Abstract
Introduction
There were few studies on the mortality of severe community-acquired pneumonia (SCAP) in elderly people. Early prediction of 28-day mortality of hospitalized patients will help in the clinical management of elderly patients (age ≥65 years) with SCAP, but a prediction model that is reliable and valid is still lacking.
Methods
The 292 elderly patients with SCAP met the criteria defined by the American Thoracic Society from 33 hospitals in China. Clinical parameters were analyzed by the use of univariable and multivariable logistic regression analysis. A nomogram to predict the 28-day mortality in elderly patients with SCAP was constructed and evaluated using the area under the receiver operating characteristic curve (AUC) and internally verified using the Bootstrap method.
Results
A total of 292 elderly patients (227 surviving and 65 died within 28 days) were included in the analysis. Age, Glasgow score, blood platelet, and blood urea nitrogen values were found to be significantly associated with 28-day mortality in elderly patients with SCAP. The AUC of the nomogram was 0.713 and the calibration curve for 28-day mortality also showed high coherence between the predicted and actual probability of mortality.
Conclusion
This study provides a nomogram containing age, Glasgow score, blood platelet, and blood urea nitrogen values that can be conveniently used to predict 28-day mortality in elderly patients with SCAP. This model has the potential to assist clinicians in evaluating prognosis of patients with SCAP.
Introduction
Community acquired pneumonia (CAP) continues to be a major cause of morbidity and mortality in adults despite advances in antibiotic treatment.Citation1,Citation2 Elderly people with pneumonia had high incidence and risk of death, almost one-half of the total hospitalizations for CAP occurred in patients over 65 years old in developed countries.Citation3 The severity of CAP also increases with age, which is primarily due to impaired gag reflex, decreased mucociliary function, waning immunity and degrees of cardiopulmonary dysfunction.Citation4 Three criteria including age, the severity of pneumonia at presentation and the presence of underlying diseases proposed by the American Thoracic Association are used to define appropriate empirical treatment of CAP.Citation5 However, some elderly patients with lower respiratory tract infection have atypical symptoms,Citation6 increasing the chance of delay in diagnosis and treatment which leads to disease progression.Citation7,Citation8
Previous study reported that the annual cost of treating patients aged ≥65 years with pneumonia was $4.8 billion, as $3.6 billion for those aged <65 years with pneumonia.Citation9 Few previous studies focused on elderly patients, although they represented a significant portion of patients with CAP, ie, 11.0–25.3%.Citation10–12 Early identification of factors related to poor prognosis might prevent severe or critical outcomes, such as death, and provide related data for physicians to make optimal clinical decisions. Therefore, there is an urgent need to establish a model for predicting prognosis in elderly patients with severe community-acquired pneumonia (SCAP).
The nomogram represents a visual statistical model that integrates multiple risk factors for predicting the outcome of a disease.Citation13 The aim of our study was to establish a nomogram that could incorporate demographics, clinical characteristics and laboratory parameters to predict the prognosis of elderly patients (≥65 years) admitted for SCAP.
Methods
Patients
Subjects were derived from a randomized, double-blind, placebo-controlled trial consisting of 710 patients with SCAP who met SCAP criteria defined by the American Thoracic Society,Citation14 between October 2014 and January 2016, at 33 public tertiary teaching hospitals in China. The methods and main results of this study were previously published.Citation15 Briefly, patients were eligible for inclusion if the following criteria were met: 18–75 years old adults, clinical symptoms suggestive of CAP (cough and other upper respiratory symptoms, etc.), acquired outside of the hospital or less than 48 hours after hospital admission, met SCAP criteria, fulfilled three or more of the following criteria: respiratory rate greater than or equal to 30 breaths/min, PaO2/FiO2 ratio less than or equal to 250 mm Hg, blood urea nitrogen greater than 20mg/dL, leukopenia (WBC count <4000 cells/mm3) not due to other causes, thrombocytopenia (platelet count <100,000 cells/mm3), hypothermia (core temperature <36°C), new onset mental confusion, receiving treatment with vasopressors at therapeutic doses after adequate fluid resuscitation, or radiographic findings of new pulmonary infiltrate(s) consistent with CAP diagnosis. Patients whose life expectancy was less than 48 hours or who were pregnant, lactating or diagnosed with severe primary diseases were excluded. Subjects were randomized (1:1) into groups receiving either XBJ or placebo (within 24 h of diagnosis of SCAP) for 5–7 days with a 28-day follow-up using a central randomization system. In post hoc analysis, patients were divided into survival and non-survival groups based on 28-day survival, and regression analysis was used to analyze the risk factors in the cohort. All patients enrolled in the trial received a standard therapy chosen by the attending physician according to the 2007 American Thoracic Society/Infectious Diseases Society of America guideline.Citation14
The participants were followed for 28 days after randomization, 28-day mortality (died within 28 days), the time of mechanical ventilation, and total duration of intensive care unit (ICU) stay were recorded.
