Abstract
Osteoarthritis (OA) is an inflammatory and degenerative joint disease with severe effects on individuals, society, and the economy that affects millions of elderly people around the world. To date, there are no effective treatments for OA; however, there are some treatments that slow or prevent its progression. Polyfunctional nanosystems have many advantages, such as controlled release, targeted therapy and high loading rate, and have been widely used in OA treatment. Previous mechanistic studies have revealed that inflammation and ROS are interrelated, and a large number of studies have demonstrated that ROS play an important role in different types of OA development. In this review article, we summarize third-generation ROS-sensitive nanomaterials that scavenge excessive ROS from chondrocytes and osteoclasts in vivo. We only focus on polymer-based nanoparticles (NPs) and do not review the effects of drug-loaded or heavy metal NPs. Mounting evidence suggests that polyfunctional nanosystems will be a promising therapeutic strategy in OA therapy due to their unique characteristics of being sensitive to changes in the internal environment.
Introduction
Osteoarthritis (OA), characterized by the progressive destruction of articular cartilage and joint dysfunction, is currently the second leading cause of disability in patients after cardiovascular disease.Citation1,Citation2 The increase in the elderly population and the prevalence of obesity across the world have incited a rising incidence of OA.Citation3 According to an epidemiological investigation, the incidence of OA in the population over 65 years old can reach 50%, and the incidence among individuals over 80 years old is as high as 80%.Citation4 Approximately 80% of OA patients have different degrees of movement impairment, and 25% have difficulties performing the activities in daily life.Citation5 Consequently, OA has caused a huge burden on society, the economy, and individuals.
Figure 2 A schematic diagram of reactive oxygen species including production and conversion.
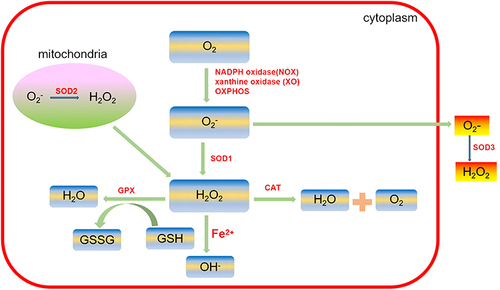
Figure 3 Schematic diagram showing the fabrication of hyaluronic (HA) and dextran (Dex) Schiff base hydrogel loaded with dexamethasone acetate (DA)-encapsulated PEG-PTK-PEG micelles (PDM) for OA therapy in vivo.
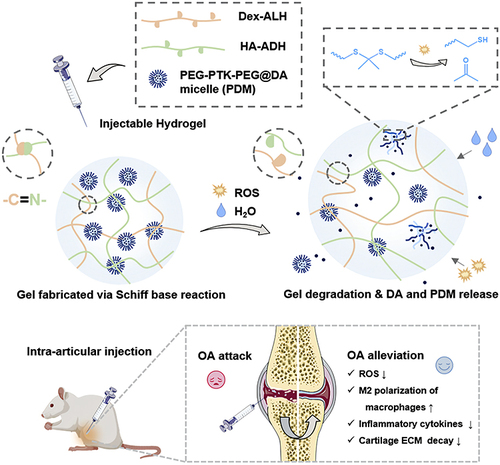
Figure 4 Schematic illustration of the self-assembly of ROS-responsive NPs for bioimaging and targeted therapy of OA in vivo.
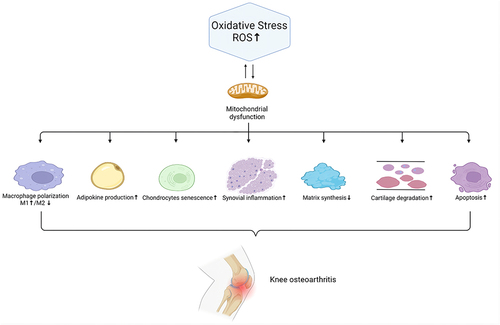
OA has a complex pathophysiological process. The main pathological manifestations are cartilage degeneration, bone remodeling, osteophyte formation and joint inflammation.Citation6 OA can affect almost any joint, but it is most common in the hands, knees, hips and feet. Among them, excessive oxidative stress is an important factor in the pathological progression of OA. Mechanistically, reactive oxygen species (ROS), as a secondary messenger, gradually aggravate OA inflammation by activating the ROS/phosphoinositol-3 kinase (PI3K)/serine/threonine kinase (AKT),Citation7 ROS/mitogen-activated protein kinase (MAPK)Citation8 and reactive nitrogen species (RNS)/tumor necrosis factor-α (TNF)-α/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways.Citation9,Citation10 Excessive ROS can not only lead to the senescence and death of chondrocytes and destroy chondrocytes and the extracellular matrix (ECM) but also induce subchondral bone dysfunction. Moreover, ROS can promote M1 macrophage polarization. Synovial inflammation driven by M1 macrophages is thought to be one of the key causes of OA development and progression.Citation11 M1 macrophages can secrete IL-1β, activating NF-κB to promote the secretion of TNF-α by chondrocytes, and this process is enhanced by interferon-γ via the JAK/STAT pathway.Citation12–14 Furthermore, TNF-α regulates CXCL1 through 2 different pathways, one through JNK and its downstream AP-1 transcription factor influencing the expression of genes and the other affecting cellular CXCL1 secretion by p38 MAPK and PI3K activation.Citation14 High CXCL1 expression leads to neutrophil infiltration,Citation15 which can enhance the inflammatory response.
In addition, other joint tissues, such as the infrapatellar fat pad, synovial membrane and meniscus, all generate inflammatory changes after the onset of OA, contributing to the progression of OA. Increasing evidence has shown that synovial macrophage activation is one of the major destructive factors during the progression of OA.Citation16 M1 macrophages, which promote synovial inflammation, secrete several proinflammatory cytokines, including IL-1β, IL-6, IL-12, and TNF-α, which cause cartilage degeneration.Citation17 Moreover, advanced oxidation protein products (AOPPs), which are a sign of oxidative stress, upregulate the expression of MMP3 and MMP13 in the synovium, contributing to cartilage degradation.Citation18 In addition, the meniscus is an important soft tissue in the etiology of knee OA (KOA). It has been reported that the severity of meniscal insufficiency is linked with the advancement of OA.Citation19 IL-1β can promote meniscal cell apoptosis by inactivating c-JNK2 and suppressing autophagy, the production of which is elevated in meniscal cells under pathological conditions.Citation20–22 Damage to the meniscus causes overload on the cartilage and thus accelerates the degeneration of chondrocytes. Furthermore, the infrapatellar pad is a new source of inflammatory factors and adipokines that contribute to KOA progression.Citation23 The combination of inflammatory pathways and ROS signaling is well recognized in adipose dysfunction and metabolic syndrome.Citation24 Lipid peroxidation produces a wide range of hydroperoxide and aldehyde compounds that are highly reactive with cell membrane and ECM components.Citation25,Citation26 Lipid radicals are also intracellular signaling molecules that affect a variety of cellular activities.Citation27,Citation28 For example, malondialdehyde (MDA), a hazardous aldehydic end product of lipid peroxidation, facilitates cartilage collagen oxidation, leading to cartilage degradation.Citation29 The interactions of ROS with OA joint tissues are shown in .
The most common clinical symptoms of OA are joint aches, swelling and stiffness. Therefore, the main objectives of OA treatment focus on easing pain, relieving joint swelling, improving the mobility of the joint, and minimizing disability. At present, conservative nonsurgical management of OA is divided into two main categories: pharmacological and physical intervention.Citation30 Pharmacological intervention generally refers to oral or intra-articular injection of nonsteroidal anti-inflammatory drugs (NSAIDs) and intra-articular injection of corticosteroids and hyaluronan to suppress joint inflammation and relieve joint pain.Citation31 However, oral drugs often cause adverse reactions such as gastrointestinal reactions and osteonecrosis, while intra-articular topical administration is shortened by the fact that drugs are rapidly cleared in synovial joints, resulting in a shortened duration of efficacy.Citation32 Currently, there are still no effective therapeutic drugs for treating or slowing the progression of OA. Physical therapy consists mainly of interventions such as moderate exercise, weight loss, or occupational therapy.Citation33,Citation34 However, when patients present with severe joint damage and conservative treatment is insufficient to control symptoms, surgical treatment, such as arthroplasty and osteotomy, is usually the only option. As a result, there is an urgent need to clarify how the pathologically driven process of OA can be effectively suppressed. Drug delivery systems with controlled release can not only reduce the side effects and normal tissue exposure in OA treatment but also prolong the duration of drugs in the joint and avoid frequent drug administration through its sensitivity to changes in the microenvironment and cell targeting.
Therefore, we first emphasized the key role of ROS in the development of OA; then, we introduced the targeting effect of ROS-sensitive nanomaterials on chondrocytes and osteoclasts in the treatment of OA; finally, we summarized the advantages of nanotherapy in the treatment of OA to illustrate that multifunctional nanosystems will be a promising treatment strategy for OA in the future.
