Abstract
Inflammasome dysfunction may be responsible for underlying inflammatory diseases, which include renal and urological pathologies. Five inflammasomes have been described, including nucleotide-binding domain leucine-rich repeat (NLR), NL pyrin domain containing receptor 1(NLPR1), NLRP3, NLR and caspase recruitment domain containing receptor 4 (NLRC4), and the AIM2-like receptor. The purpose of this study was to review literature sources regarding how innate immunity and inflammasomes contribute to urologic disease and infection. A literature search of PubMed/MEDLINE, EMBASE and Google Scholar articles. Articles were selected for review if their content included (1) inflammasomes and (2) urology in the adult population. The initiation of specific cytokine cascades, which include IL-1β and IL-18, appear responsible for a repertoire of urologic pathologies. Inflammation mediates a wide range of uropathies (urologic disorders and infections) which are found in the bladder, prostate, or kidney and inflammasomes appear to be particularly responsible for urological and renal pathologies. Understanding the role of inflammasomes in urologic disorders can help improve treatment and overall quality of life in patients with these disorders.
Introduction
The innate immune system is the principal mode of defense in combating microbial pathogens.Citation1 Pattern Recognition Receptors (PRRs), which can be found in the cytoplasm, cell surface, and endosomal membrane, contribute to infection detection.Citation1 Membrane-bound toll-like receptors (TLRs) identify suitable ligands in the extracellular and endosomal environment; nucleotide-binding oligomerization domain plus leucine-rich-repeat containing receptors nod like receptors (NLRs), AIM2-like receptors (ALRs), as well as retinoic acid-inducible gene-I-like receptors, RIG-I-like receptors (RLRs) identify suitable ligands in the cytoplasm in order to illicit a proper immune response.Citation1 The inflammasome receptors detect microbial as well as damage associated molecular patterns which can be triggered by infections or stress.Citation2 Both NLRs and ALRs can trigger inflammasome formation.Citation2,Citation3 Inflammasomes are defined as pattern recognition receptors, which illicit the innate immune response to target pathogens and trigger inflammation.Citation4
The aim of the present study was to compare literature sources regarding the role of inflammasomes in mediating urologic disease and infection. Many urological diseases and disorders affecting the bladder, prostate, or kidneys are mediated by inflammation. It is known that urological pathologies diminish the quality of life of patients. To help improve the specific treatment and quality of life for patients, it is important to study and understand the role of inflammation in urological disorders.Citation5 Research on genes could also improve the field of personalized medicine; genetic information can be used to develop personalized disease prevention strategies and aid in therapeutic interventions.Citation6
Methods
Adherence to PRISMA guidelines was conducted as previously described.Citation7,Citation8 This review investigated the role of inflammasomes in mediating urologic pathologies and possible subsequent treatment options to mitigate their effects. Sixty articles published between the years 2015 and 2021 were reviewed; 31 papers were included for the final study. Database searches consisted of PubMed/MEDLINE, EMBASE and Google Scholar.
A multitude of studies were investigated to incorporate various methodologies as well as qualitative research. This included systematic reviews, case series/reports, cross-sectional studies, randomized control studies, cohort studies, and case–control studies. Regarding the role of inflammasomes in generating pathology, it is vital to inspect numerous studies and information in order to combat their sequelae, many of which can be reversible. English articles that were published in peer-reviewed journals were ultimately what was incorporated in the final review. Subject headings as well as text words relating to inflammasomes were included as part of the search strategy. No methodological search filters were incorporated as a means to enhance sensitivity and were incorporated into all of the databases previously mentioned.
Two distinct reviewers screened the title as well as abstracts in results that came from database searches for relevance as well as inclusion. The authors then made a final decision concerning inclusion. Inclusion was based on date of publication (10 years), language of publication (English), age of subjects (adults) and study methodology (quantitative, qualitative or mixed method). Full-text articles were obtained from studies that were included for inclusion. The two review authors surveyed the risk of bias in all of the studies using the risk of bias assessment and it was addressed at the study level. All data were depicted independently and the results were not combined. Due to the nature of this review, institutional ethical approval was not required.