Statistical Analysis
Statistical analysis was performed by R software (version 4.0.3). Categorized values were expressed by frequencies or percentage and continuous variables were expressed as mean ± standard deviation. All statistical tests were bilateral. The categorical variables were compared using Pearson’s χ2-test or Fisher’s exact test. The continuous variables were analyzed using Student’s t-test or Mann Whitney U-test, as appropriate. Variables with a P-value of ≤0.05 were included in the log-binomial model, and these independent risk factors were used to establish the predictive model. Univariate and multivariate regression analyses were used to analyze the risk factors in the cohort. A nomogram on the basis of the multivariate logistic regression model was constructed.
The internal verification of the model’s prediction effect adopted bootstrap self-sampling method, and the verification results were presented in the calibration curve. The odds ratios (OR) and 95% confidence interval (CI) were calculated. The discrimination of the nomogram was evaluated using the area under the receiver operating characteristic (ROC) curve. The calibration curve was constructed to determine whether the prediction result was consistent with the observation result or not.
Results
A total of 292 elderly patients with SCAP were enrolled at 33 centers from October 2014 to January 2016. Among them, 227 (77.74%) patients survived, while 65 (22.27%) patients did not survive. Demographic and basal clinical characteristics of all participants are listed in which was classified according to survival or non-survival. We used univariate and multivariate analyses to identify the prognostic factors. There were significant differences in age, Glasgow score (Coma Index), neutrophil, blood platelet, alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), serum creatinine, prothrombin time (PT), fibrinogen and activated partial thromboplastin time (APTT) at admission between the survival and non-survival groups (P < 0.05) ().
Table 1 Clinical Characteristics and Laboratory Parameters of Patients Aged ≥65 Years with Severe Community-Acquired Pneumonia in the Survival and Non-Survival Groups
Considering the existence of co-linearity, 7 potential risk factors of age, Glasgow score, neutrophil, blood platelet, ALT, BUN and PT were included in the log-binomial model analysis after the univariate analyses. Finally, 4 variables, including age (OR: 1.138, 95% CI: 1.037–1.253), Glasgow score (OR: 0.908, 95% CI: 0.838–0.985), blood platelet (OR: 0.996, 95% CI: 0.993–0.999), and BUN (OR: 1.061, 95% CI: 1.021–1.102) (), were used to build the model.
Table 2 Odds Ratios and 95% Confidence Intervals of the 4 Predictors in the Model
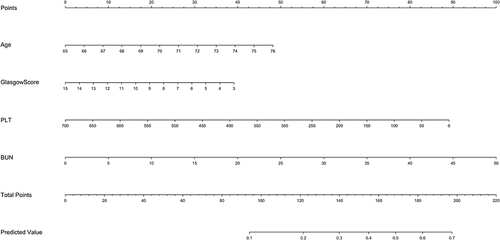
Then, the clinical model was developed based on the above independent variable. The nomogram model for predicting the 28-day mortality in elderly patients with SCAP was formed using the identified four risk factors ().
Figure 1 A nomogram for predicting 28-day mortality in elderly patients with severe community-acquired pneumonia. Instructions for using the nomogram: (1) Draw a vertical line based on the value of each variable to obtain the corresponding point; (2) Add all seven points to obtain the total point; (3) Draw a vertical line based on the total point to determine the estimated survival probability.