The Critical Role of ROS in the Development of OA
Excessive ROS production that is associated with oxidative stress plays an important role in OA. Oxidative stress has long been considered a significant factor in the induction of chondrocyte senescence and apoptosis in OA.Citation35,Citation36 The excess production of free radicals under oxidative stress is considered to be the main mechanism of cartilage damage,Citation37 and therefore, the role of ROS in the pathogenesis of OA has become a primary concern of researchers.Citation38,Citation39
Sources of ROS and Related Enzymes Involved in Oxidative/Antioxidative Systems
ROS are an important factor that influences intracellular redox status. Common biologically relevant ROS include superoxide anion (O2−), hydroxyl free radical (HO−), and hydrogen peroxide (H2O2).
Endogenous and physiological ROS are mainly generated in the oxidative reaction process of the mitochondrial respiratory chain as byproducts of normal cellular metabolism. In animals, mitochondria and plasma membrane-bound nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) are the major sources of ROS, playing a central role in the induction of cell death.Citation40 ROS can induce mitochondrial dysregulation by increasing mitochondrial membrane permeability, which further increases ROS production and leads to the release of ROS into the cytosol.Citation41 Therefore, it is now well established that mitochondria are the major producers of ROS and the main targets of ROS. Defective mitochondrial function has been implicated in degenerative joint diseases such as OA.Citation42 In addition to mitochondria, another source of cytoplasmic ROS is the cytochrome P450 enzyme (CYP) system, which responds to toxic substances. Moreover, the endoplasmic reticulum (ER) is also the source of ROS. ER oxidoreductase 1 (ERO1), which is associated with the inner ER membrane, has been shown to catalyze the formation of disulfide bonds in secretory proteins and associated H2O2 formation.Citation43
Basic levels of ROS are essential for cellular signal transduction and physiological function. Excessive ROS that are caused by the imbalance between ROS production and antioxidants may induce severe damage to various biomolecules, such as protein oxidation, lipid peroxidation, and DNA fragmentation, which are associated with various inflammatory diseases, such as OA.Citation9 These enzymes, including the NADPH oxidase family of enzymes (NOX), xanthine oxidase (XO), myeloperoxidase (MPO), and lipoxygenases, are responsible for the essential sources of ROS:Citation44
In eukaryotes, critical enzymes for preventing ROS formation are superoxide dismutases (SODs), glutathione peroxidases (GPx), catalase (CAT) and glutathione reductase (GR). SOD and CAT are the best antioxidants in vivo. SODs can be classified into cytosolic CuZn-SOD (SOD1), mitochondrial Mn-SOD (SOD2) and extracellular SOD (SOD3). SOD can catalyze O2− into O2 and H2O2.Citation45 GPxs reduce lipid hydroperoxides to their corresponding alcohols and mediate the breakdown of H2O2 to H2O. CAT, located mainly in peroxisomes, also mediates the decomposition of H2O2 to H2O and O2.Citation46 GR catalyzes the reduction of glutathione disulfide (GSSG) to the sulfhydryl form of glutathione (GSH), which is a critical molecule in resisting oxidative stress.Citation47 Recovering the appropriate ROS concentration by regulating ROS production or neutralizing ROS is a potentially effective means of preventing and treating diseases related to oxidative stress.Citation48 The production and conversion of ROS are summarized in .
Cell Function is Impaired by ROS at the Cellular and Molecular Levels
ROS are produced mainly by NOXs at low levels in normal articular chondrocytes. However, many pathological factors, such as inflammatory factors and mechanical pressure, will cause a high concentration of ROS production. Excessive ROS production can induce irreversible damage to articular cartilage and apoptosis of chondrocytes, which is the main cause of OA.Citation49–51 The downstream molecular targets of ROS include nucleic acids, proteins, and lipids. Elevated ROS levels lead to hyperperoxidation, protein carbonylation and DNA damage, leading to the functional loss of chondrocytes.Citation39,Citation52 A recent study suggested that cell senescence was also closely related to OA development.Citation53 Chondrocytes from human cartilage explants cultured in the presence of hydrogen peroxide exhibited senescence hallmarks.Citation54 Exogenous senescence is induced by a variety of stresses, such as DNA damage and oxidative stress.Citation55
Furthermore, studies have demonstrated that elevated ROS levels can promote the catabolism of chondrocytes by increasing the production of MMPs, resulting in the destruction of the cartilage matrix, including collagen, proteoglycans and hyaluronan, in OA.Citation39,Citation56 Therefore, studies have shown that targeting ROS effectively suppresses the severity of OA in vivo.Citation57 Bardoxolone methyl (BM), a semisynthetic triterpenoid, has been proven to alleviate OA progression by preventing oxidative stress-induced chondrocytes apoptosis and ECM degradation, such as aggrecan and collagen II degradation.Citation58,Citation59 In addition, irisin, an 8-pentenyl flavonoid glycoside, repressed inflammation-mediated oxidative stress and extracellular matrix underproduction by retaining mitochondrial biogenesis, dynamics and autophagic programs.Citation60
The Mutual Influences Between Inflammation and Oxidative Stress
ROS and inflammation are interdependent and interact with each other, and thus, they are both ideal targets for the treatment of OA. Local inflammatory responses along with aging and/or mechanical load can contribute to increased oxidative stress with the accumulation of ROS, O2−, H2O2, and concomitant failure in the expression of antioxidant enzymes and ROS scavenging systems.Citation61 Various proinflammatory mediators, such as cytokines TNF-α and interleukin (IL)-1β, −8, −6, −15, −17, −18, −21, and −33), are increased in the joint tissue of OA patients and have been shown to induce oxidative stress, apoptosis and catabolic gene expression in OA individuals.Citation62,Citation63 These proinflammatory cytokines stimulate chondrocytes and osteoclasts to activate cartilage degradation pathways, thereby driving OA pathogenesis.Citation64
The NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3) inflammasome is one of the most studied inflammasome sensors and a master regulator of inflammation in OA.Citation65 A recent study suggested that intracellular ROS are elevated by various NLRP3 stimulators and that enhanced ROS in turn activate the NLRP3 inflammasome.Citation66 ROS production has also been reported to stimulate NLRP3 inflammatory body assembly in a ROS-sensitive manner.Citation67 Accumulated ROS also activate the NLRP3 inflammasome in synovial membrane macrophages and increase the expression of the proinflammatory cytokines IL-18 and IL-1β.Citation68
Some compounds have exhibited both antioxidant and anti-inflammatory effects in the treatment of OA. Engeletin is a natural compound with anti-inflammatory and antioxidant effects on other diseases. Pretreatment with engeletin alleviated TNF-α-induced inhibition of ECM synthesis (collagen II and aggrecan) and upregulation of matrix catabolic enzymes (MMP9 and MMP3) and scavenged intracellular ROS by activating the Nrf2 pathway.Citation69 Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl) has excellent antioxidant and anti-inflammatory effects by decreasing superoxide ion levels in LPS-induced macrophages.Citation70 In addition, resveratrol has a strong protective effect against B[a]P-induced intracellular ROS generation by increasing mitochondrial content and function.Citation71 Furthermore, ascorbic acid reduces H2O2 production and lipid peroxidation by inhibiting the NF-κB pathway.Citation72
ROS Play an Important Role in OA
OA can be classified as primary and secondary. Aging plays a major role in primary OA, while obesity and joint injury are the two most relevant risk factors for secondary OA.Citation73
Aged-related NOXs are rarely detected in young individuals, but the levels increase with age. The degree of cartilage degradation is correlated with the activity of NOXs in age-related OA.Citation74 Advanced oxidation protein products (AOPPs) are also considered biomarkers of oxidative stress, and intra-articular injections accelerate cartilage degeneration in rabbit OA models.Citation18 AOPPs have been reported to activate the NOXs-mediated oxidative stress pathway in oxidation-associated diseases.Citation75 The excessive production of ROS is attributed to mitochondrial dysfunction, and the decline in antioxidant enzymes such as catalase (CAT) and superoxide dismutases (SODs) with age has also been implicated in the development of aging-related OA.Citation76,Citation77
In addition, type II diabetes mellitus (T2DM) is the most common subtype of diabetes in diabetic patients, accounting for >90% of the total number, and most of the patients are obese. In recent years, the incidence of T2DM with OA has gradually increased. The symptoms in OA patients with diabetes are more severe than those in nondiabetic patients with OA.Citation78 The possible reason may be linked to the increased proinflammatory cytokines that are secreted by adipocytes and invading macrophages in adipose tissue.Citation79 Adipokines increase the number of activated macrophages that generate proinflammatory cytokines, such as IL-1β, TNF-α and ROS, by acting on the synovial membrane of the joint.Citation80,Citation81 Moreover, chronic hyperglycemia induces the production of ROS, leading to increased ROS and reduced synthesis of type II collagen (COL-II).Citation82
Moreover, the aberrant overproduction of ROS is also a potential component of the biochemical inflammatory response to joint injury. In the inflammatory response, overproduction of ROS is potentially harmful to tissues and may sensitize the joint to the development of posttraumatic osteoarthritis (PTOA).Citation83 After joint injury, the inflammatory mediators in synovial fluid increased significantly and subsequently decreased gradually. Within 24 hours of acute ligament tear, cytokine (IL-1β, TNF-α) and keratin sulfate levels rise rapidly and remain high for 7 days.Citation84,Citation85 These inflammatory factors could contribute to oxidative stress.Citation86,Citation87 For example, IL-1β has been shown to reduce ATP-binding cassette transporter A (ABCA-1) mRNA expression and enhance intracellular lipid retention to promote M1 macrophage polarization.Citation88 Therefore, inhibiting aberrant ROS signaling in the inflammatory response after joint injury may have the potential to protect normal joint tissues and decrease early changes associated with the development of PTOA.Citation83
Chondrocytes and Osteoclasts as Targets for OA Treatment
Synovitis is one of the typical manifestations of OA. The synovial tissue is mainly composed of synovial macrophages and synoviocytes. Macrophage-mediated synovial inflammation is the main driving factor for the occurrence and progression of OA. The increased number of macrophages results in an imbalance between the synthesis and degradation of the ECM in cartilage by upregulating the expression levels of proinflammatory factors, thus damaging the function of the cartilage.Citation33,Citation34
However, the inflammatory response in OA depends mainly on activated osteoclasts, which can secrete ROS molecules, and persistent oxidative stress is the source of inflammation.Citation64 Recent studies have also indicated that ROS are important components regulating the differentiation process of osteoclasts.Citation89,Citation90 In a hypoxic environment, the accumulation of mitochondrial ROS is essential for osteoclast differentiation.Citation91,Citation92 NOX has been identified as one of the key sources of ROS. It has also been reported that the decreased production of ROS by silencing NOX1 (a member of the NOX family) in bone marrow mononuclear macrophages inhibited osteoclasts differentiation.Citation93 In addition to osteoclasts from synovial tissue, osteoclasts from subchondral bone also play an important role in OA. The abnormal mechanical overload caused by anterior cruciate ligament tears leads to an increase in osteoclast activity and bone resorption in subchondral bone, which finally triggers the occurrence of OA.Citation94 Inhibition of hyperactivated osteoclast activity in subchondral bone can ameliorate OA severity.Citation95 Betaine maintains the structural integrity of subchondral bone and slows the progression of OA by reducing the production of ROS and inhibiting the activity of osteoclasts.Citation96 Therefore, osteoclasts are an important target in the treatment of OA.
