9 and CD14 genes in asthma were affected by environmental factors, such as house dust endotoxin level and living place.Citation10,Citation22–24 Gene–environment interactions, which have rarely been analyzed in previous studies of AR, may provide new insight into AR pathogenesis. One study on AR children has shown no significant evidence of gene–environment interactions between traffic-related air pollution and GSTP1, TNF, TLR2, and TLR4 genes.Citation17 At present, there is no literature reporting gene–gene and gene–environment interactions in AR based on a Chinese population. This study was the first to identify the associations of candidate genes and environmental factors with genetic predisposition to AR in the Chinese Han population.
According to the data about weather, air quality, transportation and lifestyle in East China, we selected several environmental factors that may be related to AR. We screened 10 SNPs of TLR2, TLR4 and CD14 genes in the TLR signaling pathway, and conducted a case–control study in East China Han population to explore the association between polymorphisms and AR, as well as gene–gene and gene–environment interactions.
Methods
Subjects
A total of 452 AR cases (302 males and 150 females) and 495 healthy controls (303 males and 192 females) were recruited from the First Affiliated Hospital of Nanjing Medical University, Nanjing, China. All subjects were Han Chinese in Jiangsu and Anhui provinces in eastern China. The diagnosis of AR was established according to the “Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update”Citation1 and the “Chinese Society of Allergy Guidelines for Diagnosis and Treatment of Allergic Rhinitis”.Citation3 The medical history and clinical data were collected from outpatients’ medical records and face-to-face questionnaire surveys. The cases did not use glucocorticoids within 4 weeks, and antihistamines, leukotriene receptor antagonists and other anti-allergic drugs within 2 weeks before blood sampling. The controls were recruited from the hospital seeking health care or routine health examinations, and were frequency-matched with the cases in age (±5 years) and sex. The selection criteria for controls:Citation25,Citation26 (1) no symptoms and medical history of AR and nasal diseases; (2) no symptoms and medical history of other allergic diseases, such as asthma, eczema and urticaria; (3) negative blood test for serum allergen-specific IgE; (4) no history of AR or other allergic diseases in the immediate family. All the study subjects were divided into < 18-year-old group and ≥ 18-year-old group for further stratification analysis.
Clinical Parameters
The questionnaire survey covered general information, demographic characteristics, disease symptoms and scores, physical signs, medical history, accompanying diseases, and environmental factors, including smoking, residence (floors), sunlight exposure, in-house chemicals, near-residence pollutants, near-residence traffic network, radiation exposure to computers, oil fume exposure and pet-keeping. A visual analogue scale (VAS) ranging from 0 cm (not bothersome at all) to 10 cm (extremely bothersome) was used to assess the patient’s subjective perception of nasal symptoms, including rhinorrhea, sneezing, nasal congestion, itchy nose and eyes, and total nasal symptoms. VAS scores < 5 were diagnosed as mild AR, and VAS scores ≥ 5 as moderate-to-severe AR.Citation27
About 5 mL of peripheral venous blood was collected from each subject for in vitro allergen detection. The levels of eosinophil cationic protein (ECP), serum total IgE and specific IgE were measured with ImmunoCAP assays (Phadia, Uppsala, Sweden). Total IgE and ECP were determined in all subjects. Specific IgE antibodies to common inhalant allergens including Dermatophagoides pteronyssinus (Der p; d1), Dermatophagoides farinae (Der f; d2), cat dander (e1), dog dander (e5), Blatella germanica (i6), Alternaria alternata (m6), Ambrosia elatior (w1), and Artemisia vulgaris (w6) were determined in AR cases. We collected patients allergic to dust mites (d1 and/or d2), one of the most common allergens in eastern China. The positive rates of other aeroallergens (e1, e5, i6, m6, w1 and w6) were low, and they were not the main allergens causing symptoms.
SNPs Selection and Genotyping
A total of 10 SNPs from TLR2, TLR4 and CD14 genes in the TLR signaling pathway were obtained: rs7656411 (G/T), rs76112010 (A/G) and rs7682814 (A/G) of TLR2 gene; rs10983755 (A/G), rs11536889 (G/C), rs1927914 (A/G) and rs7873784 (G/C) of TLR4 gene; rs2563298 (A/C), rs2569190 (A/G) and rs2569191 (C/T) of CD14 gene (). The data of 10 SNPs were selected by using genotype data of Han Chinese in Beijing (CHB) and Japanese in Tokyo (JPT) from the 1000 Genomes Project (March 2012) and based on previously published studies.Citation12–17,Citation28,Citation29 Distributions of all genotypes in the control subjects were consistent with those estimated according to the minor allele frequency (MAF) > 0.05 and P-value of Hardy-Weinberg equilibrium (HWE) > 0.05. In addition, web-based tools were used to predict putative functions of genetic variants, including SNPinfo (https://snpinfo.niehs.nih.gov/), HaploReg (https://pubs.broadinstitute.org/mammals/haploreg/haploreg_v4.php), RegulomeDB (https://www.regulomedb.org/regulome-search/), MirSNP (http://bioinfo.bjm-u.ed-u.cn/mirsnp/search/), and RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/). Secondary structural changes and minimum free energy (MFE) changes caused by different SNP genotypes were predicted by using RNAfold (http://rna.tbi.univie.ac.at/).
Table 1 Gene Frequency Distribution of TLR2, TLR4 and CD14 Alleles
Genomic DNA was purified from peripheral blood leukocytes using a commercial kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s instructions, and stored at –70°C until usage. Genotyping was performed with the TaqMan SNP Genotyping Assay using the 384-well ABI 7900HT Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). More than 15% of the samples were randomly selected for confirmation, and the discordance rate between genotypes was below 0.3%.
