Abstract
Objective
To determine the role of nerve injury-induced protein 1 (NINJ1) introduced plasma membrane rupture (PMR) and damage-associated molecular patterns (DAMPs) release in the pathogenesis and progression of gout and to explore the potential of NINJ1 as a therapeutic target in gout.
Methods
Both peripheral blood mononuclear cells (PBMCs) and serum sample from gout patients (n = 58) and healthy controls (n = 16) were collected and processed to NINJ1 expression, lactate dehydrogenase (LDH) detection, NINJ1 inhibition, and NINJ1 expression experiments, respectively. NINJ1 knockdown was carried out by lentivirus in a monosodium urate (MSU) induced rat model, and NINJ1 neutralizing antibody was applied in a MSU induced mouse model.
Results
Our results found that NINJ1 was upregulated during a gout flare, and the resulting induction of PMR correlated with gout progression. NINJ1 knockdown significantly reduced the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation and joint swelling in the rat model, and NINJ1 neutralizing antibody also significantly reduced gout flare in the mouse model and PBMCs. Moreover, NINJ1 expression is under NLRP3 inflammasome produced interleukin (IL)-1β control.
Conclusion
These results support the notion of a pathogenic role of NINJ1 introduced PMR in gout and provide a detailed mechanism for gout pathogenesis involving inflammatory cell death and DAMPs release introduced by IL-1β. In addition, targeting NINJ1 might be a potential therapeutic approach for gout.
Introduction
Gout is an inflammatory disease resulting from MSU crystal deposition in joints and other tissues. Patients with gout always suffer from recurrent joints swelling and a great deal of pain.Citation1 According to the recurrence of a gout flare, gout is subdivided into acute phase and chronic phase, acute gout possesses less recurrence and always self-remission within 7–10 days, chronic gout possesses higher recurrence and lost self-remission.Citation2 Both genetic and environmental factors affect urate metabolism and can lead to hyperuricemia. This physiological change can result in MSU crystal formation, and activate the NLRP3 inflammasome inside of inflammatory cells synergistically with other stimuli.Citation3–7 Activated NLRP3 inflammasomes result in caspase-1 activation, which then cleave pro-IL-1β and Gasdermin-D (GSDMD), after which the cleaved GSDMD amino-terminal can insert into the plasma membrane and oligomerize to form a pore, allowing the release of small molecules, such as mature IL-1β to initiate inflammation.Citation8 IL-1β, in co-ordination with other cytokines such as tumor necrosis factor (TNF)-α and IL-6, promotes the recruitment of neutrophils at the inflamed joint, after which neutrophils were activated by MSU crystals further amplifying the inflammatory process.Citation9 Once GSDMD was cleaved, inflammatory cells either experience pyroptosis or survive and became super activated with inflammatory cytokines consistently secreted.Citation10 Pyroptosis occurs with PMR and the release of large molecules including abundant DAMPs, which further activate the NLRP3 inflammasome and other inflammatory pathways promoting inflammation.Citation11 In addition, the NLRP3 inflammasome can also trigger other types of cell death including necrosis,Citation12 MSU could also induce necrosis in a mixed lineage kinase domain-like protein (MLKL) dependent manner.Citation13,Citation14 Putatively, these inflammatory cell death results in vast PMR and DAMP release, leading to the hyperinflammation responsible for extremely painful gout flare. PMR plays a central role in this inflammation amplification.
NINJ1 was first identified as an adhesion molecule mainly expressed in neurons and Schwann cells and upregulated after nerve injury to induce neurite outgrowth.Citation15 Later studies found that it was also expressed in other cell types and involved in many diseases including cancer and autoimmune diseases.Citation16–21 NINJ1 was expressed in myeloid cells and mediates endothelial adhesion in the brains of experimental autoimmune encephalomyelitis (EAE) rats.Citation21 NINJ1 was upregulated in macrophage to increase cell–cell and cell–matrix adhesion of macrophages in hyaloid vascular system.Citation20 NINJ1 plays a key role in the transmigration of inflammatory antigen-presenting cells across the blood–brain barrier.Citation22 NINJ1 expression results in macrophage activation in intestinal inflammatory conditions.Citation23 Blocked of NINJ1 protected diabetic endothelial cells from high-glucose induced apoptosis in diabetes mellitus.Citation24 Besides, NINJ1 asp110ala single nucleotide polymorphism is associated with protection in leprosy nerve damage.Citation19 The role of NINJ1 in inflammation has long owe to its function in inflammatory cell adhesion and migration.Citation25,Citation26 Until recently, it was reported that NINJ1 is a key regulator of PMR during pyroptosis and other types of cell death, and knockout NINJ1 could prevent PMR and macromolecule release.Citation27 PMR has long been considered a passive process following plasma membrane permeability change, it is now identified as an automatic process tightly controlled by NINJ1. Specifically, the N-terminal extracellular α-helical of NINJ1 responsible for NINJ1 oligomerizes to form a huge pore on the cell membrane to induce PMR, while factors that induce NINJ1 oligomerization still need further study.Citation27 At present, clinical treatments for gout are focused on colchicine, glucocorticoids and nonsteroidal anti-inflammatory drugs (NSAIDs),Citation28,Citation29 which all have side effects and even yield poor outcomes in serious cases.Citation30 Hence, there is an urgent need for new targets and drugs. A previous study showed that genetic deletion of GSDMD and MLKL did not prevent MSU crystal induced cell death and inflammation,Citation31 which suggest target GSDMD and MLKL could not inhibit cell death in gout. Whether target NINJ1 could inhibit MSU induced cell death and gout inflammation still need further study.