Chondrocytes, the only cell type present in articular cartilage, are responsible for cartilage homeostasis by maintaining a balance between the synthesis and degradation of ECM comprising primarily type II collagen and aggrecan. It has been reported that ROS components detected in OA joints that can directly destroy hyaline include O2−, HO−, peroxide, and hydroxyproline.Citation97 H2O2 inhibited proteoglycan production in chondrocytes by inhibiting mitochondrial oxidative phosphorylation and ATP generation and subsequently induced chondrocyte cell death in a dose-dependent manner.Citation98,Citation99 Furthermore, oxidative stress causes apoptosis in superficial cartilage areas, resulting in hypertrophic chondrocytes in deeper places and affecting normal endochondral ossification and matrix repair.Citation100 Hence, early intervention for oxidative stress is necessary to prevent cartilage damage. Antioxidative agents hold great potential in OA treatment for a considerably high level of oxidative stress that was associated with the development of OA.Citation39,Citation101 Free radical scavengers with excellent biocompatibility and lower cytotoxicity will expand their further applications in OA therapy.
Polyfunctional Nanosystems 3.0 means that the third generation of nanocarriers was stimuli-responsive or activated by local environmental changes such as decreased pH, imbalanced redox state, temperature, and external stimuli such as light, ultrasound and magnetic field. Here, we focus on the third generation of nanomaterials that are ROS-responsive in treating OA.
The ROS-responsive nanomaterials for targeting chondrocytes and osteoclasts in OA are shown in .
Table 1 The ROS Responsive Biomaterials for OA Therapy
ROS-Responsive Nanomaterials for Osteoclasts
Dexamethasone sodium phosphate (DEX-P) is widely used in the treatment of OA to relieve inflammation and prevent cartilage ECM loss.Citation102 The main difficulty of clinical application is that soluble drugs (DEX-P) are easy to clear; thus, frequent administration of DEX-P (once daily) is required.Citation103 Poly (D,L-lactic acid-co-glycolic acid) (PLGA) has been extensively used in drug delivery, and its drug release is relatively slow in the absence of initiating factors, which will affect the therapeutic effect.Citation104 The ultrasensitive ROS-responsive hollow PLGA microspheres (HM) contain the anti-inflammatory drugs DEX-P, ethanol, ferrous chloride (FeCl2), and sodium bicarbonate (SBC) as bubble-generating agents. Excessive H2O2 converted encapsulated ethanol into acetic acid in the presence of Fe2+ by the Fenton reaction to form an acidic milieu (acetic acid) in OA tissue. The SBC is decomposed into CO2 in an acidic environment, which subsequently destroys the PLGA shell and releases the internal DEX-P.Citation105
It has been found that macrophage polarization is a crucial component of the joint inflammatory microenvironment, which is a therapeutic target for OA.Citation106,Citation107 Other researchers synthesized a novel polythioketal urethane (PTKU) using diisocyanate and polythioketal (PTK), which is sensitive to ROS. PTKU, as a drug delivery carrier, is loaded with the anti-inflammatory drug DEX to form NPs (PTKU@DEXNPs). It can significantly inhibit the intracellular level of ROS in the articular cavity and reduce the destruction of oxidative stress, which results in a lower ratio of inflammatory M1 macrophages to anti-inflammatory M2 macrophages. PTKU@DEXNPs show good cartilage protection and elimination of inflammation in a monosodium iodoacetate (MIA)-induced OA model.Citation108
Based on the ROS-responsive property of PTK, a ROS-scavenging and drug-release platform was synthesized by encapsulating dexamethasone acetate (DA)-loaded ROS-erasable poly(ethylene glycol)-b-polythioketal-b-poly(ethylene glycol) (PEG-PTK-PEG) micelles (PDM) into an injectable hydrogel. To achieve a self-healing property for viscosupplementation, the hydrogel (HDH@PDM) was created using a Schiff base reaction between hydrazide-grafted hyaluronic acid (HA-ADH) and aldehyde-modified dextran (Dex-ALH). Intra-articular injections of a multifunctional hydrogel potently reduced inflammation through the depletion of ROS, the suppression of inflammatory cytokines, and the downregulation of the proinflammatory M1 macrophage ratio in a rat OA modelCitation109 ().
A possible enhancement of OA therapy could be achieved by using ROS clearance to stimulate anti-inflammatory drug release in response to oxidation. The therapeutic effect is further increased by targeting the membrane receptor in macrophages and subsequently releasing anti-inflammatory drugs. Here, a multifunctional anti-inflammatory drug (CPH) was constructed by physically encapsulating CO release molecules 401(CORM-401) as a CO donor, folic acid (FA)-modified hyaluronic acid (HA) as the targeting ligand, and peptide dendrimers nanogels (PDNs) as a carrier. The oxidation-responsive release of CO from CORM-401 is significantly accelerated in the presence of relevant oxidants, such as H2O2. CPHs enter activated macrophages via FA-modified HA and rapidly release large amounts of CO that suppress the secretion of inflammatory factors by inhibiting cell proliferation, protect articular cartilage and suppress the degradation of ECM by deleting ROS in OA joints and inhibit proinflammatory signaling pathways.Citation110
Boronate-stabilized polyphenol–poloxamer NPs are used as drug delivery systems (PPNPs). Dexamethasone is loaded into PPNP to form nanodrugs (DEX@PPNP) for OA treatment that exhibit ROS-responsive drug release behavior and ROS scavenging capabilities. In lipopolysaccharide-activated RAW264.7 macrophages, nanodrugs inhibit ROS and nitric oxide production efficiently and modulate M2 polarization. Treating monosodium iodoacetate-induced OA mice with this nanodrug reduced the inflammation associated with angiogenesis and arthritis scores and inhibited cartilage degradation and bone erosion in the joints.Citation111
ROS-Responsive Nanomaterials for Chondrocytes
Melanin can scavenge ROS due to its abundant reductive functional groups, such as catechol, amine, and imine. Artificial melanin-like NPs prepared by dopamine polymerization also exhibit the potential to neutralize ROS and reduce joint inflammation.Citation112–114 A smart dual responsive hybrid micelle with free radical scavenger melanin in the micellar core and polydopamine (PDA) on the shell (DHMP/M) has been developed for photoacoustic imaging-guided OA microenvironmental regulation. The PDA shell on the surface of the HM/M core is responsive to ROS and slowly degraded by endogenous ROS. Subsequently, this drug delivery system will gradually release melanin for a long time under chronic inflammation and be manually activated explosively by near-infrared (NIR) irradiation during acute inflammation. These two methods are very important for personalized therapy at specific time points.Citation115
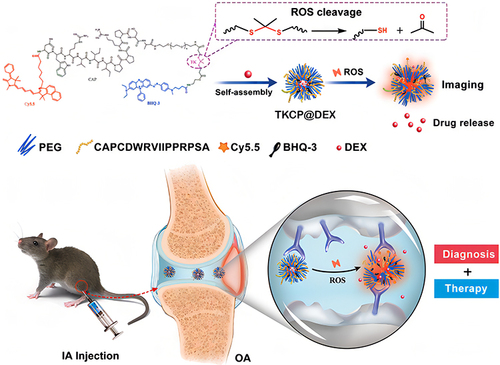
Recently, the ROS-responsive linker thioketal has attracted considerable interest for its sensitivity and responsiveness to downregulate endogenous ROS. TKCP is formed by Cy5.5-modified cartilage-targeting peptide (CAP, DWRVIIPPRPSA) and PEG-modified oxidation-responsive thioketal linkers (TK), which contain Black Hole Quencher 3 (BHQ-3) as a quencher for Cy5.5, and then encapsulated with DEX to form TKCP@DEX NPs. The advantage of TKCP@DEX Ns is that they specifically target chondrocytes through CAP, cleaving the thioketal linkages in response to high levels of ROS and resulting in the gradual dissociation of the polymer to release Cy5.5 and DEX in inflamed tissues. The released Cy5.5 from the polymer makes it a long distance between the quencher (BHQ-3) and Cy5.5, which enables a stronger fluorescence signal from Cy5.5 without the quenching action from BHQ-3. Conversely, the low level of ROS in normal tissue does not cleave thioketal linkers. BHQ-3 played an important role in quenching Cy5.5 over a short distance. This smart cartilage-targeting ROS-responsive theranostic nanoprobe is promising for OA therapyCitation116 ().