Statistical Analysis
The values of total IgE, specific IgE and ECP were transformed by log logarithm. The Student’s t-test was performed to analyze continuous variables, and the χ2 test to analyze categorical variables and the genotype distributions of SNPs in two groups. HWE of the genotype distribution in controls was tested by a goodness-of-fit χ2 test. The odds ratios (OR) and 95% confidence intervals (CI) were calculated by logistic regression analysis after adjustment for gender and age, to quantify the association between the polymorphisms and risk of AR. Gene–gene interactions were analyzed using multifactor dimensionality reduction (MDR) software (http://sourceforge.net/projects/mdr/). Logistic regression analyses were performed to explore gene–environment interactions, with adjustment for gender and age. Calculations were carried out using Statistical Analysis System software (version 9.1.3; SAS Institute, Cary, NC, USA). A P-value < 0.05 was considered statistically significant, and all statistical tests were two-sided. The Bonferroni correction (P < 0.05 divided by the number of SNPs analyzed, P < 0.005) was applied to adjust for multiple comparisons.
Results
Characteristics of Subjects
The demographic characteristics of the study subjects are summarized in . There was no significant difference in the distribution of age (P = 0.597) and sex (P = 0.073). Apparently, serum levels of total IgE (264.0 [120.6–593.5] kU/L) and ECP (12.7 [5.2–28.7] μg/L) in AR cases were significantly higher (P < 0.001) than those in controls (26.8 [11.1–52.2] kU/L and 4.6 [3.1–7.5] μg/L, respectively). In AR cases, the serum levels of allergen-specific IgE against Der p and Der f were 29.0 (4.6–72.5) kUA/L and 24.1 (5.0–69.9) kUA/L, respectively. A total of 247 (54.6%) cases presented mild (VAS < 5) and 194 (42.9%) presented moderate-to-severe (VAS ≥ 5) AR, with a mean VAS score of 5.25 ± 2.39; 118 (26.1%) cases reported to have concomitant asthma. According to the questionnaire, asthma information missed in 63 (13.9%) patients, for the related questions in their questionnaires were not answered.
Table 2 Distribution of Selected Variables Among Cases and Controls
Association Between SNPs and AR Risk in Single-Locus Analyses
The genotype and allele distributions of the 10 SNPs and their associations with AR risk are presented in . The single-locus analyses revealed that the genotype frequencies of two SNPs rs2563298 (A/C) and rs2569191 (C/T) in CD14 were significantly different between the cases and the controls (rs2563298: P = 0.003; rs2569191: P = 0.008). Multivariate logistic regression analysis indicated that the variant CA and AA genotypes of CD14 rs2563298 were associated with a significantly decreased risk of AR, compared with the wild-type CC genotype (adjusted OR = 0.65, 95% CI = 0.48–0.89 for CA and adjusted OR = 0.40, 95% CI = 0.18–0.89 for AA). For the CD14 rs2569191, compared with the CC genotype, the CT and TT genotypes were associated with a significantly decreased risk of AR (adjusted OR = 0.65, 95% CI = 0.48–0.87 for CT and adjusted OR = 0.64, 95% CI = 0.44–0.95 for TT). We also found that the dominant models of CD14 rs2563298 (CA/AA vs CC) and rs2569191 (CT/TT vs CC) were significantly associated with AR risk (adjusted OR = 0.62, 95% CI = 0.46–0.83 for rs2563298 and 0.65, 0.49–0.85 for rs2569191). The genotype and allele frequencies of TLR2 rs7656411 showed no significant difference between cases and controls; however, compared with the GG genotype, the GT and GT/TT genotypes were associated with a significantly increased risk of AR (adjusted OR = 1.41, 95% CI = 1.04–1.91 for GT and adjusted OR = 1.35, 95% CI = 1.01–1.79 for GT/TT). No significant difference was found between the associations of genotype and allele frequencies with AR susceptibility in the other seven SNPs (P > 0.05).
Table 3 Genotype and Allele Frequencies in TLR2, TLR4 and CD14 Polymorphisms Among Cases and Controls
Association of Stratification Analysis Between the SNPs and AR
In the stratification analysis (), compared with the controls, the dominant model of TLR2 rs7656411 (GT/TT vs GG) exhibited a significantly increased risk of AR in the subgroups of males (adjusted OR = 1.60, 95% CI = 1.12–2.30) and concomitant asthma (adjusted OR = 1.61, 95% CI = 1.13–2.28). Furthermore, a significantly decreased risk of AR was found in the dominant model of CD14 rs2563298 (CA/AA vs CC) in the subgroups of < 18-year-old, females, lower total IgE, mild symptoms, moderate-to-severe symptoms, and asthma (with/without). This decreased risk was also more pronounced in the dominant model of CD14 rs2569191 (CT/TT vs CC) among all the subgroups of age, gender, asthma, VAS, and total IgE, compared with that in the controls. However, only the associative significance of CD14 rs2563298 in the subgroup of < 18-year-old, females, without asthma and lower total IgE, and that of CD14 rs2569191 in the subgroup of without asthma, moderate-to-severe symptoms and lower total IgE remained statistically evident after Bonferroni correction.