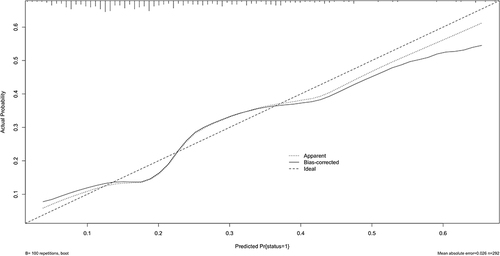
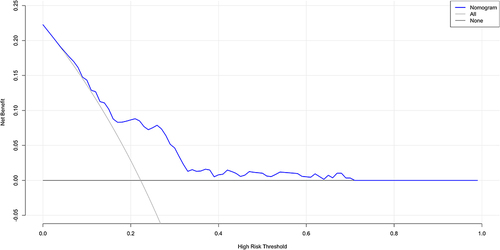
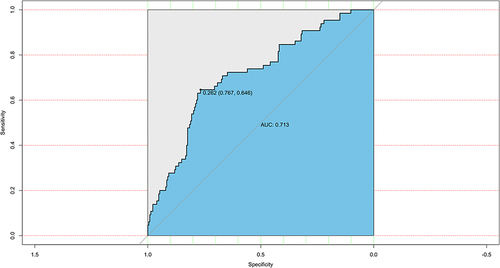
The ROC curve was generated for the nomogram, with an AUC of 0.713 (95% CI: 0.642–0.713) () showing good clinical predictive value. The bootstrap method was used for internal verification, and the results were presented in the calibration curve (). This revealed that the prediction probability of the nomogram was consistent with the actual situation (). The decision curve showed that if the threshold probability was 5%–70%, using the nomogram to predict the 28-day mortality of elderly patients with SCAP would add more benefit.
Figure 2 The receiver operating characteristic (ROC) curve for the established nomogram.
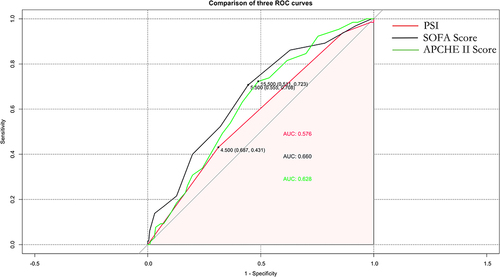
PSI, SOFA score and APCHE II score are widely used to evaluate the prognosis of patients with pneumonia. The comparison of PSI, Total PSI score, SOFA score and APCHE II score in elderly patients is listed in . ROC curves were used to evaluate the prediction effect of the model (), and AUC and 95% confidence interval were calculated (). Area under the curve for 28-day mortality was 0.576 (95% CI: 0.507–0.645) for PSI, 0.660 (95% CI: 0.586–0.733) for SOFA score, and 0.628 (95% CI: 0.556–0.700) for APCHE II score. The prediction effects of these models were all worse than the nomogram constructed in the article with the predicting value of 0.713 (95% CI: 0.642–0.713).
Table 3 Comparison of PSI, Total PSI Score, SOFA Score and APCHE II Score in Elderly Patients
Table 4 Area Under the Receiver Operating Characteristic (ROC) Curve for Prediction
Discussion
In the present study, 292 elderly patients with SCAP, who were admitted in 33 public tertiary teaching hospitals in China were analyzed. The multivariate logistic regression analysis identified age, Glasgow score, PLT and BUN as independent factors for the 28-day mortality of elderly patients with SCAP. The predictor variables are all explicitly defined and can be readily assessed at the time of patient presentation, and a nomogram was established based on these factors with excellent performance.
Previous studies showed that the mortality of patients with CAP aged 65 or above was higher than that of those younger than 65 (10.3 versus 2.2%).Citation10 Studies in Asia revealed consistent result with the 30-day mortality of 7.3–8.6%.Citation16,Citation17 As expected in our study, 28-day mortality was significantly positively associated with older age. Both univariate and multivariate analyses showed that age ≥65 years old was an independent factor that affected the prognosis of elderly patients with SCAP. The mortality rate of elderly patients with SCAP increased with age. This may be because aging will lead to a decline in immunity and organ function, which are all related to the increase in mortality.Citation7,Citation18
The symptoms of patients with CAP are often atypical in elderly patients; this may be due to immunosenescence reducing the ability of elderly patients to respond to an infection.Citation19 Altered mental status, fatigue and lethargy are the most frequent symptoms associated with pneumonia in older patients.Citation20 Delirium may be the only manifestation of pneumonia in those patients.Citation9 This could be an explanation that as the score increases, the mortality of patients decreases, which might be conducive to the early identification and intervention of critically ill patients. Physicians should be alert to the diagnosis of pneumonia in elderly patients in order to avoid the adverse consequences associated with delayed diagnosis.