In addition, the NPs named DLNPs were composed of SeSe-group, DEX and cartilage-derived-morphogenetic-protein-1 (CDMP-1). The SeSe group, as the ROS response component, will be fractured when encountering a high concentration of oxygen free radicals in arthritis lesions, and then DEX and CDMP-1, as the main pharmacophore, will be released to eliminate joint inflammation and induce cartilage repair.Citation117 The drug-loaded micelles exhibited an anti-inflammatory effect by inhibiting the proliferation of activated macrophages, inducing macrophage apoptosis, and promoting bone marrow mesenchymal stem cells (BMSC) differentiation into chondrocytes.
Previous studies proved that phenylboronic acid is an efficient ROS-responsive group.Citation118 Dex-pPADN NPs are constructed by modifying L-DOPA with phenylboronic acid and PEGylating to self-assemble into NPs (pPADN) and subsequently loading and dual-responsive delivery of Dex,Citation119 inducing the inhibition of proinflammatory factors, the scavenging of ROS, and the activation of a NIR photoacoustic (PA) signal. In a rat OA model, Dex-pPADN markedly alleviated synovial inflammation and inhibited joint destruction and cartilage matrix degradation. Moreover, the structural transformation of pPADN makes it a suitable tool to monitor therapeutic effects such as photoacoustic imaging.
ROS-Responsive Nanomaterials for Other Cells
Other intra-articular tissues, such as the infrapatellar pad, meniscus and synovium, in OA have contributed to the progression of OA,Citation16,Citation19,Citation28 but relatively little research using NPs has been done in these tissues.
To control intracellular gases, researchers created encapsulated S-methylisothiourea hemisulfate salt (SMT) and CAT ZIF-8 NPs. These NPs were additionally modified with anti-CD16/32 antibody (Ab) to target M1 macrophages and extend the retention duration of NPs in the synovium. ZIF-8 NPs breakdown into the acidic environment of endosomes after being ingested by M1 macrophages and release CAT and SMT. In addition, CAT may breakdown extracellular H2O2 to produce O2, which is required for mitochondrial respiration.Citation120 Hypoxia-inducible factor 1α (HIF1α), which is crucial for M1 macrophage polarization, can also be inhibited by O2Citation121. More notably, modified NPs prevented cartilage degradation in vivo and inhibited macrophage conditioned medium (CM)-induced chondrocyte hypertrophy in vitro.
The Advantages of Nanotherapy in OA
Traditional injection or oral intake of antioxidants usually cannot manifest therapeutic effects because of rapid clearance, degradation, and low bioavailability. For example, intra-articular injection takes at least once a week for 8 weeks, but effectiveness cannot be guaranteed due to the rapid clearance and degradation of drug molecules.Citation122,Citation123 However, systematic administration often requires a high dosage and frequent drug administration to ensure curative effects, resulting in obvious side effects.Citation33,Citation124 Nanotechnology refers to the development and use of small nanometer-sized objects based on their various properties, the size of which ranges from 1–100 nm or their volume-specific surface area is larger than 60 m2/cm2. NPs have been used as drug delivery systems, where they are capable of reaching targeted organs or sites by recognizing receptors on the target cells’ cellular pathways.Citation125 The advantages of nanodrug delivery systems in the treatment of OA are summarized as follows.
Increased Bioavailability in vivo by Improving Drug Solubility
Polyethylene glycol (PEG) has been proven to increase drug solubility and is widely used in polymer-drug conjugates due to its negligible toxicity and immunogenicity.Citation126 Formononetin (FMN), a phytoestrogen from natural herbal plants, can effectively decrease proteoglycan loss in IL-1β-induced catabolic chondrocytes.Citation127 PEGylation of FMN followed by coupling with cartilage-targeting peptide (CollBP) showed good anti-inflammatory and chondroprotective effects in IL-1β-stimulated chondrocytes and OA rat joints.Citation128 However, the bioavailability of FMN is low due to its poor water solubility, but the loading rate of FMN in PEG-CollBP-FMN (PCFMN) is nearly 9%.
High Loading Rate of Drugs
Numerous studies have shown that a higher drug loading of biomaterials is linked to a higher pore surface area. The mesoporous structure in the NPs was constructed to enhance the drug loading, such as CaP coated mesoporous polydopamine nanoparticles with responsive membrane permeation ability realizing high siRNA loading capacity (10 wt%) and mesoporous polydopamine nanoparticles (MPDA) have properties of a high payload of doxorubicin hydrochloride (up to 2000 μg mg−1).Citation129–131 The superiority of improving the loading rate of the drug in NPs can effectively reduce the number of intra-articular injections, which will avoid further damage to cartilage.
Precise Drug Targeting to Organ or Tissue
Synovial inflammation is a major indication of OA. In particular, macrophages in the synovium have attracted considerable attention.Citation132–134 Several targeting peptides have been described in recent studies. Liposomes containing phosphatidylserine (PS) (PS-containing liposomes; PSL), as a biomaterial carrier, show the ability to target macrophages and anti-inflammatory function both in vivo and in vitro.Citation132 A 4-phenyl borate-cyclodextrin biomaterial loaded with dexamethasone (Dex/Oxi-αCDNPs) reduced joint swelling and cartilage destruction by targeting synovial macrophages.Citation133 CEL-loaded PRNPs (CEL-PRNPs), which were composed of celastrol (CEL), enzyme-responsive NPs (termed PRNPs) and RGD-modified NPs (termed RNPs) covered with cleavable PEG chains, selectively induced the apoptosis of osteoclasts and inflammatory macrophages in arthritic joints.Citation134
Controlled Release for NPs
External environmental factors, such as near-infrared light (NIR, λ= 700–1100 nm), can be used as influencing factors for precise and controllable drug release. NIR can penetrate inflammatory tissue, the treatment of which is of particular interest because it is relatively harmless.Citation135,Citation136
The intra-articular drug delivery nanosystem MoS2@CS@Dex (MCD) was constructed by using chitosan (CS)-modified molybdenum disulfide (MoS2) nanosheets as NIR photoresponsive carriers loaded with the anti-inflammatory drug dexamethasone (Dex). MCD was in response to NIR light both in vitro and in vivo and released Dex through photothermal conversion. Injecting MCD into the articular cavity followed by NIR radiation downregulated the secretion levels of TNF-α and IL-1β, thus protecting chondrocytes and articular cartilage from injury.Citation137
Loading and Delivering Multiple Drugs Simultaneously
The onset and development of OA are affected by multiple factors. Therefore, different drugs that target different factors are essential in OA treatment to achieve remarkable effects. For example, RB@MPMW refers to a metal organic framework (MOF)-decorated mesoporous polydopamine (MPDA)-based dual-drug delivery system that has bilirubin (Br) loaded onto the MOF shell and rapamycin (Rap) loaded into the mesopores, which are responsible for enhancing autophagy activation and scavenging reactive oxygen species (ROS), respectively.Citation138 Rapamycin, an autophagy activator, protects chondrocytes from harmful stressors and apoptosis,Citation138 and bilirubin inhibits the polarization of M1 macrophages, which release proinflammatory cytokines.Citation139,Citation140 Furthermore, as little as 10 nM bilirubin protects against 10,000-fold higher concentrations of H2O2.Citation141
Theranostic NPs for OA
The nanoprobe with the features of bioimaging and targeted therapy in treating OA shows different fluorescence intensities depending on the physiologic state. The fluorescent signal from the ROS-responsive nanoprobe following intra-articular injections was strong in the OA microenvironment but extremely weak in normal chondrocytes and joints, indicating that the level of the fluorescence signal correlates with the ROS content. This smart cartilage-targeting ROS-responsive nanoprobe with diagnosis and treatment is especially suitable for OA therapy.Citation116
The OA microenvironment includes excessive inflammation and the overproduction of ROS, which mainly destroys ECM synthesis in the development of OA.Citation142 The abnormality of the antioxidant defense system in OA patients is an important factor that makes tissues susceptible to oxidative stress and causes joint oxidative pathological damage.Citation117,Citation138,Citation143 Hence, ROS might be a potential therapeutic target in clinical application. Ideal treatment should be sensitive and activated by changes in the tissue microenvironment, leading to a controlled release of the drug and satisfying OA therapy.Citation144
Although nanotherapy has many advantages over conventional treatment, it still has many limitations. 1) NPs can be transferred via the bloodstream throughout the body and then accumulate in various organs, affecting their functions after entering the body,Citation145 which is prevalent, as is the case for ROS-responsive NPs. 2) The synthesis of some NPs is complicated, and this method requires high cost, such as liposomes.Citation146 The manufacturing process, reproducibility, and quality are difficult to control.Citation146 3) Some NPs become unstable in close to neutral pH circumstances, such as chitosan.Citation147 4) The NPs may be present in the joint cavity for only a short amount of time due to rapid clearance.Citation148 The advantages and disadvantages of Nanotherapy in OA are summarized in .
