Abstract
Abstract
Procalcitonin is an inflammatory marker that had shown marked potential as an antimicrobial stewardship tool for administering antibiotics when needed in patients with pneumonia as it raises concurrently with other inflammatory markers, yet no systematic review has assessed its potential in COVID-19 patients. This systematic review aimed to assess the potential appropriateness of procalcitonin as an antimicrobial stewardship tool in COVID-19 patients with superimposed bacterial and non-bacterial infections. All study designs published after 2019 were included in this systematic review. We included all studies that had reported procalcitonin levels in COVID-19 patients with suspected superimposed secondary infection(s). We searched MEDLINE, Scopus, and the Directory for Open Access Journal from April 2022 to May 2022 and retrieved all related articles for screening with no restrictions on language. We conducted risk of bias assessment according to the Critical Appraisal Skills Programme (CASP) criteria for cohort and case–control studies. Results were presented according to procalcitonin cut-off values, gold standard test used to confirm infection, and overall study conclusion(s), among other variables. This systematic review included 18 articles with 7196 patients in 8 countries. Despite different cut-off values of procalcitonin used, thirteen studies had indicated the appropriateness of using procalcitonin as antimicrobial stewardship tool in COVID-19 patients. We urge physicians to take this into account when treating COVID-19 patients suspected of superimposed infections and we look forward to further studies with standardized procalcitonin cut-off values that may provide appropriate quantitative data that can contribute to clinical guidelines.
Registration (PROSPERO)
CRD42022315013.
Introduction
COVID-19 SARS-CoV-2, also known as coronavirus disease 2019, is an upper respiratory tract infection that has caused a global pandemic. According to the World Health Organization (WHO) COVID-19 Dashboard, as of April 19th 2022, there have been over 500 million confirmed cases of COVID-19 including over 6 million deaths worldwide. The disease presents with a multitude of symptoms, mainly respiratory, but can also present with fatal complications, such as acute respiratory distress syndrome (ARDS) and respiratory failure. Patients with COVID-19 can also present with superimposed infections. Superimposed bacterial infections are prevalent in approximately 10% of hospitalized COVID-19 patients.Citation1 This low rate, while optimal, makes it challenging for healthcare providers when trying to suspect the underlying insulting organism to decide the treatment regimen. In the first year of the pandemic; due to lack of proper evidence-based management, there was a widespread abuse of antibiotics in the treatment of COVID-19 patients irrespective of whether a bacterial coinfection was confirmed or not. This unnecessary administration of antimicrobial agents could further drive antibiotic resistance and increase the likelihood for medication side effects to manifest.Citation2 Thus, the need for a minimally invasive biomarker which indicates the presence of a superimposed bacterial infection in COVID-19 patients arises.
Procalcitonin is an amino acid glycoprotein produced by neuroendocrine thyroid parafollicular C cells. In an average healthy individual, procalcitonin is below plasma levels of 0.1 ng/mL. During inflammation, procalcitonin increases proportionately to the severity of infection in response to pro-inflammatory cytokines such as tumor necrosis factor (TNF)-alpha, interleukin-1, interleukin-6, and endotoxins which are associated with bacterial infections; and it is inhibited by interferon (IFN)-gamma which is associated with viral infections.Citation1–4 These properties favored the utilization of procalcitonin as a tool to distinguish between bacterial and nonbacterial superimposed infections in COVID-19 patients.
There are studies which reported that COVID-19 patients with procalcitonin higher than normal cutoff values had significantly more frequent superimposed infections, they were more likely to develop bacteremia, their ICU stay was more likely to be longer, and their risk of mortality was higher compared to COVID-19 patients with lower procalcitonin levels.Citation1–5
One of the arguments against using procalcitonin as a biomarker for bacterial co-infection in COVID-19 patients is that despite its great sensitivity, it is less specific for bacterial infections as it can also be elevated in other syndromes such as acute renal failure. For that reason, it has been proposed to use procalcitonin in combination with other inflammatory markers namely C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR) for a more accurate diagnosis. CRP, in particular, was found significantly higher in COVID-19 patients with bacterial superimposed infection, COVID-19 pneumonia, bacteremia, as well as in patients who died.Citation4,Citation6–8
In this paper, we reviewed observational studies that had assessed the usefulness of procalcitonin levels in COVID-19 patients with superimposed infections to distinguish between bacterial or non-bacterial causes. If proven effective, this could help in antimicrobial drug choice and early intervention, which could improve the clinical outcomes in COVID-19 patients.