Table 4 Stratification Analyses of TLR2, TLR4 and CD14 Polymorphisms in the Dominant Model in Cases and Controls
Locus–Locus Interactions of SNPs in AR
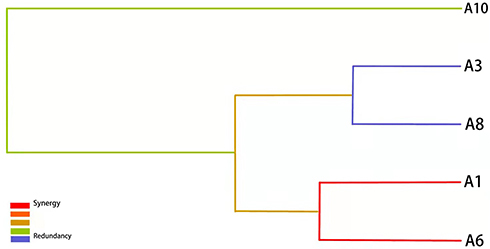
We investigated the locus–locus interactions of 10 SNPs in TLR2, TLR4 and CD14 genes using MDR analysis. As shown in , CD14 rs2569191 polymorphism was a significant single-locus model, with a cross-validation consistency (CVC) of 8/10 and a test accuracy of 53.52% (P = 0.0036), and the best interaction model was the three-factor model (TLR2 rs7656411, TLR4 rs1927914 and CD14 rs2563298), with a testing accuracy to 53.08% and a CVC of 6/10 (P < 0.0001), as determined empirically by the permutation testing.
Table 5 Multifactor Dimensionality Reduction Models for Locus–Locus Interactions
Besides, we applied the interaction dendrogram to determine whether there was a synergistic relationship among these polymorphisms in the best model (). The closer distance between TLR2 rs7656411 and TLR4 rs1927914 in the diagram indicated a stronger synergistic interaction, which showed that there may be a gene–gene synergistic interaction between TLR2 and TLR4.
Figure 1 Tree diagram of the best genotype models. The distance between single nucleotide polymorphisms (SNPs) indicates the intensity of the interactions. The color indicates the type of interactions. Red, orange, and green denote strong, moderate and weak interactions, respectively. A1: rs7656411; A3: rs7682814; A6: rs1927914; A8: rs2563298; A10: rs2569191.
Gene–Environment Interactions in AR Cases
As shown in , a total of 237 patients in the case group were asked to answer questionnaires covering environmental factors, including smoking, living or working floors, sunlight exposure, recently renovated or purchased furniture, polluting enterprises nearby, distance to main road, time of using computer, cooking fumes and pet keeping. Since these environmental data are difficult to be obtained from the controls, we only questionnaire-surveyed the cases, and explored gene–environment interactions through the logistic regression in a case-only study.Citation30
Table 6 Statistics of Environmental Factors in AR Case Group
The correlations between environmental factors and polymorphisms of TLR4 and CD14 were evident in AR patients (). Compared with homozygous wild type GG, the proportion of AR patients with TLR4 rs10983755 GA/AA genotype living or working on the ≥ 4 floor was significantly less than that on the < 4 floor (OR = 0.51, 95% CI = 0.27–0.99), indicating a negative correlation between TLR4 rs10983755 and floors (P = 0.048). TLR4 rs11536889 had also a negative correlation with pet keeping (P = 0.043), while AR cases in the pet group were significantly less than those in the non-pet group (OR = 0.12, 95% CI = 0.02–0.93) under the dominant model (GC/CC vs GG). In addition, sunlight exposure exhibited significantly positive correlation with three SNPs (OR = 2.66, 95% CI = 1.12–6.34 for TLR4 rs7873784; 2.22, 1.00–4.90 for CD14 rs2569190 and 2.56, 1.15–5.73 for CD14 rs2569191). However, none of these interactions withstood Bonferroni correction. No significant interactions were observed between genotypes of other SNPs and environmental factors (data not shown).
Table 7 Association Between Polymorphisms and Environmental Factors in the Dominant Model Among AR Case Group
Functional Assessment of SNPs
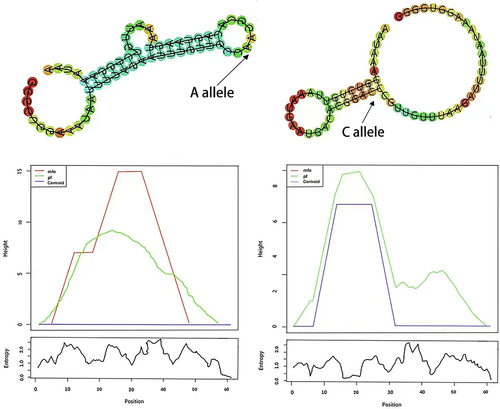
As shown in , using SNPinfo, HaploReg, RegulomeDB and MirSNP, we performed a functional annotation analysis of these 10 SNPs and estimated that TLR2 rs7656411, CD14 rs2563298 and CD14 rs2569191 all possessed promoter histone marks, enhancer histone marks, changed motifs, and selected expression quantitative trait locus (eQTL) hits. Moreover, for CD14 rs2563298 located in the 3ʹUTR region, using MirSNP and RNAhybrid, we predicted that miR-451 can be bound to rs2563298 A-allele but not to rs2563298 C-allele. RNAfold predicted that rs2563298 A to C substitution led to alteration of CD14 secondary structure, with the MFE decreasing from −6.8 kcal/mol to −8.0 kcal/mol ().
Table 8 Functional Annotation Information of 10 SNPs
Discussion
TLRs modulate Th1/Th2 immune balance via a variety of cells closely related to AR, such as dendritic cells, mast cells and regulatory T-cells (Treg). In this study, we explored the associations of genetic variations and environmental factors with the susceptibility to AR in a Chinese Han population. The selected genes, TLR2, TLR4 and CD14, are implicated in the pathogenesis of allergy. We found that polymorphisms of rs2563298 (A/C) and rs2569191 (C/T) in CD14, and rs7656411 (G/T) in TLR2 were significantly associated with AR. In addition, gene–gene and gene–environment interactions may contribute to the development of AR.