Table 2 Advantages and Disadvantages of Nanotherapy in OA
Discussion and Future Perspectives
Recently, ROS-responsive nanomaterials have attracted considerable attention in basic research of many diseases, such as cancer, inflammation and neurodegenerative diseases. Remarkable progress has been made in ROS-responsive biomaterials for cancer treatment. However, relatively few studies are available on OA treatment with ROS-responsive biomaterials. Previous studies have demonstrated that inflammation stimulates oxidative stress and vice versa. Theoretically, anti-inflammatory drugs such as DEX also possess the property of removing ROS. In this review, we found that DEX has been used in almost all ROS-responsive nanodrug delivery systems for clearing excess free radicals in OA tissue.
However, DEX has limited clinical applications due to its side effects at high doses in short-term use or side effects at low doses in long-term use, which often cause muscular atrophy, bone fractures, and osteonecrosis. Controlling the release of DEX precisely and responsively in OA treatment is still a great challenge. Melanin, a natural antioxidative metabolite, has also been applied in OA treatment, which efficiently diminishes inflammatory cytokine release and cartilage degradation.Citation112 Nevertheless, melanin-based/like biomaterials are linked to poor stability due to the autoxidation of catechol in aqueous solution. Given the unsatisfactory clinical efficacy of OA treatment, it is urgent to discover novel drugs targeting inflammation and ROS.
Compared with unresponsive delivery systems, ROS-responsive systems remain stable in normal tissue, preventing their release to noninflamed tissues, which suggests their specificity in inflammation sites. OA is characterized by the degeneration of the whole joint, which is especially suitable for intra-articular injection of ROS-responsive nanomedicine, realizing controlled release and targeted therapy without affecting other tissues or organs through blood circulation. Generally, this approach entailed the endogenous or exogenous stimulus-responsive release of bioactive agents to the target tissue and reduced the side effects.Citation149,Citation150
There are currently a number of clinical trials using NPs. For example, a Phase 3 controlled trial using doxorubicin-loaded NPs for patients with advanced hepatocellular carcinoma after sorafenib treatment failure (RELIVE) showed the dose-dependent toxicity of doxorubicin, and this first phase 3 study did increase overall survival.Citation151 However, there are few clinical trials using NPs in OA. For example, a randomized controlled trial showed that P. amarus NP gel was effective for reducing pain and improving the results of the six-minute walk test (6-MWT) in patients with OA knees.Citation152 This means that NPs are very promising in the treatment of OA, and there is a lot of work to do.
However, difficulties in clinical transformation still exist. 1) The effectiveness and safety of ROS-responsive nanomaterials need to be considered for long-term use. The best choice for clinical application is the drug carrier from natural polymers such as chitosan, dextran, alginate, pullulan, and HA, all of which are FDA-approved natural polymers for their noncytotoxicity, nonimmunogenicity, biocompatibility, and biodegradability. In particular, biomaterials from the components of cartilage tissue (HA) may be more preferred. 2) The biological mechanism underlying OA should be well understood. OA is a multifactorial disease for which it is difficult to achieve the desired therapeutic effect using single-modal therapy. Multimodal therapy is an attractive treatment method, especially when paired with precise treatments. 3) The changes in physiological behavior and tissue structure in experimental animals are different from those in humans, and different patients respond differently to ROS-responsive nanomedicines because of the complexity of biological systems.
In addition, all these platforms, including the delivery efficiency of current drug carriers for scavenging ROS, in vivo therapeutic effects and controlled drug release profiles of the current drug delivery system, are still unsatisfactory due to their insufficient responsiveness and rapid joint clearance.
Although NPs can respond to the microenvironment when they enter the human body, there is a lack of long-term, comprehensive analysis of the in vivo toxicity of nanomaterials. Future work should focus on chemistry, new biomaterials, specific targets for therapy, and effective free radical scavenging drugs. Moreover, mitochondria are responsible for the main source of ROS. Conventional antioxidants fail to ameliorate the severity of ROS-related diseases, which may be a result of their inability to concentrate in mitochondria. Thus, it seems to be a worthwhile strategy to develop mitochondria-targeted antioxidants in the future. Furthermore, considering the rapid clearance of the joint cavity, NPs should be designed to maximize the retention time in the joint cavity.
As previously mentioned, the infrapatellar pad, meniscus and synovium promote the development of OA. However, there are very few studies using ROS-responsive nanoparticles in these tissues. Therefore, novel nanomaterials that target these tissues can be considered.
Conclusion
ROS-responsive polyfunctional nanosystems 3.0 are a promising therapy for the treatment of OA. Given the effects proven by the current study, this is very prospective for clinical applications.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
Acknowledgments
We thanked National Natural Science Foundation of China (81902306, 82174406, 81873328), the National Key R&D Program of China (Grant No. 2018YFC2001600) and Engineering Research Center of Traditional Chinese Medicine Intelligent Rehabilitation, Ministry of Education for the support. The help about revised manuscript from Zhi-Heng Zhu, Jia-Ying Ding and Yong-Jia Song, from Shanghai University of Traditional Chinese Medicine, are greatly appreciated.
References
- Machado GC, Maher CG, Ferreira PH., et al.. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. Brit Med J. 2015;350. doi:10.1136/bmj.h1225
- Qin J, Barbour KE, Murphy LB, et al. Lifetime risk of symptomatic hand osteoarthritis: the Johnston County osteoarthritis project. Arthritis Rheumatol. 2017;69(6):1204–1212. doi:10.1002/art.40097
- Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi:10.1016/S0140-6736(19)30417-9
- da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and Hip osteoarthritis: a network meta-analysis. Lancet. 2016;387(10033):2093–2105. doi:10.1016/S0140-6736(16)30002-2
- World Health Organization. Chronic Rheumatic Conditions. Geneva, Switzerland: World Health Organization; 2016.
- Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr Cartilage. 2015;23(8):1233–1241. doi:10.1016/j.joca.2015.03.036
- Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23(10):1515–1527. doi:10.1016/j.cellsig.2011.05.004
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi:10.1016/j.cub.2014.03.034
- Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26(4):249–261. doi:10.1016/j.tcb.2015.12.002
- Lepetsos P, Papavassiliou KA, Papavassiliou AG. Redox and NF-kappaB signaling in osteoarthritis. Free Radic Biol Med. 2019;132:90–100. doi:10.1016/j.freeradbiomed.2018.09.025
- Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi:10.1016/S0140-6736(14)60802-3
- Mendes AF, Caramona MM, Carvalho AP, Lopes MC. Role of mitogen-activated protein kinases and tyrosine kinases on IL-1-Induced NF-kappaB activation and iNOS expression in bovine articular chondrocytes. Nitric Oxide. 2002;6(1):35–44. doi:10.1006/niox.2001.0378
- Mendes AF, Carvalho AP, Caramona MM, Lopes MC. Role of nitric oxide in the activation of NF-kappaB, AP-1 and NOS II expression in articular chondrocytes. Inflamm Res. 2002;51(7):369–375. doi:10.1007/pl00000317
- Lo HM, Lai TH, Li CH, Wu WB. TNF-alpha induces CXCL1 chemokine expression and release in human vascular endothelial cells in vitro via two distinct signaling pathways. Acta Pharmacol Sin. 2014;35(3):339–350. doi:10.1038/aps.2013.182
- Campbell SJ, Wilcockson DC, Butchart AG, Perry VH, Anthony DC. Altered chemokine expression in the spinal cord and brain contributes to differential interleukin-1beta-induced neutrophil recruitment. J Neurochem. 2002;83(2):432–441. doi:10.1046/j.1471-4159.2002.01166.x
- Zhang H, Lin C, Zeng C, et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. 2018;77(10):1524–1534. doi:10.1136/annrheumdis-2018-213450
- Kalaitzoglou E, Griffin TM, Humphrey MB. Innate immune responses and osteoarthritis. Curr Rheumatol Rep. 2017;19(8):45. doi:10.1007/s11926-017-0672-6
- Yu H, Ye WB, Zhong ZM, Ding RT, Chen JT. Effect of advanced oxidation protein products on articular cartilage and synovium in a rabbit osteoarthritis model. Orthop Surg. 2015;7(2):161–167. doi:10.1111/os.12179
- Qiong J, Xia Z, Jing L, Haibin W. Synovial mesenchymal stem cells effectively alleviate osteoarthritis through promoting the proliferation and differentiation of meniscus chondrocytes. Eur Rev Med Pharmacol Sci. 2020;24(4):1645–1655. doi:10.26355/eurrev_202002_20338
- Cake MA, Read RA, Appleyard RC, Hwa SY, Ghosh P. The nitric oxide donor glyceryl trinitrate increases subchondral bone sclerosis and cartilage degeneration following ovine meniscectomy. Osteoarthritis Cartilage. 2004;12(12):974–981. doi:10.1016/j.joca.2004.08.012
- Evans DM, Ralston SH. Nitric oxide and bone. J Bone Miner Res. 1996;11(3):300–305. doi:10.1002/jbmr.5650110303
- Gupta T, Zielinska B, McHenry J, Kadmiel M, Haut Donahue TL. IL-1 and iNOS gene expression and NO synthesis in the superior region of meniscal explants are dependent on the magnitude of compressive strains. Osteoarthritis Cartilage. 2008;16(10):1213–1219. doi:10.1016/j.joca.2008.02.019
- Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280(5365):898–902. doi:10.1126/science.280.5365.898
- Galleron S, Borderie D, Ponteziere C, et al. Reactive oxygen species induce apoptosis of synoviocytes in vitro. Alpha-tocopherol provides no protection. Cell Biol Int. 1999;23(9):637–642. doi:10.1006/cbir.1999.0424
- Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22(1–2):287–305. doi:10.1016/s0891-5849(96)00327-9
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi:10.1016/0891-5849(91)90192-6
- McIntyre TM, Zimmerman GA, Prescott SM. Biologically active oxidized phospholipids. J Biol Chem. 1999;274(36):25189–25192. doi:10.1074/jbc.274.36.25189
- Duplus E, Glorian M, Forest C. Fatty acid regulation of gene transcription. J Biol Chem. 2000;275(40):30749–30752. doi:10.1074/jbc.R000015200
- Tiku ML, Allison GT, Naik K, Karry SK. Malondialdehyde oxidation of cartilage collagen by chondrocytes. Osteoarthritis Cartilage. 2003;11(3):159–166. doi:10.1016/s1063-4584(02)00348-5
- McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartilage. 2014;22(3):363–388. doi:10.1016/j.joca.2014.01.003
- Bjordal JM, Klovning A, Ljunggren AE, Slordal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: a meta-analysis of randomised placebo-controlled trials. Eur J Pain. 2007;11(2):125–138. doi:10.1016/j.ejpain.2006.02.013
- Kang ML, Im GI. Drug delivery systems for intra-articular treatment of osteoarthritis. Expert Opin Drug Deliv. 2014;11(2):269–282. doi:10.1517/17425247.2014.867325
- Hermann W, Lambova S, Muller-Ladner U. Current treatment options for osteoarthritis. Curr Rheumatol Rev. 2018;14(2):108–116. doi:10.2174/1573397113666170829155149
- Sen R, Hurley JA. Osteoarthritis. In: StatPearls. Treasure Island: Stat Pearls Publishing Copyright; 2022.