Methods
Registration
This study was registered on PROSPERO under the registration ID CRD42022315013. We reported findings in this systematic review according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
Eligibility Criteria
All observational studies as well as randomized (or quasi-experimental) clinical trials that had documented procalcitonin levels in confirmed COVID-19 patients with suspected superimposed bacterial and/or non-bacterial infections, defined as
a secondary infection that occurs during an existing infection, or immediately following a previous infection, especially when caused by microorganisms that are resistant or have become resistant to the antibiotics used earlier,Citation9
were deemed eligible for inclusion in this systematic review. All articles published prior to 2019 were excluded to avoid confusion with earlier pandemics caused by other strains of coronaviruses, such as the Middle East Respiratory Syndrome (MERS).
Study Identification
We searched MEDLINE, Scopus, and the Directory of Open Access Journals for all related articles using the MeSH terms (or keywords when applicable) “procalcitonin” AND “COVID-19” OR “coronavirus” OR “corona virus” OR “acute respiratory distress syndrome” OR “severe acute respiratory syndrome” OR “SARS.” Articles were screened by title and abstract by two independent reviewers and discrepancies were resolved by consensus and a third independent reviewer. Two independent reviewers then screened the full-text of the screened articles to confirm eligibility and discrepancies were resolved by consensus and a third independent reviewer.
Data Extraction
Two independent reviewers extracted information related to gold standard tests utilized to confirm diagnosis, procalcitonin cut-off levels, and overall conclusion, amongst other variables.
Evaluation of Study Quality
Studies included in this systematic review were assessed using the Critical Appraisal Skills Programme (CASP) criteria for cohortCitation10 and case–controlCitation11 studies by sex independent reviewers. The CASP criteria mainly focuses on three domains in its assessment, including the study design, research methodology, and study results. As recommended by the authors of the CASP criteria, no points are allocated to studies assessed using the criteria.
Results
Study Selection
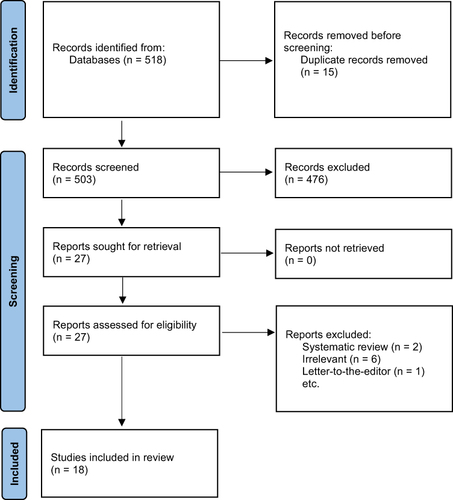
An extensive search yielded a total of 518 studies. Fifteen duplicates were identified and two of the authors independently screened the titles and abstracts of all of the remaining 503 studies using the specified inclusion and exclusion criteria to assess for eligibility. Of these, 476 studies were excluded, and 27 studies were included with inter-author conflict being resolved by a third author. The two reviewers then independently assessed the 27 studies with discrepancies resolved by consensus and a third independent reviewer. Overall, 18 studies were later deemed eligible for inclusion in this systematic review ().
Studies’ Characteristics
In this review, there were a total of 7196 patients included from all 18 studies. The included studies were from 8 different countries, and most of the studies were conducted in the United Kingdom (n=6, 33.3%). There was a total of four study types, of which 11 (61%) were retrospective observational, 4 (22.2%) were retrospective cohort, 2 (11.11%) were prospective cohort, and 1 (5.5%) was a descriptive cross-sectional study. Furthermore, procalcitonin cut-off levels varied between studies; however, the two most common cut-off values were 0.5/0.55 ng/mL (n=10, 55.5%) and 0.2/0.25 ng/mL (n=6, 33.33%). In order to diagnose bacterial co-infections, the different studies used various methods for detection. Out of the 18 studies, 9 (50%) used blood and sputum cultures as the gold standard for bacterial detection. Further details are found in and .