TLR2 has a wide recognition spectrum, which can recognize bacterial peptidoglycans, lipoproteins, viral envelope proteins and other microbial components, activate signal transduction pathways, and induce adaptive immunity. In this study, the GT/TT genotype of rs7656411 in TLR2 was associated with AR risk (P = 0.035). The rs7656411 was located in the 3ʹnear gene region, which may participate in mRNA transcription and affect gene expression. TLR2 rs7656411 was first reported to be associated with AR, while this rs7656411 has been confirmed to be associated with asthma in Chinese population.Citation7 Few studies have investigated the role of TLR2 SNPs in the etiology of AR.Citation15,Citation17 The results of studies on TLR2 SNPs and the risk of asthma in different populations are contradictory.Citation22,Citation31–35 Moreover, meta-analyses analyzed the correlation of four SNPs (rs5743708, rs3804099, rs3804100 and rs4696480) of TLR2 to susceptibility of asthma, suggesting that rs4696480 and rs3804099 were associated with asthma.Citation9,Citation11
TLR4 acts as a surface receptor on macrophages for bacterial endotoxin or lipopolysaccharide (LPS) in a dose-dependent manner, and activates macrophages to produce cytokines that affect Th1/Th2 balance. A recent study reported that polymorphisms of rs4986790 and rs4986791 in TLR4 were associated with COVID-19 severity, cytokine storm, and mortality,Citation36 while the association between these two SNPs with asthma was almost negative result.Citation10,Citation31,Citation32 In this study, four SNPs of TLR4 (rs10983755, rs11536889, rs1927914 and rs7873784) were not significantly associated with AR risk. A lack of association between TLR4 SNPs and asthma risk was also reported in a Chinese population with two SNPs (rs10983755 and rs1927914),Citation12 and a European population with rs11536889;Citation13 whereas rs10759930 in TLR4 was associated with AR risk.Citation37 However, more negative results have demonstrated that TLR4 gene polymorphisms may not be strongly associated with allergic diseases; this may be explained by the effect of endotoxin level, the race of the population, and the complexity of the allergic mechanism.
In this study, three SNPs of CD14 were located in the functional region: rs2563298 was located in the 3ʹUTR region, which may participate in the translation of mRNA regulated by microRNA; rs2569190 and rs2569191 were located in the 5ʹUTR region and 5ʹnear gene region, which may be involved in the expression of promoter region. Our study first explored that the polymorphisms of rs2563298 and rs2569191 in CD14 significantly decreased the risk of AR in Chinese population. Studies in EgyptCitation14 and PakistanCitation28 confirmed that rs2569191 polymorphism was associated with asthma. No association between rs2569190 polymorphism and AR was also reported in a meta-analysis.Citation29 However, a study from northern ChinaCitation16 found that the TT genotype of rs2569190 was associated with AR. This contradiction may arise from the low reliability of the results in that study with a small sample size (92 cases and 72 controls) and the environmental differences in regions between northern and eastern China.
In this study, 118 patients (26.1%) with AR had concomitant asthma. AR and asthma are interrelated in many aspects, including epidemiology, physiology, histology, immunopathology and therapeutic principles. Since upper and lower respiratory tract diseases usually coexist in one patient in an interdependent manner,Citation38 the concept of “one airway, one disease” has been proposed.Citation1 In the stratification analysis, the mutation heterozygous/homozygous genotypes of rs2563298 and rs2569191 in CD14 gene could reduce the risk of AR with and without asthma, and the AR without asthma demonstrated higher associative significance to withstand the Bonferroni correction. We speculate that AR combined with asthma may bring about more serious allergic symptoms than simple AR, suggesting that the genetic polymorphisms do play a protective role in the allergic diseases. A study of French population has suggested that the heterozygous/homozygous genotype of CD14 rs2563298 can reduce the risk of allergic asthma.Citation22 Another study in a Chinese population has shown that the frequency of CD14 rs2569191 A-allele in allergic asthma is significantly lower than that in non-allergic asthma.Citation39 Moreover, we believe that although AR and asthma share many similarities, the inhibitory effects of genetic variations in TLR pathway on AR, asthma or other allergic diseases may differ to some extent, as Micheal et alCitation28 confirmed that in Pakistani adults, the CD14 rs2569191 is significantly associated with atopic asthma, but not AR.
In the stratification analysis, the mutation heterozygous/homozygous of rs2563298 (CA/AA) and rs2569191 (CT/TT) in CD14 reduced the risk of AR in patients with lower IgE levels, while rs2569190 was not associated with total IgE levels. However, the association between CD14 rs2569190 and atopy has been confirmed in studies of diverse populations including French,Citation40 AustralianCitation41 and Chinese,Citation42 but not in GermanCitation43 and JapaneseCitation44 populations. The controversial results may be explained by the various alleles frequencies among races. Furthermore, Martinez et alCitation21 have reported that the CD14 rs2569190 C-allele is a risk factor for allergic phenotypes at low levels of endotoxin exposure, whereas the T-allele is a risk factor at high levels of exposure, suggesting CD14 polymorphisms may be associated with allergic sensitization and environmental endotoxin exposure. After Bonferroni correction, this study still showed that CD14 rs2569191 was associated with AR severity evaluated by VAS score. The associations of SNPs with allergic disease severity vary across studies.Citation33,Citation45 VAS is a tool to evaluate AR severity using subjective indexes which may depend on individual cognition and sensitivity. Therefore, the correlation with disease severity cannot be fully explained from the perspective of SNPs alone, since the severity of AR is related to many other factors, such as quality of life, sleep status, daily activities, workload, course of disease, and so on.