- Choudhary D, Adhikary S, Ahmad N, et al. Prevention of articular cartilage degeneration in a rat model of monosodium iodoacetate induced osteoarthritis by oral treatment with Withaferin A. Biomed Pharmacother. 2018;99:151–161. doi:10.1016/j.biopha.2017.12.113
- Khan NM, Ahmad I, Ansari MY, Haqqi TM. Wogonin, a natural flavonoid, intercalates with genomic DNA and exhibits protective effects in IL-1beta stimulated osteoarthritis chondrocytes. Chem Biol Interact. 2017;274:13–23. doi:10.1016/j.cbi.2017.06.025
- Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73–82. doi:10.1016/j.freeradbiomed.2018.08.038
- Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, Haqqi TM. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic Biol Med. 2017;106:288–301. doi:10.1016/j.freeradbiomed.2017.02.041
- Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576–591. doi:10.1016/j.bbadis.2016.01.003
- Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi:10.1016/j.redox.2015.09.005
- Ansari MY, Khan NM, Ahmad I, Haqqi TM. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26(8):1087–1097. doi:10.1016/j.joca.2017.07.020
- Goetz JE, Coleman MC, Fredericks DC, et al. Time-dependent loss of mitochondrial function precedes progressive histologic cartilage degeneration in a rabbit meniscal destabilization model. J Orthop Res. 2017;35(3):590–599. doi:10.1002/jor.23327
- Ozgur R, Turkan I, Uzilday B, Sekmen AH. Endoplasmic reticulum stress triggers ROS signalling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. J Exp Bot. 2014;65(5):1377–1390. doi:10.1093/jxb/eru034
- Burtenshaw D, Hakimjavadi R, Redmond EM, Cahill PA. Nox, reactive oxygen species and regulation of vascular cell fate. Antioxidants. 2017;6(4). doi:10.3390/antiox6040090
- He L, Eslamfam S, Ma X, Li D. Autophagy and the nutritional signaling pathway. Front Agr Sci Eng. 2016;3(03):222–230.
- Pudlarz AM, Czechowska E, Ranoszek-Soliwoda K, et al. Immobilization of recombinant human catalase on gold and silver nanoparticles. Appl Biochem Biotech. 2018;185(3):717–735. doi:10.1007/s12010-017-2682-2
- Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta. 2013;1830(5):3217–3266. doi:10.1016/j.bbagen.2012.09.018
- Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45(23):6597–6626. doi:10.1039/c6cs00271d
- Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–26054. doi:10.3390/ijms161125943
- Hosseinzadeh A, Kamrava SK, Joghataei MT, et al. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J Pineal Res. 2016;61(4):411–425. doi:10.1111/jpi.12362
- Charlier E, Relic B, Deroyer C, et al. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int J Mol Sci. 2016;17(12). doi:10.3390/ijms17122146
- Portal-Nunez S, Esbrit P, Alcaraz MJ, Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem Pharmacol. 2016;108:1–10. doi:10.1016/j.bcp.2015.12.012
- Hou A, Chen P, Tang H, et al. Cellular senescence in osteoarthritis and anti-aging strategies. Mech Ageing Dev. 2018;175:83–87. doi:10.1016/j.mad.2018.08.002
- Yudoh K, Nguyen VT, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7(2):R380–91. doi:10.1186/ar1499
- McCulloch K, Litherland GJ, Rai TS. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16(2):210–218. doi:10.1111/acel.12562
- Collins JA, Diekman BO, Loeser RF. Targeting aging for disease modification in osteoarthritis. Curr Opin Rheumatol. 2018;30(1):101–107. doi:10.1097/BOR.0000000000000456
- Coleman MC, Goetz JE, Brouillette MJ, et al. Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis. Sci Transl Med. 2018;10(427). doi:10.1126/scitranslmed.aan5372
- Pang Z, Jiang Z, Zhu R, et al. Bardoxolone-methyl prevents oxidative stress-mediated apoptosis and extracellular matrix degradation in vitro and alleviates osteoarthritis in vivo. Drug Des Devel Ther. 2021;15:3735–3747. doi:10.2147/DDDT.S314767
- Jiang W, Liu H, Wan R, Wu Y, Shi Z, Huang W. Mechanisms linking mitochondrial mechanotransduction and chondrocyte biology in the pathogenesis of osteoarthritis. Ageing Res Rev. 2021;67:101315. doi:10.1016/j.arr.2021.101315
- Wang FS, Kuo CW, Ko JY, et al. Irisin mitigates oxidative stress, chondrocyte dysfunction and osteoarthritis development through regulating mitochondrial integrity and autophagy. Antioxidants. 2020;9(9). doi:10.3390/antiox9090810
- Gavriilidis C, Miwa S, von Zglinicki T, Taylor RW, Young DA. Mitochondrial dysfunction in osteoarthritis is associated with down-regulation of superoxide dismutase 2. Arthritis Rheumatol. 2013;65(2):378–387.