Table 1 Characteristics of the Included Studies
Table 2 Summary of Statistics
After an in-depth review of all the articles, 10 of the 18 studies concluded that increased procalcitonin levels is indeed an indicator of bacterial co-infection in COVID-19 patients. Additionally, 6 articles reported that procalcitonin can be used as a tool for antimicrobial stewardship to aid in the early discontinuation of antibiotics when they are no longer necessary. Further, 2 articles stated that decreased procalcitonin levels can definitely rule-out bacterial co-infection in COVID-19 patients; however, high procalcitonin levels is not diagnostic of a super-added bacterial infection. On the contrary, there were 4 studies that found no significant association between procalcitonin levels and bacterial co-infection. Interestingly, 6 of the studies deduced that elevated procalcitonin levels is a strong predictor of mortality (, Supplementary Table 1).
Table 3 Studies’ Overall Conclusion(s)
Heterogeneity and Bias
Regarding possible causes of heterogeneity, this systematic review included articles with 4 different study designs. Additionally, the variation in the cut-off values of procalcitonin may also contribute to the variability of the included articles. Some of the studies in this review had missing data which might increase the risk of bias; examples of missing data included procalcitonin cut-off value and gold standard tests for the diagnosis of bacterial co-infection. The CASP criteria was used to assess for the quality of the included articles ( and ).
Table 4 Risk of Bias, Cohort Studies
Table 5 Risk of Bias, Case–Control Studies
Discussion
Procalcitonin (PCT) is a biomarker used by clinicians as a tool to determine the etiology of lower respiratory tract infections, with serum levels reflecting the severity of the infection. PCT is also used to guide the administration and duration of antibiotic therapy Citation22 In this systematic review, more than half the studies reported PCT serum values to be a marker for concurrent bacterial infections in patients affected with COVID-19.
To differentiate between bacterial inflammation and non-infective inflammation, different inflammatory biomarkers have been used, including PCT and C-reactive protein (CRP). Nevertheless, it was shown that PCT is more specific and sensitive than CRP in diagnosing bacterial infection.Citation23,Citation24 It was reported that serum interferon-gamma synthesis is amplified during respiratory viral infections, and it actively inhibits PCT secretion.Citation23 Hence, PCT is used to distinguish bacterial from viral pneumonia and is found to be a more accurate diagnostic tool than CRP, IL-6, and leukocyte count.Citation23,Citation24 Furthermore, it’s anticipated that PCT levels remain normal during uncomplicated COVID-19 infection whereas enormous elevation is indicative of bacterial co-infection in severely COVID-19 infected patients.Citation26 The majority of studies in our systematic review confirm that PCT is an essential indicator of bacterial co-infection in COVID-19 patients. However, four studies in our systematic review denied any association between procalcitonin level and bacterial co-infection.
The current study findings reported that PCT-guided antibiotic therapy aids in the early discontinuation of antibiotics when they are no longer necessary. PCT has been widely recruited to guide antibiotic management in a process known as PCT-guided antibiotic stewardship.Citation25,Citation27–29 A recent meta-analysis demonstrated that PCT-guided antibiotic stewardship offered to critically ill patients who suffer from bacterial infection will create greater modulating effects in increasing survival and reducing antibiotic treatment duration. Furthermore, PCT-guided antibiotic stewardship in acute respiratory tract infections significantly lowers the risk of mortality and reduces the consumption and side effects of antibiotics.Citation27 Another study confirmed that PCT-guided antibiotic stewardship reduced the exposure to antibiotic therapy in COVID-19 patients.Citation16
Several studies considered PCT levels to be a predictor of mortality in COVID-19 patients with bacterial co-infection. In a study by Hughes et al, patients with lower levels of PCT had significantly lower mortality rates, length of hospital stay, and intensive care unit (ICU) admission.Citation15 Furthermore, another study reported that hospitalized patients and patients deceased by the 28th admission day had higher PCT levels compared to those discharged from the hospital.Citation2
Meanwhile, a study by Richards et al reported that among patients who passed away in the ICU, over 85% had raised PCT levels. However, there was no significant association between PCT elevation and subsequent mortality in a logistic regression analysis.Citation3 Similarly, although the length of stay was longer in patients with higher PCT levels, the effect of PCT rise was also not statistically significant.