Using MDR model, we discovered that the locus–locus interactions of TLR2, TLR4 and CD14 genes might be associated with the susceptibility to AR. In the presence of CD14, LPS can activate TLR2 or TLR4 signaling pathways to enhance Th2 type immune response.Citation46 The interactions between CD14 and allergy-related genes were also found in the Philippine allergic population (CD14, IL4, FCER1B, IL4RA and ADRB2)Citation47 and in Korean children with asthma (CD14, IL4RA, IL13, IL13RA1 and CTLA4).Citation48
A large number of studiesCitation49–52 have confirmed that environmental factors, including rural farm environment, tobacco smoke, traffic pollution, climate change, nutrition and occupational factors, are related to IgE levels, atopic and allergic diseases. At present, there are many studies on the role of gene–environment interaction in the pathogenesis of asthma in TLR pathway,Citation10,Citation22–24 but few on TLR pathway with AR.Citation17 In this study, 237 patients providing data about environmental factors were collected in a case-only study.Citation30 The premise of the application is that genotype and environmental exposure should occur as independent factors in a general population. In this study, we can only evaluate the factor–factor associations in cases but not the main effect between cases and controls. Although none of these interactions withstood Bonferroni correction, the results of the case-only analysis still suggest a possible interaction between gene and environment during the development of AR.
Most of the AR patients were collected from two provinces with a humid climate in East China. Asthma and allergic symptoms are associated with mold and humidity.Citation53 A study of 400 Swedish children showed that low home ventilation rate in combination with moldy odor from the building structure increased the risk of allergic symptoms in children.Citation54 Compared with the higher floors, the lower floors are more humid, moldy and ventilated, which may increase the susceptibility to AR. We found that the patients carrying TLR4 rs10983755 GA/AA genotype living on higher floors (≥ 4 F) were less than those living on lower floors (< 4 F), but this result did not pass the Bonferroni correction. According to the function annotation query (), TLR4 rs10983755 is located in the 5ʹnear gene region, which may enrich DNase hypersensitivity sites, eQTL hits and changed motifs and affect the expression of TLR4 protein. In the TLR signaling pathway, the binding of TLR4 to bacterial endotoxin or LPS was affected by the environmental humidity and moldiness in a dose-dependent manner. Therefore, we suspected that the mutation of TLR4 rs10983755 may have a synergistic effect with environmental humidity and moldiness in the pathogenesis of AR. Similarly, Chen et alCitation55 have found gene–environment interactions between the polymorphism rs769214 and moldy odor in AR children. However, more functional research is needed to verify this interaction.
Moreover, we deduced a positive interaction between gene polymorphisms and sunlight exposure in AR. We found that AR patients carrying CD14 rs2569191 CT/TT genotype were more prone to sunshine exposure, whereas CD14 rs2569191 T-allele decreased the risk of AR in this study. The lack of environmental data in the controls may cause data deviation. Therefore, the interaction between CD14 rs2569191 and sunlight exposure in AR, and related TLR pathways, needs to be further studied. Ultraviolet radiation (UVR) from sunlight stimulates anti-inflammatory and immunosuppressive pathways in the skin that modulate psoriasis, atopic dermatitis and some systemic diseases (such as multiple sclerosis, type 1 diabetes and asthma) through the actions of UVR-induced regulatory cells and mediators, including 1,25-dihydroxy vitamin D3, IL-10, and nitric oxide.Citation56 The potential beneficial effects of UVR in controlling allergic airways disease have been evaluated in murine asthma models showing that UVR on the skin can repress Th2 response to asthma.Citation57 Rueter et alCitation58 have reported that exposure to UVR can reduce the risk of eczema in early childhood but has undefined associations with other allergic disease outcomes. Furthermore, it has been discovered that vitamin D, from either sunshine exposure or oral intake, may function in the process of allergic diseases.Citation59 However, the association between vitamin D level and AR risk is still controversial.Citation60
In addition, pet-keeping seems to protect against AR in this study, though this result failed to challenge Bonferroni correction. Opinions differ a lot about whether pet-keeping can prevent allergic diseases.Citation61,Citation62 It has been established that the CD14 rs2569190 polymorphism is associated with increased total and specific serum IgE levels in children with frequent contact with pets.Citation63 Interestingly, another study has found that the sensibility to asthma is different between adults keeping cats and dogs.Citation64 Thereby, the effects of pet-keeping are influenced by various aspects, like pet species, individual allergic sensitivity and wide environmental exposure to allergen.
Several limitations exist in our study. First, this study only collected AR patients sensitive to dust mites in eastern China, but did not include patients allergic to other allergens (such as pollen, cockroach, and molds), for dust mites were the major allergens in this area. However, other allergens may also arouse various gene–environment interactions. Second, only analysis of gene–environment interactions in AR cases may not be powerful enough to cement the present evidence. A larger sample size and environmental data for healthy controls were required. Finally, functional experiments, especially combined with environmental factors, are required to further explain the correlation between genetic polymorphisms in TLR signaling pathway and AR pathogenesis from the perspective of the mechanism.
Conclusions
The polymorphisms of TLR2 and CD14 and gene–gene interactions in TLR signaling pathway were associated with susceptibility to AR in the Han Chinese population. However, the present results were limited to support the association between gene-environment interactions and AR. Moreover, more environmental data in the general population and functional studies are needed to warrant these findings.
Abbreviations
AR, allergic rhinitis; ARIA, allergic rhinitis and its impact on asthma; CHB, Han Chinese in Beijing; CI, confidence intervals; CVC, cross-validation consistency; DC, dendritic cell; ECP, eosinophil cationic protein; eQTL, expression quantitative trait locus; HWE, Hardy-Weinberg equilibrium; JPT, Japanese in Tokyo; LPS, lipopolysaccharide; MAF, minor allele frequency; MDR, multifactor dimensionality reduction; MFE, minimum free energy; OR, odds ratios; PAMP, pathogen-associated molecular patterns; SNPs, single nucleotide polymorphisms; TLR, toll-like receptor; Treg, regulatory T-cells; UTR, untranslated region; UVR, ultraviolet radiation; VAS, visual analogue scale.