- Haseeb A, Haqqi TM. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146(3):185–196. doi:10.1016/j.clim.2012.12.011
- Poulet B, Staines KA. New developments in osteoarthritis and cartilage biology. Curr Opin Pharmacol. 2016;28:8–13. doi:10.1016/j.coph.2016.02.009
- Tao H, Ge G, Liang X, et al. ROS signaling cascades: dual regulations for osteoclast and osteoblast. Acta Biochim Biophys Sin. 2020;52(10):1055–1062. doi:10.1093/abbs/gmaa098
- Murakami T, Nakaminami Y, Takahata Y, Hata K, Nishimura R. Activation and function of NLRP3 inflammasome in bone and joint-related diseases. Int J Mol Sci. 2022;23(10). doi:10.3390/ijms23105365
- He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41(12):1012–1021. doi:10.1016/j.tibs.2016.09.002
- Chen Z, Zhong H, Wei J, et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther. 2019;21(1):300. doi:10.1186/s13075-019-2085-6
- Hughes MM, O’Neill LAJ. Metabolic regulation of NLRP3. Immunol Rev. 2018;281(1):88–98. doi:10.1111/imr.12608
- Wang H, Jiang Z, Pang Z, et al. Engeletin protects against TNF-alpha-induced apoptosis and reactive oxygen species generation in chondrocytes and alleviates osteoarthritis in vivo. J Inflamm Res. 2021;14:745–760. doi:10.2147/JIR.S297166
- Calabrese G, Ardizzone A, Campolo M, Conoci S, Esposito E, Paterniti I. Beneficial effect of tempol, a membrane-permeable radical scavenger, on inflammation and osteoarthritis in in vitro models. Biomolecules. 2021;11(3). doi:10.3390/biom11030352
- Omidian K, Rafiei H, Bandy B. Increased mitochondrial content and function by resveratrol and select flavonoids protects against benzo[a]pyrene-induced bioenergetic dysfunction and ROS generation in a cell model of neoplastic transformation. Free Radic Biol Med. 2020;152:767–775. doi:10.1016/j.freeradbiomed.2020.01.021
- Peng Y, Kwok KH, Yang PH, et al. Ascorbic acid inhibits ROS production, NF-kappa B activation and prevents ethanol-induced growth retardation and microencephaly. Neuropharmacology. 2005;48(3):426–434. doi:10.1016/j.neuropharm.2004.10.018
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi:10.1016/S0140-6736(11)60243-2
- Kim MJ, Kim HJ, Hong YH, et al. Age-related NADPH Oxidase (arNOX) activity correlated with cartilage degradation and bony changes in age-related osteoarthritis. J Korean Med Sci. 2015;30(9):1246–1252. doi:10.3346/jkms.2015.30.9.1246
- Liao CR, Wang SN, Zhu SY, et al. Advanced oxidation protein products increase TNF-alpha and IL-1beta expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2020;28:101306. doi:10.1016/j.redox.2019.101306
- Collins JA, Wood ST, Nelson KJ, et al. Oxidative stress promotes peroxiredoxin hyperoxidation and attenuates pro-survival signaling in aging chondrocytes. J Biol Chem. 2016;291(13):6641–6654. doi:10.1074/jbc.M115.693523
- Koike M, Nojiri H, Ozawa Y, et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci Rep. 2015;5:11722. doi:10.1038/srep11722
- Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi:10.1016/S0140-6736(17)30058-2
- Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3(12):716–724. doi:10.1038/ncprheum0674
- de Boer TN, van Spil WE, Huisman AM, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20(8):846–853. doi:10.1016/j.joca.2012.05.002
- Lee AS, Ellman MB, Yan D, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–447. doi:10.1016/j.gene.2013.05.069
- Veronese N, Cooper C, Reginster JY, et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum. 2019;49(1):9–19. doi:10.1016/j.semarthrit.2019.01.005
- Wegner AM, Campos NR, Robbins MA, et al. Acute Changes in NADPH Oxidase 4 in Early Post-Traumatic Osteoarthritis. J Orthop Res. 2019;37(11):2429–2436. doi:10.1002/jor.24417
- Cuellar VG, Cuellar JM, Golish SR, Yeomans DC, Scuderi GJ. Cytokine profiling in acute anterior cruciate ligament injury. Arthroscopy. 2010;26(10):1296–1301. doi:10.1016/j.arthro.2010.02.011
- Sward P, Frobell R, Englund M, Roos H, Struglics A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)–a cross-sectional analysis. Osteoarthritis Cartilage. 2012;20(11):1302–1308. doi:10.1016/j.joca.2012.07.021
- Teshima S, Nakanishi H, Nishizawa M, et al. Up-regulation of IL-1 receptor through PI3K/Akt is essential for the induction of iNOS gene expression in hepatocytes. J Hepatol. 2004;40(4):616–623. doi:10.1016/j.jhep.2003.12.018
- Teng XW, Zhang HF, Snead C, Catravas JD. Molecular mechanisms of iNOS induction by IL-1 beta and IFN-gamma in rat aortic smooth muscle cells. Am J Physiol Cell Ph. 2002;282(1):C144–C152. doi:10.1152/ajpcell.2002.282.1.C144
- Bhandary B, Marahatta A, Kim HR, Chae HJ. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14(1):434–456. doi:10.3390/ijms14010434
- Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33(4):359–370. doi:10.1007/s00774-015-0656-4
- Schroder K. NADPH oxidases in bone homeostasis and osteoporosis. Free Radic Biol Med. 2019;132:67–72. doi:10.1016/j.freeradbiomed.2018.08.036
- Knowles HJ. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia. 2015;3:73–82. doi:10.2147/HP.S95960
- Morten KJ, Badder L, Knowles HJ. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J Pathol. 2013;229(5):755–764. doi:10.1002/path.4159
- Lee NK, Choi YG, Baik JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106(3):852–859. doi:10.1182/blood-2004-09-3662
- Khorasani MS, Diko S, Hsia AW, et al. Effect of alendronate on post-traumatic osteoarthritis induced by anterior cruciate ligament rupture in mice. Arthritis Res Ther. 2015;17:30. doi:10.1186/s13075-015-0546-0
- Geurts J, Patel A, Hirschmann MT, et al. Elevated marrow inflammatory cells and osteoclasts in subchondral osteosclerosis in human knee osteoarthritis. J Orthop Res. 2016;34(2):262–269. doi:10.1002/jor.23009
- Yajun W, Jin C, Zhengrong G, et al. Betaine attenuates osteoarthritis by inhibiting osteoclastogenesis and angiogenesis in subchondral bone. Front Pharmacol. 2021;12:723988. doi:10.3389/fphar.2021.723988
- Kawai Y, Kubota E, Okabe E. Reactive oxygen species participation in experimentally induced arthritis of the temporomandibular joint in rats. J Dent Res. 2000;79(7):1489–1495. doi:10.1177/00220345000790071001
- Johnson K, Jung A, Murphy A, Andreyev A, Dykens J, Terkeltaub R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000;43(7):1560–1570. doi:10.1002/1529-0131(200007)43:7<1560::AID-ANR21>3.0.CO;2-S
- Khan IM, Gilbert SJ, Caterson B, Sandell LJ, Archer CW. Oxidative stress induces expression of osteoarthritis markers procollagen IIA and 3B3(-) in adult bovine articular cartilage. Osteoarthritis Cartilage. 2008;16(6):698–707. doi:10.1016/j.joca.2007.10.004
- Morita K, Miyamoto T, Fujita N, et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med. 2007;204(7):1613–1623. doi:10.1084/jem.20062525
- Arra M, Swarnkar G, Ke K, et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat Commun. 2020;11(1):3427. doi:10.1038/s41467-020-17242-0
- Crielaard BJ, Rijcken CJF, Quan LD, et al. Glucocorticoid-loaded core-cross-linked polymeric micelles with tailorable release kinetics for targeted therapy of rheumatoid arthritis. Angew Chem Int Edit. 2012;51(29):7254–7258. doi:10.1002/anie.201202713
- Elron-Gross I, Glucksam Y, Biton IE, Margalit R. A novel Diclofenac-carrier for local treatment of osteoarthritis applying live-animal MRI. J Control Release. 2009;135(1):65–70. doi:10.1016/j.jconrel.2008.12.005
- Ke CJ, Su TY, Chen HL, et al. Smart multifunctional hollow microspheres for the quick release of drugs in intracellular lysosomal compartments. Angew Chem Int Ed Engl. 2011;50(35):8086–8089. doi:10.1002/anie.201102852
- Chung MF, Chia WT, Wan WL, Lin YJ, Sung HW. Controlled release of an anti-inflammatory drug using an ultrasensitive ROS-responsive gas-generating carrier for localized inflammation inhibition. J Am Chem Soc. 2015;137(39):12462–12465. doi:10.1021/jacs.5b08057
- Hu Y, Gui Z, Zhou Y, Xia L, Lin K, Xu Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic Biol Med. 2019;145:146–160. doi:10.1016/j.freeradbiomed.2019.09.024
- Ni L, Lin Z, Hu S, et al. Itaconate attenuates osteoarthritis by inhibiting STING/NF-kappaB axis in chondrocytes and promoting M2 polarization in macrophages. Biochem Pharmacol. 2022;198:114935. doi:10.1016/j.bcp.2022.114935
- Zhang HL, Xiong H, Ahmed W, et al.. Reactive oxygen species-responsive and scavenging polyurethane nanoparticles for treatment of osteoarthritis in vivo. Chem Eng J. 2021;409. doi:10.1016/j.cej.2020.128147
- Tong ZA, Hao XB, Sw A, et al.. An injectable hydrogel dotted with dexamethasone acetate-encapsulated reactive oxygen species-scavenging micelles for combinatorial therapy of osteoarthritis-science direct. Mater Today Nano. 2021;17:100164.