Results and Discussion
Overview of Inflammasomes
The inflammasome was discovered in 2002. The first discovered inflammasome was NLRP1, whose expression was upregulated by a select few number of triggers, such as Bacillus anthracis and Toxoplasma Gondii, by sensing lethal toxin and muramyl dipeptide, which may play a role in prostate inflammation.Citation9 Moreover, the NLRP6 inflammasome is believed to attenuate NF-KB response as well as mitogen-activated protein kinase pathways.Citation10 While its function has been restricted to maintaining gut health, its ability to stimulate mucus production is hypothesized to protect against bladder disorders that include urinary tract infections, interstitial cystitis, calculus production, and cancers.Citation11
The NLRP7 inflammasome is upregulated by microbial acylated lipopeptides, which are often seen in bacteria, such as pseudomonas, which commonly cause urinary tract infections.Citation4 It also produces IL-1β as well as IL-18, which helps prevent bacteria replication in human macrophages and is believed to control bacterial replication in chronic cystitis.Citation12 Gram-negative bacteria have dedicated protein secretion systems numbered Type 1 through Type VI, with each system transporting a specific protein.Citation12 The NLRP12 inflammasome relies on a Type 3 secretion system, which allows certain microbes such as pseudomonas to invade host cells, and is believed to be responsible for sterile inflammatory pathologies such as interstitial cystitis.Citation13 The Type 3 secretion system also induces NLRC4 inflammasome formation, which may mediate bladder cancer and urinary tract infections, and is activated by bacterial virulence factors like flagellin (which activates caspase-1) and inner rod protein PrgJ.Citation13 Moreover, AIM-2 enhances inflammasome formation by binding to bacterial and viral double stranded DNA, allowing it to readily acknowledge uropathogenic bacteria that transmit their DNA to the cytoplasm.Citation13 While there is a basic blueprint laid out regarding how inflammasomes function, many of the inflammasomes and their mechanisms are not well understood.Citation13
Another inflammasome, NLRP3, is relevant for understanding inflammation dynamics because of its relation to numerous fields of health and disease, including uropathies.Citation2 This inflammasome senses stressors and signals, which include adenosine triphosphate (ATP), reactive oxygen species (ROS), saturated fatty acid, and amyloid polypeptides.Citation2 NLRP3 is composed of three domains, which include a leucine rich repeat, which senses danger signals at the C-terminal, a central nucleotide binding and oligomerization domain NACHT, which possesses ATPase activity, as well as a pyrin domain, PYD at the N-terminal.Citation3 NLRP3 is activated via canonical as well as noncanonical pathways.Citation2
Canonical Pathway
The canonical pathway involves two steps, priming and activation.Citation2 Priming is the first step in the pathway; this step uses a ligand (eg, lipopolysaccharide) in order to interact with a receptor that is not NLR (eg, TLR4). This then upregulates inflammasome parts such as NLRP3, as well as caspase-1, as well as apoptosis-associated speck-like protein containing a CARD (ASC), pro-IL-1 β, IL-18 and activates NLRP3 that is already present.Citation2 Activation is the second step in the pathway; activation is triggered when ATP interacts with a receptor that is purinergic in nature, along with reactive oxygen species, in addition to crystals that aggravate the cell membrane.Citation2 This then stimulates potassium efflux, reactive oxygen species production, and NLRP3 to move to the mitochondria, releasing cardiolipin and mitochondrial DNA, as well as cathepsin mediated lysosomal damage.Citation2 As a result, NLRP3 undergoes oligomerization, causing nucleation of ASC proteins to create long filaments, which then associate with pro-caspase-1 to form caspase-1 via induced proximity and then go on to cleave pro-IL-1 β and pro-IL-18 into IL-1 β and IL-18 respectively.Citation2 Caspase-1 cleaves gasdermin D, inducing lytic cell death in a process known as pyroptosis, which releases IL-1 β and IL-18 as well as DAMPS that include ATP, uric acid, as well as high mobility group box 12. This induces inflammasome formation in the local environment and further stimulates inflammation.Citation2
Noncanonical Pathway