Ethics Approval and Informed Consent
The research protocol complying with the Declaration of Helsinki was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2015-SRFA-122) and written informed consent was obtained from all participants.
Consent for Publication
We have obtained the informed consent from all patients or their legal guardians.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. All authors took part in drafting, revising, or critically reviewing the article; have agreed on the journal to which the article has been submitted; gave final approval for the version to be published; and agreed to be accountable for the contents of the article.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We are grateful to the participants in this study and all staff involved in the study throughout the years. We also thank associate professor Yong-Ke Cao at the College of Foreign Languages of Nanjing Medical University for professional English-language proofreading of the manuscript.
Additional information
Funding
References
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA (2) LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. doi:10.1111/j.1398-9995.2007.01620.x
- Wang XD, Zheng M, Lou HF, et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71(8):1170–1180. doi:10.1111/all.12874
- Cheng L, Chen J, Fu Q, et al. Chinese Society of Allergy guidelines for diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2018;10(4):300–353. doi:10.4168/aair.2018.10.4.300
- Warner JO. Asthma/rhinitis (The United Airway) and allergy: chicken or egg; which comes first? J Clin Med. 2020;9(5):1483. doi:10.3390/jcm9051483
- Parker LC, Prince LR, Sabroe I. Translational mini-review series on Toll-like receptors: networks regulated by Toll-like receptors mediate innate and adaptive immunity. Clin Exp Immunol. 2007;147(2):199–207. doi:10.1111/j.1365-2249.2006.03203.x
- Michels KR, Lukacs NW, Fonseca W. TLR activation and allergic disease: early life microbiome and treatment. Curr Allergy Asthma Rep. 2018;18(11):61. doi:10.1007/s11882-018-0815-5
- Qian FH, Zhang Q, Zhou LF, Jin GF, Bai JL, Yin KS. Polymorphisms in the toll-like receptor 2 subfamily and risk of asthma: a case-control analysis in a Chinese population. J Investig Allergol Clin Immunol. 2010;20(4):340–346.
- Korppi M, Törmänen S. Toll-like receptor 1 and 10 variations increase asthma risk and review highlights further research directions. Acta Paediatr. 2019;108(8):1406–1410. doi:10.1111/apa.14795
- Zhao J, Shang H, Cao X, et al. Association of polymorphisms in TLR2 and TLR4 with asthma risk: an update meta-analysis. Medicine. 2017;96(35):e7909. doi:10.1097/MD.0000000000007909
- Lau MY, Dharmage SC, Burgess JA, et al. The interaction between farming/rural environment and TLR2, TLR4, TLR6 and CD14 genetic polymorphisms in relation to early- and late-onset asthma. Sci Rep. 2017;7:43681. doi:10.1038/srep43681
- Gao Y, Xiao H, Wang Y, Xu F. Association of single-nucleotide polymorphisms in toll-like receptor 2 gene with asthma susceptibility: a meta-analysis. Medicine. 2017;96(20):e6822. doi:10.1097/MD.0000000000006822
- Zhang Q, Qian FH, Zhou LF, et al. Polymorphisms in toll-like receptor 4 gene are associated with asthma severity but not susceptibility in a Chinese Han population. J Investig Allergol Clin Immunol. 2011;21(5):370–377.
- Castro-Giner F, Künzli N, Jacquemin B, et al. Traffic-related air pollution, oxidative stress genes, and asthma (ECHRS). Environ Health Perspect. 2009;117(12):1919–1924. doi:10.1289/ehp.0900589
- Nabih ES, Kamel HF, Kamel TB. Association between CD14 polymorphism (−1145G/A) and childhood bronchial asthma. Biochem Genet. 2016;54(1):50–60. doi:10.1007/s10528-015-9699-4
- Kang I, Oh YK, Lee SH, Jung HM, Chae SC, Lee JH. Identification of polymorphisms in the Toll-like receptor gene and the association with allergic rhinitis. Eur Arch Otorhinolaryngol. 2010;267(3):385–389. doi:10.1007/s00405-009-1100-y
- Han D, She W, Zhang L. Association of the CD14 gene polymorphism C-159T with allergic rhinitis. Am J Rhinol Allergy. 2010;24(1):e1–3. doi:10.2500/ajra.2010.24.3411
- Fuertes E, Brauer M, MacIntyre E, et al. Childhood allergic rhinitis, traffic-related air pollution, and variability in the GSTP1, TNF, TLR2, and TLR4 genes: results from the TAG Study. J Allergy Clin Immunol. 2013;132(2):342–352. doi:10.1016/j.jaci.2013.03.007
- Henmyr V, Carlberg D, Manderstedt E, et al. Genetic variation of the Toll-like receptors in a Swedish allergic rhinitis case population. BMC Med Genet. 2017;18(1):18. doi:10.1186/s12881-017-0379-6
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi:10.1136/bmj.299.6710.1259
- von Mutius E. Allergies, infections and the hygiene hypothesis-the epidemiological evidence. Immunobiology. 2007;212(6):433–439. doi:10.1016/j.imbio.2007.03.002
- Martinez FD. CD14, endotoxin, and asthma risk: actions and interactions. Proc Am Thorac Soc. 2007;4(3):221–225. doi:10.1513/pats.200702-035AW
- Smit LA, Siroux V, Bouzigon E, et al. CD14 and toll-like receptor gene polymorphisms, country living, and asthma in adults. Am J Respir Crit Care Med. 2009;179(5):363–368. doi:10.1164/rccm.200810-1533OC
- Carnes MU, Hoppin JA, Metwali N, et al. House dust endotoxin levels are associated with adult asthma in a U.S. farming population. Ann Am Thorac Soc. 2017;14(3):324–331. doi:10.1513/AnnalsATS.201611-861OC
- Eder W, Klimecki W, Yu L, et al. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113(3):482–488. doi:10.1016/j.jaci.2003.12.374
- Chen RX, Lu WM, Lu MP, et al. Polymorphisms in MicroRNA target sites of TGF-beta signaling pathway genes and susceptibility to allergic rhinitis. Int Arch Allergy Immunol. 2021;182(5):399–407. doi:10.1159/000511975
- Zhu XJ, Lu MP, Chen RX, et al. Polymorphism −509C/T in TGFB1 promoter is associated with increased risk and severity of persistent allergic rhinitis in a Chinese population. Am J Rhinol Allergy. 2020;34(5):597–603. doi:10.1177/1945892420913441
- Bousquet PJ, Combescure C, Neukirch F, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. 2007;62(4):367–372. doi:10.1111/j.1398-9995.2006.01276.x
- Micheal S, Minhas K, Ishaque M, Ahmed F, Ahmed A. Promoter polymorphisms of the CD14 gene are associated with atopy in Pakistani adults. J Investig Allergol Clin Immunol. 2011;21(5):394–397.