Exacerbation of pneumonia is often accompanied by a decrease in PLT,Citation21 and this change is consistent with the pathological process of DIC.Citation22 A large amount of over-consumed coagulation factors could cause a significant negative impact on prognosis.Citation23 This suggests that attention should be paid to anticoagulation therapy for elderly patients and monitoring of coagulation function might be necessary.
Acute kidney injury (AKI) is considered a marker of severe pneumonia and a negative prognostic factor for survival.Citation24–26 Earlier studies showed that non-survivors of CAP had higher BUN levels.Citation27–29 Because reabsorption of urea by the kidneys is increased in the dehydrated condition, elevated blood urea nitrogen level is frequently observed in patients with water deficiency, for example, resulted from fever in pneumonia.Citation30 Therefore, the poorer prognosis of patients with elevated BUN is understandable.
Previous study showed that the prognostic prediction scores such as CURB-65 and pneumonia severity index (PSI) were not excellent in elderly patients (age ≥65 years) with pneumonia.Citation31,Citation32 This study evaluated the effects of PSI, sequential organ failure assessment (SOFA) score and acute physiology and chronic health evaluation (APCHE II) score to predict 28-day mortality in elderly patients with SCAP. Our findings demonstrated that PSI, SOFA score and APCHE II score might not be reliable prognostic predictors in elderly patients with SCAP. The lower AUC of PSI, SOFA score and APCHE II score might be due to the patients in our study belong to a special group of age ≥65 years and critically ill. By contrast, ROC curve analysis showed that the nomogram we established might be a more efficient model for predicting 28-day mortality in elderly patients with SCAP.
There existed several limitations in our study. Firstly, although our data was from a well-designed randomized controlled trial (RCT), the sample size was small so that the results might not be considered totally confirmative. Secondly, as external verification is lacking in this study, the predicting effect needs to be further verified. Further studies are required to evaluate the effects of the nomogram in elderly patients with SCAP.
Conclusions
We constructed a nomogram to predict the 28-day mortality in elderly patients with SCAP for better identifying the prognosis of these patients. The proposed nomogram considered four independent risk factors of age, Glasgow score, blood platelet and blood urea nitrogen. Our data demonstrated that this nomogram showed excellent discrimination and clinical availability.
Abbreviations
CAP, community-acquired pneumonia; SCAP, severe community-acquired pneumonia; RCT, randomized controlled trial; PSI, pneumonia severity index; ICU, intensive care unit; CRP, C-reactive protein; APCHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; ARDS, acute respiratory distress syndrome; PLT, platelet; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PT, prothrombin time; APTT, activated partial thromboplastin time; OR, odds ratios; AUC, area under the curve; 95% CI, 95% confidence interval.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available as there are still relevant studies to be conducted in the future but are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The trial was approved by the Medical Ethics Committee of Zhongshan Hospital, Fudan University (2011–2038 [3]). Written informed consent was obtained from all patients or their guardians before enrolment into the study. The study was conducted in accordance with the principles established in the Declaration of Helsinki and the International Council for Harmonisation guidelines for good clinical practice.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
Acknowledgments
We gratefully acknowledge all of the study centers and study participants who have devoted their time and effort to this trial.