- Yang GZ, Fan MN, Zhu JW, et al.. A multifunctional anti-inflammatory drug that can specifically target activated macrophages, massively deplete intracellular H2O2, and produce large amounts CO for a highly efficient treatment of osteoarthritis. Biomaterials. 2020;255. doi:10.1016/j.biomaterials.2020.120155
- Li X, Wang X, Liu Q, et al. ROS-responsive boronate-stabilized polyphenol-poloxamer 188 assembled dexamethasone nanodrug for macrophage repolarization in osteoarthritis treatment. Adv Healthc Mater. 2021;10(20):e2100883. doi:10.1002/adhm.202100883
- Ju KY, Lee Y, Lee S, Park SB, Lee JK. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules. 2011;12(3):625–632. doi:10.1021/bm101281b
- Zhong G, Yang X, Jiang X, et al. Dopamine-melanin nanoparticles scavenge reactive oxygen and nitrogen species and activate autophagy for osteoarthritis therapy. Nanoscale. 2019;11(24):11605–11616. doi:10.1039/c9nr03060c
- Bao X, Zhao J, Sun J, Hu M, Yang X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano. 2018;12(9):8882–8892. doi:10.1021/acsnano.8b04022
- Ruan JH, Yu QL, Cui HM, et al.. A smart ROS/NIR dual-responsive melanin delivery platform for photoacoustic imaging-guided osteoarthritis therapy. Appl Mater Today. 2021;25. doi:10.1016/j.apmt.2021.101216
- Shen C, Gao M, Chen H, et al. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J Nanobiotechnology. 2021;19(1):395. doi:10.1186/s12951-021-01136-4
- Wu XD, Li PP, Cheng J, et al.. ROS-sensitive nanoparticles co-delivering dexamethasone and CDMP-1 for the treatment of osteoarthritis through chondrogenic differentiation induction and inflammation inhibition. Front Bioeng Biotech. 2021;9. doi:10.3389/fbioe.2021.608150
- Xie C, Zhen X, Lyu Y, Pu K. Nanoparticle regrowth enhances photoacoustic signals of semiconducting macromolecular probe for in vivo imaging. Adv Mater. 2017;29(44). doi:10.1002/adma.201703693
- Zhao C, Chen J, Ye J, et al. Structural transformative antioxidants for dual-responsive anti-inflammatory delivery and photoacoustic inflammation imaging. Angew Chem Int Ed Engl. 2021;60(26):14458–14466. doi:10.1002/anie.202100873
- Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys. 2014;68(3):475–478. doi:10.1007/s12013-013-9750-1
- Wang T, Liu H, Lian G, Zhang SY, Wang X, Jiang C. HIF1alpha-induced glycolysis metabolism is essential to the activation of inflammatory macrophages. Mediators Inflamm. 2017;2017:9029327. doi:10.1155/2017/9029327
- Chen Q, Shao X, Ling P, Liu F, Han G, Wang F. Recent advances in polysaccharides for osteoarthritis therapy. Eur J Med Chem. 2017;139:926–935. doi:10.1016/j.ejmech.2017.08.048
- Vinatier C, Merceron C, Guicheux J. Osteoarthritis: from pathogenic mechanisms and recent clinical developments to novel prospective therapeutic options. Drug Discov Today. 2016;21(12):1932–1937. doi:10.1016/j.drudis.2016.08.011
- Zhang W, Robertson WB, Zhao J, Chen W, Xu J. Emerging trend in the pharmacotherapy of osteoarthritis. Front Endocrinol. 2019;10:431. doi:10.3389/fendo.2019.00431
- Albrahim T, Alonazi MA. Role of beetroot (beta vulgaris) juice on chronic nanotoxicity of silver nanoparticle-induced hepatotoxicity in male rats. Int J Nanomedicine. 2020;15:3471–3482. doi:10.2147/IJN.S248078
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi:10.1038/nrd1033
- Cho IA, Kim TH, Lim H, et al. Formononetin antagonizes the interleukin-1beta-induced catabolic effects through suppressing inflammation in primary rat chondrocytes. Inflammation. 2019;42(4):1426–1440. doi:10.1007/s10753-019-01005-1
- Xiong W, Lan Q, Liang X, et al. Cartilage-targeting poly(ethylene glycol) (PEG)-formononetin (FMN) nanodrug for the treatment of osteoarthritis. J Nanobiotechnology. 2021;19(1):197. doi:10.1186/s12951-021-00945-x
- Zhao W, Wang H, Wang H, et al. Light-responsive dual-functional biodegradable mesoporous silica nanoparticles with drug delivery and lubrication enhancement for the treatment of osteoarthritis. Nanoscale. 2021;13(13):6394–6399. doi:10.1039/d0nr08887k
- Wang Z, Wang L, Prabhakar N, et al. CaP coated mesoporous polydopamine nanoparticles with responsive membrane permeation ability for combined photothermal and siRNA therapy. Acta Biomater. 2019;86:416–428. doi:10.1016/j.actbio.2019.01.002
- Xing Y, Zhang J, Chen F, Liu J, Cai K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale. 2017;9(25):8781–8790. doi:10.1039/c7nr01857f
- Toita R, Kawano T, Murata M, Kang JH. Anti-obesity and anti-inflammatory effects of macrophage-targeted interleukin-10-conjugated liposomes in obese mice. Biomaterials. 2016;110:81–88. doi:10.1016/j.biomaterials.2016.09.018
- Ni R, Song G, Fu X, et al. Reactive oxygen species-responsive dexamethasone-loaded nanoparticles for targeted treatment of rheumatoid arthritis via suppressing the iRhom2/TNF-alpha/BAFF signaling pathway. Biomaterials. 2020;232:119730. doi:10.1016/j.biomaterials.2019.119730
- Deng C, Zhang Q, He P, et al. Targeted apoptosis of macrophages and osteoclasts in arthritic joints is effective against advanced inflammatory arthritis. Nat Commun. 2021;12(1):2174. doi:10.1038/s41467-021-22454-z
- Ao L, Wu C, Liu K, et al. Polydopamine-derivated hierarchical nanoplatforms for efficient dual-modal imaging-guided combination in vivo cancer therapy. ACS Appl Mater Interfaces. 2018;10(15):12544–12552. doi:10.1021/acsami.8b02973
- Cen Y, Deng WJ, Yang Y, Yu RQ, Chu X. Core-shell-shell multifunctional nanoplatform for intracellular tumor-related mRNAs imaging and near-infrared light triggered photodynamic-photothermal synergistic therapy. Anal Chem. 2017;89(19):10321–10328. doi:10.1021/acs.analchem.7b02081
- Zhao Y, Wei C, Chen X, et al. Drug delivery system based on near-infrared light-responsive molybdenum disulfide nanosheets controls the high-efficiency release of dexamethasone to inhibit inflammation and treat osteoarthritis. ACS Appl Mater Interfaces. 2019;11(12):11587–11601. doi:10.1021/acsami.8b20372
- Xue S, Zhou X, Sang W, et al. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact Mater. 2021;6(8):2372–2389. doi:10.1016/j.bioactmat.2021.01.017
- Takei R, Inoue T, Sonoda N, et al. Bilirubin reduces visceral obesity and insulin resistance by suppression of inflammatory cytokines. PLoS One. 2019;14(10):e0223302. doi:10.1371/journal.pone.0223302
- Hinds TD, Creeden JF, Gordon DM, Stec DF, Donald MC, Stec DE. Bilirubin nanoparticles reduce diet-induced hepatic steatosis, improve fat utilization, and increase plasma beta-hydroxybutyrate. Front Pharmacol. 2020;11:594574. doi:10.3389/fphar.2020.594574
- Dore S, Takahashi M, Ferris CD, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96(5):2445–2450. doi:10.1073/pnas.96.5.2445
- Ahmad N, Ansari MY, Haqqi TM. Role of iNOS in osteoarthritis: pathological and therapeutic aspects. J Cell Physiol. 2020;235(10):6366–6376. doi:10.1002/jcp.29607
- Zhao WW, Wang H, Han Y, Wang HM, Sun YL, Zhang HY. Dopamine/phosphorylcholine copolymer as an efficient joint lubricant and ROS scavenger for the treatment of osteoarthritis. Acs Appl Mater Inter. 2020;12(46):51236–51248. doi:10.1021/acsami.0c14805
- Zhang Y, Wu XW, Hou CX, et al. Dual-responsive dithio-polydopamine coated porous CeO2 nanorods for targeted and synergistic drug delivery. Int J Nanomed. 2018;13:2161–2173. doi:10.2147/Ijn.S152002
- Ajdary M, Moosavi MA, Rahmati M, et al. Health concerns of various nanoparticles: a review of their in vitro and in vivo toxicity. Nanomaterials. 2018;8(9). doi:10.3390/nano8090634
- Garcia-Santana MA, Duconge J, Sarmiento ME, et al. Biodistribution of liposome-entrapped human gamma-globulin. Biopharm Drug Dispos. 2006;27(6):275–283. doi:10.1002/bdd.511
- Jonassen H, Kjoniksen AL, Hiorth M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules. 2012;13(11):3747–3756. doi:10.1021/bm301207a
- Ren X, Liu H, Wu X, Weng W, Wang X, Su J. Reactive Oxygen Species (ROS)-responsive biomaterials for the treatment of bone-related diseases. Front Bioeng Biotechnol. 2021;9:820468. doi:10.3389/fbioe.2021.820468
- Agas D, Laus F, Lacava G, et al. Thermosensitive hybrid hyaluronan/p(HPMAm-lac)-PEG hydrogels enhance cartilage regeneration in a mouse model of osteoarthritis. J Cell Physiol. 2019;234(11):20013–20027. doi:10.1002/jcp.28598
- Chen X, Liu Y, Wen Y, et al. A photothermal-triggered nitric oxide nanogenerator combined with siRNA for precise therapy of osteoarthritis by suppressing macrophage inflammation. Nanoscale. 2019;11(14):6693–6709. doi:10.1039/c8nr10013f
- Merle P, Blanc JF, Phelip JM, et al. Doxorubicin-loaded nanoparticles for patients with advanced hepatocellular carcinoma after sorafenib treatment failure (RELIVE): a phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4(6):454–465. doi:10.1016/S2468-1253(19)30040-8
- Pinkaew D, Kiattisin K, Wonglangka K, Awoot P. Phonophoresis of Phyllanthus amarus nanoparticle gel improves functional capacity in individuals with knee osteoarthritis: a randomized controlled trial. J Bodyw Mov Ther. 2020;24(1):15–18. doi:10.1016/j.jbmt.2019.04.013