- Chen ML, Zhao H, Huang QP, Xie ZF. Single nucleotide polymorphisms of IL-13 and CD14 genes in allergic rhinitis: a meta-analysis. Eur Arch Otorhinolaryngol. 2018;275(6):1491–1500. doi:10.1007/s00405-018-4975-7
- Greenland S. A unified approach to the analysis of case-distribution (case-only) studies. Stat Med. 1999;18(1):1–15. doi:10.1002/(SICI)1097-0258(19990115)18:1<1::AID-SIM961>3.0.CO;2-L
- Smit LA, Bongers SI, Ruven HJ, et al. Atopy and new-onset asthma in young Danish farmers and CD14, TLR2, and TLR4 genetic polymorphisms: a nested case-control study. Clin Exp Allergy. 2007;37(11):1602–1608. doi:10.1111/j.1365-2222.2007.02831.x
- Lachheb J, Dhifallah IB, Chelbi H, Hamzaoui K, Hamzaoui A. Toll-like receptors and CD14 genes polymorphisms and susceptibility to asthma in Tunisian children. Tissue Antigens. 2008;71(5):417–425. doi:10.1111/j.1399-0039.2008.01011.x
- Hussein YM, Awad HA, Shalaby SM, Ali AS, Alzahrani SS. Toll-like receptor 2 and Toll-like receptor 4 polymorphisms and susceptibility to asthma and allergic rhinitis: a case-control analysis. Cell Immunol. 2012;274(1–2):34–38. doi:10.1016/j.cellimm.2012.02.006
- Ortiz-Martínez MG, Frías-Belén O, Nazario-Jiménez S, López-Quintero M, Rodríguez-Cotto RI, Jiménez-Vélez BD. A case-control study of innate immunity pathway gene polymorphisms in Puerto Ricans reveals association of toll-like receptor 2 +596 variant with asthma. BMC Pulm Med. 2016;16(1):112. doi:10.1186/s12890-016-0272-7
- Dębińska A, Danielewicz H, Drabik-Chamerska A, Kalita D, Boznański A. Genetic polymorphisms in pattern recognition receptors are associated with allergic diseases through gene-gene interactions. Adv Clin Exp Med. 2019;28(8):1087–1094. doi:10.17219/acem/104538
- Taha SI, Shata AK, Baioumy SA, et al. Toll-like receptor 4 polymorphisms (896A/G and 1196C/T) as an indicator of COVID-19 severity in a convenience sample of Egyptian patients. J Inflamm Res. 2021;14:6293–6303. doi:10.2147/JIR.S343246
- He S, Chen J. [Correlation between TLR4 gene polymorphisms and allergic rhinitis]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;31(13):991–994. Chinese. doi:10.13201/j.issn.1001-1781.2017.13.005
- Sedaghat AR, Phipatanakul W, Cunningham MJ. Prevalence of and associations with allergic rhinitis in children with chronic rhinosinusitis. Int J Pediatr Otorhinolaryngol. 2014;78(2):343–347. doi:10.1016/j.ijporl.2013.12.006
- Feng J, Zhang C, Wang Z, Li Q, Li J, Wang H. Association between CD14 gene promoter polymorphisms with serum total-IgE and eosinophil levels in atopic and non-atopic asthma patients in a Chinese Han population. J Asthma. 2016;53(2):119–124. doi:10.3109/02770903.2015.1080267
- Leynaert B, Guilloud-Bataille M, Soussan D, et al. Association between farm exposure and atopy, according to the CD14 C-159T polymorphism. J Allergy Clin Immunol. 2006;118(3):658–665. doi:10.1016/j.jaci.2006.06.015
- O’Donnell AR, Toelle BG, Marks GB, et al. Age-specific relationship between CD14 and atopy in a cohort assessed from age 8 to 25 years. Am J Respir Crit Care Med. 2004;169(5):615–622. doi:10.1164/rccm.200302-278OC
- Zhang YN, Li YJ, Li H, Zhou H, Shao XJ. Association of CD14 C159T polymorphism with atopic asthma susceptibility in children from Southeastern China: a case-control study. Genet Mol Res. 2015;14(2):4311–4317. doi:10.4238/2015.April.30.3
- Sengler C, Haider A, Sommerfeld C, et al. Evaluation of the CD14 C-159 T polymorphism in the German Multicenter Allergy Study cohort. Clin Exp Allergy. 2003;33(2):166–169. doi:10.1046/j.1365-2222.2003.01549.x
- Nishimura F, Shibasaki M, Ichikawa K, Arinami T, Noguchi E. Failure to find an association between CD14-159C/T polymorphism and asthma: a family-based association test and meta-analysis. Allergol Int. 2006;55(1):55–58. doi:10.2332/allergolint.55.55
- Dutta S, Mondal P, Saha NC, et al. Role of offending out-door aero-allergen and CD14 C(−159)T polymorphism in development and severity of asthma in a Kolkata patient population. Afr Health Sci. 2017;17(4):1101–1109. doi:10.4314/ahs.v17i4.18