Additional information
Funding
References
- Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313(7):677–686. doi:10.1001/jama.2015.88
- Lee JS, Giesler DL, Gellad WF, et al. Antibiotic therapy for adults hospitalized with community-acquired pneumonia: a systematic review. JAMA. 2016;315(6):593–602. doi:10.1001/jama.2016.0115
- Kelly E, macredmond RE, Cullen G, et al. Community-acquired pneumonia in older patients: does age influence systemic cytokine levels in community-acquired pneumonia? Respirology. 2009;14(2):210–216. doi:10.1111/j.1440-1843.2008.01423.x
- Cunha BA. Pneumonia in the elderly. Clin Microbiol Infect. 2001;7(11):581–588. doi:10.1046/j.1198-743x.2001.00328.x
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
- Gutiérrez F, Masiá M. Improving outcomes of elderly patients with community-acquired pneumonia. Drugs Aging. 2008;25(7):585–610. doi:10.2165/00002512-200825070-00005
- Cilloniz C, Ceccato A, San Jose A, et al. Clinical management of community acquired pneumonia in the elderly patient. Expert Rev Respir Med. 2016;10(11):1211–1220. doi:10.1080/17476348.2016.1240037
- Waterer GW, Kessler LA, Wunderink RG. Delayed administration of antibiotics and atypical presentation in community-acquired pneumonia. Chest. 2006;130(1):11–15. doi:10.1378/chest.130.1.11
- Marrie TJ. Community-acquired pneumonia in the elderly. Clin Infect Dis. 2000;31(4):1066–1078. doi:10.1086/318124
- Kothe H, Bauer T, Marre R, et al. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J. 2008;32(1):139–146. doi:10.1183/09031936.00092507
- Ko FW, Lam RKY, Li TST, et al. Sputum bacteriology in patients hospitalized with acute exacerbations of chronic obstructive pulmonary disease and concomitant pneumonia in Hong Kong. Intern Med J. 2005;35(11):661–667. doi:10.1111/j.1445-5994.2005.00956.x
- Thiem U, Niklaus D, Sehlhoff B, et al. C-reactive protein, severity of pneumonia and mortality in elderly, hospitalised patients with community-acquired pneumonia. Age Ageing. 2009;38(6):693–697. doi:10.1093/ageing/afp164
- Han MZ, Huang B, Ni SL, et al. A validated prognostic nomogram for patients with newly diagnosed lower-grade gliomas in a large-scale Asian cohort. Neuro Oncol. 2020;22(5):729–731. doi:10.1093/neuonc/noaa027
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(2):S27–72. doi:10.1086/511159
- Song Y, Yao C, Yao Y, et al. XueBiJing injection versus placebo for critically Ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med. 2019;47(9):e735–e743. doi:10.1097/CCM.0000000000003842
- Song J-H, Oh WS, Kang C-I, et al. Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int J Antimicrob Agents. 2008;31(2):107–114. doi:10.1016/j.ijantimicag.2007.09.014
- Man SY, Lee N, Ip M, et al. Prospective comparison of three predictive rules for assessing severity of community-acquired pneumonia in Hong Kong. Thorax. 2007;62(4):348–353. doi:10.1136/thx.2006.069740
- Janesch P, Stulik L, Rouha H, et al. Age-related changes in the levels and kinetics of pulmonary cytokine and chemokine responses to Streptococcus pneumoniae in mouse pneumonia models. Cytokine. 2018;111:389–397. doi:10.1016/j.cyto.2018.09.012
- Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22(11):1041–1050. doi:10.1111/j.1432-2277.2009.00927.x
- Saldías Peñafiel F, O’Brien Solar A, Gederlini Gollerino A, et al. [Community-acquired pneumonia requiring hospitalization in immunocompetent elderly patients: clinical features, prognostic factors and treatment]. Arch Bronconeumol. 2003;39(8):333–340. Spanish. doi:10.1016/S0300-2896(03)75400-3
- Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi:10.1016/j.cca.2020.03.022
- Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690. doi:10.1080/22221751.2020.1741327
- Kawano N, Wada H, Uchiyama T, et al. Analysis of the association between resolution of disseminated intravascular coagulation (DIC) and treatment outcomes in post-marketing surveillance of thrombomodulin alpha for DIC with infectious disease and with hematological malignancy by organ failure. Thromb J. 2020;18(1):2. doi:10.1186/s12959-020-0216-6
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
- Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi:10.1016/j.kint.2020.03.005
- Xu X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol. 2015;10(9):1510–1518. doi:10.2215/CJN.02140215
- Lim WS, Laing R, Boersma WG, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi:10.1136/thorax.58.5.377
- Farr BM, Sloman AJ, Fisch MJ. Predicting death in patients hospitalized for community-acquired pneumonia. Ann Intern Med. 1991;115(6):428–436. doi:10.7326/0003-4819-115-6-428
- Raz R, Dyachenko P, Levy Y, et al. A predictive model for the management of community-acquired pneumonia. Infection. 2003;31(1):3–8. doi:10.1007/s15010-002-2083-4
- Edelstein CL. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis. 2008;15(3):222–234. doi:10.1053/j.ackd.2008.04.003
- Baek MS, Park S, Choi J-H, et al. Mortality and prognostic prediction in very elderly patients with severe pneumonia. J Intensive Care Med. 2020;35(12):1405–1410. doi:10.1177/0885066619826045
- Chen JH, Chang -S-S, Liu JJ, et al. Comparison of clinical characteristics and performance of pneumonia severity score and CURB-65 among younger adults, elderly and very old subjects. Thorax. 2010;65(11):971–977. doi:10.1136/thx.2009.129627