In line with the other studies, severe COVID-19 patients had increased PCT rates upon admission to ICU and were linked with a higher risk of mortality. Interestingly, those findings were comparable to results obtained by acute physiology and chronic health evaluation II which does not require additional costs as PCT measurements.Citation6 Garrido et al have also observed elevated blood PCT values in both deceased patients and COVID-19 patients admitted to the ICU compared to those in the general ward at the hospital.Citation20 These findings showcase the value of using initial PCT serum levels as an indicator of mortality.
Although a study by Heer et al did not support the claim that PCT concentrations are a predictor of bacterial co-infection in COVID-19 patients, it had still demonstrated that raised serum values are associated with respiratory failure with prolonged mechanical ventilation and a greater risk of inpatient death.Citation17 It is crucial to note however that the remaining studies did not assess mortality rates based on PCT levels.
There are several limitations to this review. To begin with, despite using the Critical Appraisal Skills Programme (CASP) risk assessment tool, it is not possible to eliminate publication bias in the studies we have included. The use of different modalities to identify bacterial co-infection among the mentioned studies makes it challenging to generalize the results. Furthermore, there was also a variety of PCT cut-off values used to define bacterial co-infection, raising the possibility of missing PCT elevations during earlier phases of infection and making it difficult to combine results for meta-analysis. Surprisingly, 7 studies (38.8%) did not use any culture do diagnose bacterial co-infection, and they resorted to white blood cell count, inflammatory markers, and clinical diagnostic methods. Despite these limitations, we believe that this study’s results are nonetheless valid for the purpose of answering our research questions and could inspire future research on the diagnostic ability of PCT and its role in antibiotics stewardship for COVID-19 patients.
Conclusion
Out of 18 studies included in this systematic review, 13 (72.2%) had indicated the potential use of procalcitonin in ruling out superimposed bacterial infection(s) and/or its potential use as an antimicrobial stewardship tool. We urge physicians to take this into account when treating COVID-19 patients suspected of superimposed infections and we look forward to further studies with standardized procalcitonin cut-off values that may provide appropriate quantitative data that can contribute to clinical guidelines and improve clinical outcomes in COVID-19 patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- Vanhomwegen C, Veliziotis I, Malinverni S, et al. Procalcitonin accurately predicts mortality but not bacterial infection in COVID-19 patients admitted to intensive care unit. Ir J Med Sci. 2021;190:1649–1652. doi:10.1007/s11845-020-02485-z
- Atallah NJ, Warren HM, Roberts MB, et al. Baseline procalcitonin as a predictor of bacterial infection and clinical outcomes in COVID-19: a case-control study. PLoS One. 2022;17(1):e0262342. doi:10.1371/journal.pone.0262342
- Richards O, Pallmann P, King C, et al. Procalcitonin increase is associated with the development of critical care-acquired infections in COVID-19 ARDS. Antibiotics. 2021;10:1425. doi:10.3390/antibiotics10111425
- May M, Chang M, Dietz D, et al. Limited utility of procalcitonin in identifying community-associated bacterial infections in patients presenting with coronavirus disease 2019. Antimicrob Agents Chemother. 2021;65(4):e02167–20. doi:10.1128/AAC.02167-20
- Moreno-García E, Puerta-Alcalde P, Letona L, et al. Bacterial co-infection at hospital admission in patients with COVID-19. Int J Infect Dis. 2022;118:197–202. doi:10.1016/j.ijid.2022.03.003
- He S, Liu W, Jiang M, et al. Clinical characteristics of COVID-19 patients with clinically diagnosed bacterial co-infection: a multi-center study. PLoS One. 2021;16(4):e0249668. doi:10.1371/journal.pone.0249668
- Ming K, Myall AC, Hernandez B, et al. Informing antimicrobial management in the context of COVID-19: understanding the longitudinal dynamics of C-reactive protein and procalcitonin. BMC Infect Dis. 2021;21:932. doi:10.1186/s12879-021-06621-7
- Pink I, Raupach D, Fuge J, et al. C -reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection. 2021;49:935–943. doi:10.1007/s15010-021-01615-8
- National Center for Biotechnology Information.. Superimposed infection (concept ID: C0038826) - MedGen. U.S. National Library of Medicine; [cited August 21, 2022]. Available from: https://www.ncbi.nlm.nih.gov/medgen/11659#top. Accessed October 26, 2022.