- Varadaradjalou S, Féger F, Thieblemont N, et al. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33(4):899–906. doi:10.1002/eji.200323830
- de Guia RM, Echavez MD, Gaw EL, et al. Multifactor-dimensionality reduction reveals interaction of important gene variants involved in allergy. Int J Immunogenet. 2015;42(3):182–189. doi:10.1111/iji.12200
- Choi WA, Kang MJ, Kim YJ, et al. Gene-gene interactions between candidate gene polymorphisms are associated with total IgE levels in Korean children with asthma. J Asthma. 2012;49(3):243–252. doi:10.3109/02770903.2012.660294
- Sozańska B, Blaszczyk M, Pearce N, Cullinan P. Atopy and allergic respiratory disease in rural Poland before and after accession to the European Union. J Allergy Clin Immunol. 2014;133(5):1347–1353. doi:10.1016/j.jaci.2013.10.035
- Ege MJ, von Mutius E. Atopy: a mirror of environmental changes? J Allergy Clin Immunol. 2014;133(5):1354–1355. doi:10.1016/j.jaci.2014.01.031
- Wu YC, James LK, Vander Heiden JA, et al. Influence of seasonal exposure to grass pollen on local and peripheral blood IgE repertoires in patients with allergic rhinitis. J Allergy Clin Immunol. 2014;134(3):604–612. doi:10.1016/j.jaci.2014.07.010
- Silverberg JI, Braunstein M, Lee-Wong M. Association between climate factors, pollen counts, and childhood hay fever prevalence in the United States. J Allergy Clin Immunol. 2015;135(2):463–469. doi:10.1016/j.jaci.2014.08.003
- Baxi SN, Portnoy JM, Larenas-Linnemann D, Phipatanakul W, Environmental Allergens Workgroup. Exposure and health effects of fungi on humans. J Allergy Clin Immunol Pract. 2016;4(3):396–404. doi:10.1016/j.jaip.2016.01.008
- Hägerhed-Engman L, Sigsgaard T, Samuelson I, Sundell J, Janson S, Bornehag CG. Low home ventilation rate in combination with moldy odor from the building structure increase the risk for allergic symptoms in children. Indoor Air. 2009;19(3):184–192. doi:10.1111/j.1600-0668.2008.00573.x
- Chen HI, Lin YT, Jung CR, Hwang BF. Interaction between catalase gene promoter polymorphisms and indoor environmental exposure in childhood allergic rhinitis. Epidemiology. 2017;28(Suppl 1):S126–S132. doi:10.1097/EDE.0000000000000741
- Hart PH, Norval M, Byrne SN, Rhodes LE. Exposure to ultraviolet radiation in the modulation of human diseases. Annu Rev Pathol. 2019;14(1):55–81. doi:10.1146/annurev-pathmechdis-012418-012809
- Gorman S, McGlade JP, Lambert MJ, Strickland DH, Thomas JA, Hart PH. UV exposure and protection against allergic airways disease. Photochem Photobiol Sci. 2010;9(4):571–577. doi:10.1039/b9pp00136k
- Rueter K, Jones AP, Siafarikas A, Chivers P, Prescott SL, Palmer DJ. The influence of sunlight exposure and sun protecting behaviours on allergic outcomes in early childhood. Int J Environ Res Public Health. 2021;18(10):5429. doi:10.3390/ijerph18105429
- Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D’s effect on immune function. Nutrients. 2020;12(5):1248. doi:10.3390/nu12051248
- Tian HQ, Cheng L. The role of vitamin D in allergic rhinitis. Asia Pac Allergy. 2017;7(2):65–73. doi:10.5415/apallergy.2017.7.2.65
- Eller E, Roll S, Chen CM, et al. Meta-analysis of determinants for pet ownership in 12 European birth cohorts on asthma and allergies: a GA2LEN initiative. Allergy. 2008;63(11):1491–1498. doi:10.1111/j.1398-9995.2008.01790.x
- Takkouche B, González-Barcala FJ, Etminan M, Fitzgerald M. Exposure to furry pets and the risk of asthma and allergic rhinitis: a meta-analysis. Allergy. 2008;63(7):857–864. doi:10.1111/j.1398-9995.2008.01732.x
- Eder W, Klimecki W, Yu L, et al. Opposite effects of CD 14/-260 on serum IgE levels in children raised in different environments. J Allergy Clin Immunol. 2005;116(3):601–607. doi:10.1016/j.jaci.2005.05.003
- Svanes C, Heinrich J, Jarvis D, et al. Pet-keeping in childhood and adult asthma and hay fever: European community respiratory health survey. J Allergy Clin Immunol. 2003;112(2):289–300. doi:10.1067/mai.2003.1596