The noncanonical pathway utilizes caspase 11 in rodents (caspase 4 and 5 in humans) which are upregulated by binding to LPS or intracellular bacteria and further stimulates inflammasome production and assembly.Citation2 This pathway does not rely on the production of IL-1 β or IL-18 to stimulate inflammation.Citation2 Furthermore, this inflammatory protease not only closely resembles caspase 1 but is also able to bind to the Lipid A component of LPS in order to enter the cytosol leading to a pyroptotic cell death.Citation14 This caspase is able to be activated by a wide variety of microbial pathogens, including Escherichia coli, Salmonella, Shigella, and Burkholderia species.Citation14 E. coli and Shigella species possess a hexa-acylated LPS, which is able to further upregulate caspase-11 response.Citation14 Cathepsin B can also upregulate caspase 11 and the NLRP3 inflammasome complex.Citation15 It can also be activated by endogenous DAMPs, oxidative stress, tissue damage, and widespread infections.Citation14
In the literature, the non-canonical pathway is often used interchangeably with the canonical pathway but still warrants a closer look due to its other roles.Citation2 Epithelial cells in the kidney were surveyed in order to further examine the non-canonical role of the NLRP3 inflammasome.Citation16 Epithelial cells in the kidney can undergo apoptosis via the non-canonical pathway by the action of caspase 8, which combines with NLRP3 and ASC.Citation16 This provides wider insight into the role of inflammasomes in not only mediating the inflammatory cascade but also offshoots of the process, such as apoptosis. The NLRP3 inflammasome’s repertoire of roles is important to comprehend because of its ability to exacerbate renal inflammation, end-stage renal disease, and chronic kidney disease.Citation17 There are minimal options to treat chronic kidney disease, making the quest to understand the pathophysiology of the disease more important than ever.Citation18
Heat Shock Proteins and Inflammasomes
Intracellular heat shock proteins (HSP) are damage-associated molecular patterns, which can induce the inflammatory cascade via inflammasome activation.Citation19 This includes HSP90, HSP70, and calreticulin, which stimulate CD91 and release of IL-1B from antigen presenting cells, the terminal cytokine product of inflammasome activation. This release is also sensitive to gp96, which relies on the NLRP3 inflammasome. In addition, it can sense double stranded DNA, which utilizes AIM2 inflammasomes, further inducing inflammasome activity which leads to cell death and pyroptosis. NLRP3 inflammasome can be inhibited by geldanamycin which downregulates HSP90 expression and upregulates HSP70 expression.Citation20 Deficiency of HSP70 has been found to induce caspase-1 as well as IL-1B activity, which enhances apoptosis related speck-like protein in macrophages, suggesting it can hinder NLRP3 inflammasome activation.Citation21 This has many implications for a repertoire of inflammatory conditions, including uropathies.Citation2
Disease Aspect
The sequelae of inflammation seen in uropathies can negatively influence the patient’s overall quality of life. Complications can lead to repetitive and irreversible outcomes which can cause voiding problems, urinary urgency, and even cancer.Citation1 Upregulation of inflammasomes has been linked to prostate pathology encompassing infection, stress, and carcinogenesis.Citation22 Nonetheless, efforts to provide a unified mode of the role of inflammasomes in the sector of urologic health and disease are still ongoing, but necessary to ensure a better overall quality of life.Citation2 Patients with urological complications report having bladder dysfunction, voiding difficulties, sexual/erectile dysfunction, higher recurrence rates of urinary tract infections leading to septic emergencies, urinary urgency/incontinence, and cancers that often metastasize.Citation23 It is hypothesized that on a molecular level, inflammasome assembly and its components trigger the inflammatory signaling cascade and result in urological pathology, which can be largely reversible with proper treatment.
The genitourinary system is composed of many inflammasome components that are necessary in aiding the immune system in detecting various stimuli due to infection or disease.Citation2 Recent literature demonstrates that NLRP7 and IF116 are pattern recognition receptors that can upregulate inflammasome formation by enhancing caspase-1 expression.Citation13 However, there is still limited information present regarding how inflammasomes work in the host defense against infection.Citation2 Elucidating the role of inflammasome components can provide a deeper insight into the role of inflammation in mediating uropathology as well as investigating suitable therapeutics for attenuation or prevention of sequelae.