- Critical Appraisal Skills Programme. CASP Cohort Study Checklist; 2018. Available from: https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf. Accessed October 26, 2022.
- Critical Appraisal Skills Programme. CASP Case-Control Study Checklist; 2018. Available from: https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Case-Control-Study-Checklist-2018_fillable_form.pdf. Accessed October 26, 2022.
- Basnet A, Chand AB, Shrestha LB, et al. Co-infection of uropathogenic Escherichia coli among COVID-19 patients admitted to a tertiary care centre: a descriptive cross-sectional study. J Nepal Med Assoc. 2022;60(247). doi: 10.31729/jnma.7376
- Cheng K, He M, Shu Q, Wu M, Chen C, Xue Y. Analysis of the risk factors for nosocomial bacterial infection in patients with COVID-19 in a tertiary hospital. Risk Manag Healthc Policy. 2020;13:2593. doi:10.2147/RMHP.S277963
- Waris A, Din M, Iqbal N, et al. Evaluation of serum procalcitonin level as a biomarker for disease severity in COVID-19 patients. New Microbes New Infect. 2021;43:100922. doi:10.1016/j.nmni.2021.100922
- Hughes S, Mughal N, Moore LS. Procalcitonin to guide antibacterial prescribing in patients hospitalised with COVID-19. Antibiotics. 2021;10(9):1119. doi:10.3390/antibiotics10091119
- Peters C, Williams K, Un EA, et al. Use of procalcitonin for antibiotic stewardship in patients with COVID-19: a quality improvement project in a district general hospital. Clin Med. 2021;21(1):e71. doi:10.7861/clinmed.2020-0614
- Heer RS, Mandal AK, Kho J, et al. Elevated procalcitonin concentrations in severe Covid-19 may not reflect bacterial co-infection. Ann Clin Biochem. 2021;58(5):520–527. doi:10.1177/00045632211022380
- Roy A, Powers HR, Craver EC, Nazareno MD, Yarrarapu SN, Sanghavi DK. Antibiotic stewardship: early discontinuation of antibiotics based on procalcitonin level in COVID‐19 pneumonia. J Clin Pharm Ther. 2022;47(2):243–247. doi:10.1111/jcpt.13554
- Tang ML, Li YQ, Chen X, et al. Co-infection with common respiratory pathogens and SARS-CoV-2 in patients with COVID-19 pneumonia and laboratory biochemistry findings: a retrospective cross-sectional study of 78 patients from a single center in China. Med Sci Monit. 2021;27:e929783–1. doi:10.12659/MSM.929783
- Garrido P, Cueto P, Rovira C, et al. Clinical value of procalcitonin in critically ill patients infected by SARS-CoV-2. Am J Emerg Med. 2021;46:525–531. doi:10.1016/j.ajem.2020.11.011
- Heesom L, Rehnberg L, Nasim-Mohi M, et al. Procalcitonin as an antibiotic stewardship tool in COVID-19 patients in the intensive care unit. J Glob Antimicrob Resist. 2020;22:782. doi:10.1016/j.jgar.2020.07.017
- Kamat IS, Ramachandran V, Eswaran H, Guffey D, Musher DM. Procalcitonin to distinguish viral from bacterial pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2020;70(3):538–542. doi:10.1093/cid/ciz545
- Melendi GA, Laham FR, Monsalvo A, et al. Cytokine profiles in the respiratory tract during primary infection with human metapneumovirus, respiratory syncytial virus, or influenza virus in infants. Pediatrics. 2007;120(2):e410–5. doi:10.1542/peds.2006-3283
- Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39(2):206–217. doi:10.1086/421997
- Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med. 2014;190(10):1102–1110. doi:10.1164/rccm.201408-1483OC
- Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190. doi:10.1016/j.cca.2020.03.004
- Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Evid Based Child Health. 2013;8(4):1297–1371. doi:10.1002/ebch.1927
- Annane D, Maxime V, Faller JP, et al. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ open. 2013;3(2):e002186. doi:10.1136/bmjopen-2012-002186
- Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505. doi:10.1164/rccm.200708-1238OC