While there exists a general consensus that inflammation plays a pivotal role in influencing urological dysfunction in a variety of diseases, both benign and malignant, the specific function of inflammasomes as prime mediators is often overlooked.Citation24 Recent data, as evidenced by the obstruction, urinary tract infections, aging, and cyclophosphamide-induced bladder studies, suggest that this molecular component is responsible for spear-heading the inflammatory cascade and the resulting complications that patients often face. The genitourinary system provides a suitable microenvironment for agents to trigger inflammasome and subsequent inflammation activation.Citation2 The primary symptoms of inflammation are often benign; changes in urinary patterns and urinary urgency may be observed ().Citation25 However, if this persists, complications of inflammation can become malignant.Citation25
Figure 1 Inflammasome activity in rats undergoing bladder outlet obstruction. The volume and caspase activity for each variable are represented as milliliters and pg AFC/min/mg, respectively. Data from Hughes et al.Citation25
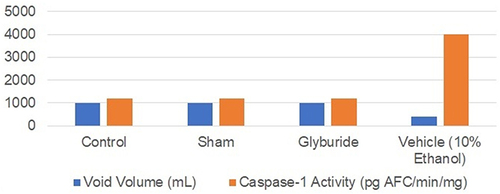
In an animal model, Hughes et. al demonstrated bladder outlet obstruction (BOO) in female rats by placing a one-millimeter outer diameter transurethral catheter.Citation25 These studies showed that urothelial inflammasome activity was enhanced by bladder outlet obstruction leading to decreased voided volume but was attenuated by glyburide administration.Citation25 In order to simulate obstructive nephropathy in animal models, one ureter was ligated to simulate an obstructed kidney and compared with the unobstructed kidney.Citation2 Mice that undergo unilateral ureteral obstruction also experienced enhanced NLRP3 priming, activation, and inflammation, a phenomenon which is not seen in the NLRP3 −/−mice, suggesting that inflammasomes are upregulated in response to obstruction.Citation2 Furthermore, obstruction induces Angiotensin II expression, which upregulates NLRP3 priming and activation, creating a vicious cycle of inflammation.Citation2 Inflammation can also be triggered by unilateral ureteral obstruction, which induces hypoxia and subsequent recruitment of NLRP3 and IL-1.Citation2
In humans, inflammatory responses were assessed in healthy women, ages 18–55 years old, with urinary tract infections.Citation11 Healthy women with uropathogenic E. coli and subsequent urinary tract infections displayed upregulation of mRNA and protein levels associated with inflammasomes and its components, which included NLRP3, NLRC4, ASC, CASPASE-1, and NAIP.Citation11 They also experienced upregulation of pro-inflammatory cytokines, which included IL-6, IL-8, IFN-Y, TNF-alpha, as well as MCP-1.Citation11 These findings suggest that inflammasomes influence urinary tract pathology. In other disease states, diabetic patients experience higher incidences of urinary tract infections and are at a greater risk of morbid complications and longer hospital stays.Citation23 Nonetheless, in the United States alone, there are over one million hospitalizations related to urinary tract infections, totaling over $1.6 billion in expenses.Citation26 Thus, understanding the pathophysiology of these infections can be lifesaving, as well as cost-effective, in the long term.
Potential Therapeutics
Current anti-inflammatory treatments have been aimed at targeting the NLRP3 inflammasome, a 115 kDa cytosolic protein found in dendritic cells, monocytes, neutrophils, osteoblasts, lymphocytes, as well as epithelial cells.Citation3 Activation of the NLPR3 inflammasome is implicated in various pathologies, not just related to urological emergencies, which include Alzheimer’s, Inflammatory Bowel Disease, and colon cancer.Citation1 This has warranted investigation of a target; the signaling cascade of the NLRP3 inflammasome which includes inhibiting NLRP3 inflammasome activation, suppressing upstream signals, interrupting assembly of inflammasomes and neutralizing its inflammatory elements, inhibiting caspase-1 activation, and blocking cleavage of pore-forming protein gasdermin D (GSDMD).Citation3
Previous studies in humans have shown that NLRP3 blocks ATP hydrolysis as well as NLRP3 inflammasome formation; mice studies have shown that this compound reduces inflammation in pulmonary and epithelial tissue.Citation3 Aspirin and ibuprofen have been shown to decrease bladder wall thickness, and subsequent dysfunction, as well as decrease levels of IL-1β, IL-6, PGE2, and decreased pantothenic acid levels, indicating biomarkers to measure inflammation in the bladder in future pharmacological studies.Citation27 Moreover, Glyburide has been able to reduce inflammatory components by 30–50%, as well as bladder physiology/cystometry initially induced by DAMPs and PAMPs such as cyclophosphamide.Citation27 Pharmacological inhibitors that target inflammasomes and signaling pathway constituents can prove to be a promising therapy to counteract inflammation and the pathology that ensues.
Recent therapeutics have aimed to target particular small-molecule inhibitors that can discern certain components of the NLRP3 pathway to inhibit the inflammatory cascade.Citation28 MCC950 can accomplish this by attenuating the chloride efflux and the subsequent inflammatory feedback loop.Citation28 Moreover, understanding the NLRP3 pathway can pave the way for future immunotherapy and its responsiveness in combating malignancy.Citation29 Even though this therapeutic is new, its benefits are promising; further studies are needed to evaluate these benefits that can improve quality of life.
Conclusion
There is increasing evidence that inflammasomes play a crucial role in inflammatory responses. Inflammasomes mediate various urological pathologies; both cell-mediated and humoral responses of the immune system contribute to inflammation. Future clinical studies are warranted to develop new therapeutics and potential effective treatments that target inflammasomes, as well as potentially improve quality of life issues.
Inflammasome activity and chronic inflammation can mediate both benign and malignant pathologies (i.e benign prostatic hyperplasia or prostate cancer).Citation30 Understanding the role of inflammasomes in mediating urologic pathology will allow for more opportune moments of intervention as well as management of symptoms. The patient-reported outcome measurement information system (PROMIS) is used to further understand how patients are experiencing urological pathologies.Citation31 PROMIS can further guide and humanize our understanding of how urologic pathologies manifest on a day-to-day basis. As more information is reported, regarding the pathophysiology and components of inflammation, preventative and chronic disease management can continue to evolve in order to ensure the overall quality of life of patients. Healthcare providers can try to improve treatment strategies through understanding specific components of the inflammatory cascade of urologic disease.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
No conflict of interest to report.
Additional information
Funding
References
- Kanneganti TD. The inflammasome: firing up innate immunity. Immunolol Rev. 2015;265(1):1–5. doi:10.1111/imr.12297
- Purves JT, Hughes FM Jr. Inflammasomes in the urinary tract: a disease-based review. Am J Physiol Renal Physiol. 2016;311(4):653–662. doi:10.1152/ajprenal.00607.2015
- Kayagaki N, Wong M, Stowe I, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi:10.1126/science.1240248
- Zahid A, Li B, Kombe J, Arnaud J, Tengchuan T. Pharmacological inhibitors of the NLRP3 inflammasome. Front Immunol. 2019;10:2538. doi:10.3389/fimmu.2019.02538
- Poli G, Egidi MG, Cochetti G, Brancorsini S, Mearini E. Relationship between cellular and exosomal miRNAs targeting NOD-like receptors in bladder cancer: preliminary results. Minerva Urol Nefrol. 2020;72(2):207–213. doi:10.23736/S0393-2249.19.03297-1
- Barbarino M, Cesari D, Bottaro M, et al. PRMT5 silencing selectively affects MTAP-deleted mesothelioma: in vitro evidence of a novel promising approach. J Cell Mol Med. 2020;24:5565–5577.
- Moher D, Shamseer L, Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. System Rev. 2014;4:1.
- Shamseer L, Moher D, Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:7647. doi:10.1136/bmj.g7647
- Inouye B, Hughes F, Sexton S, Purves JT. The emerging role of inflammasomes as central mediators in inflammatory bladder pathology. Curr Urol. 2018;11:57–72. doi:10.1159/000447196
- Baker PJ, Boucher D, Bierschenk D, et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol. 2015;45(10):2918–2926. doi:10.1002/eji.201545655
- Verma V, Gupta S, Kumar P, et al. Involvement of NLRP3 and NLRC4 inflammasome in uropathogenic e. coli mediated urinary tract infections. Front Microbiol. 2019;10:3–6. doi:10.3389/fmicb.2019.02020
- Chen KW, Demarco B, Heilig R, et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 2019;38:e101638. doi:10.15252/embj.2019101638
- Russo A, Behl B, Banerjee I, Rathinam V. Emerging insights into noncanonical inflammasome recognition of microbes. J Mol Biol. 2018;430(2):207–216. doi:10.1016/j.jmb.2017.10.003
- Chen N, Ou Z, Zhang W, et al. Cathepsin B regulates non-canonical NLRP3 inflammasome pathway by modulating activation of caspase-11 in Kupffer cells. Cell Prolif. 2018;51(6):e12487. doi:10.1111/cpr.12487
- Wang Y, Sedlacek AL, Pawaria S, et al. Cutting edge: the heat shock protein gp96 activates inflammasome-signaling platforms in APCs. J Immunol. 2018;201(8):2209–2214. doi:10.4049/jimmunol.1800505
- Chung H, Vilaysane A, Lau A, et al. NLRP3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death Diff. 2016;23(8):1331–1346. doi:10.1038/cdd.2016.14
- Vilaysane A, Chun J, Seamone M, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–1744.
- Ermer T, Eckardt KU, Aronson PS, Knauf F. Oxalate, inflammasome, and progression of kidney disease. Curr Opin Nephrol Hypertens. 2016;25(4):363–371. doi:10.1097/MNH.0000000000000229
- Martine P, Rébé C. Heat shock proteins and inflammasomes. Int J Mol Sci. 2019;20(18):4508. doi:10.3390/ijms20184508
- Martine P, Chevriaux A, Derangère V, et al. HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell Death Dis. 2019;10(4):256. doi:10.1038/s41419-019-1491-7
- Augé C, Chene G, Dubourdeou M, et al. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Euro J Pharm. 2013;707(1–3):32–40. doi:10.1016/j.ejphar.2013.03.008
- Karan D, Dubey S. From inflammation to prostate cancer: the role of inflammasomes. Adv Urol. 2016;18:314–372.
- Arrellano-Valdez F, Osario M, Arroyo C, Vega E. A comprehensive review of urologic complications in patients with diabetes. Spri Plus. 2014;3(1):549–551. doi:10.1186/2193-1801-3-549
- Kesavardhana S, Kanneganti TD. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int Immunol. 2017;29(5):201–210. doi:10.1093/intimm/dxx018
- Hughes FM, Hill HM, Wood CM, et al. The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J Urol. 2016;195:1598–1605.
- Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open Forum Infect Dis. 2017;4(1):ofw281. doi:10.1093/ofid/ofw281
- Hughes FM, Vivar N, Kennis J, et al. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol Renal Physiol. 2014;306:3–14.
- Wu D, Chen Y, Sun Y, et al. Target of MCC950 in inhibition of NLRP3 inflammasome activation: a literature review. Inflamm. 2020;43(1):17–23. doi:10.1007/s10753-019-01098-8
- Ju M, Bi J, Wei Q, et al. Pan-cancer analysis of NLRP3 inflammasome with potential implications in prognosis and immunotherapy in human cancer. Brief Bioinform. 2021;22(4):bbaa345. doi:10.1093/bib/bbaa345
- Ponomareva L, Liu H, Duan X, et al. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res. 2013;11(10):1193–1202. doi:10.1158/1541-7786.MCR-13-0145
- Patel N, Brown RD, Sarkissian C, De S, Monga M. Quality of life and urolithiasis: the patient - reported outcomes measurement information system (PROMIS). Int Braz J Urol. 2017;43(5):880–886. doi:10.1590/s1677-5538.ibju.2016.0649