To answer this question, we first tested the expression and activation of NINJ1 in different gout groups. Second, we investigated whether NINJ1 induced PMR and DAMPs release mediate gout inflammation by using NINJ1 knockdown lentivirus and NINJ1 block antibody. Finally, we found that in gout patients, IL-1β induces the expression of NINJ1 and initiates inflammation. We also identified a critical role of NINJ1 mediated inflammatory cell death in the pathogenesis and progression of gout.
Materials and Methods
Participants
The study compiled with the declaration of the Helsinki and was approved by the Ethics Committee of The First Affiliated Hospital of University of Science and Technology of China. All subjects gave written informed consent. Gout patients, meeting ACR/EULAR criteria,Citation32 and healthy controls were recruited from the Department of Rheumatology and Immunology at The First Affiliated Hospital of University of Science and Technology of China between January 2018 and April 2021. Gout patients (n = 58) were recruited first and subdivided into three groups according to the recurrence and joints flare, followed by individually matching 16 healthy controls, without past gout. The characteristics of gout patients (n = 58) and health controls (n = 16) can be found in Supplementary Materials Table 1. Acute gout group: gouty patients with gout flare and the recurrence is less than six times within half a year and self-remission. Chronic gout group: gouty patients with gout flare and the recurrence is more than six times within half a year and without self-remission. Gout in diapause: gouty patients without gout flare.Citation33
Animals
All animal experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of University of Science and Technology of China and conducted in conformity with the Guiding Principles for Research Involving Animals and Human Beings. Male C57/BL6 mice weighing between 25 and 30 g and Male Sprague-Dawley rats weighing between 150 and 200 g were purchased from Hangzhou Ziyuan Experimental Animal Technology Co., Ltd. All animals were kept in pathogen-free animal facilities and were housed six per cage under standard conditions (12 h/12 h light/dark cycle and [22 ± 2] ℃ with a relative humidity of [55% ± 5%]) with free access to feed and water throughout the experiment. All animals used in the experiment were at 8 to 12 weeks of age.
Peripheral Blood Mononuclear Cell Culture
Human PBMCs were freshly isolated from Natrium Citrate-treated blood, and were then isolated by Ficoll-Hypaque (TBD science, cat#LDS1075) density-gradient centrifugation. Cells were cultivated in RPMI 1640 medium (Hyclone, cat# SH30809101) supplemented with 2 mM L-glutamine, penicillin/streptomycin (Solarbio, cat# P1400), and 10% fetal bovine serum (FBS, Hyclone, cat# SH30809.01) in Teflon bags and allowed to rest for 24 h prior to stimulation. All incubations were performed at 37℃ in humidified air with 5% CO2. PBMCs were stimulated with MSU (100ng/mL) and TLR1/2 stimulator Pam3Cys (10 μg/mL) with or without MCC950 (Topscience, cat#T3701, 5 μM) for 6 h, supernatant and cells were collected for ELISA and RT-PCR. PBMC were cultured with or without recombinant human IL-1β (Novoprotein, cat# 0331537, 5 ng/mL), cells were collected at different time points for RT-PCR.
MSU Crystals-Induced Gout Arthritis
MSU crystals were prepared under pyrogen-free conditions by dissolving 10 mg/mL uric acid (Sigma-U2875-5G) in 0.01 M NaOH (pH 7.1) solution. Filter the supersaturated uric acid solution (0.45 μm) and keep it at room temperature for 48 h. The crystals were washed with 100% ethanol and sonicated to reduce their size. The amount of endotoxin present in the injected MSU crystals was <5 pg, which determined by chromogenic Limulus amebocyte lysate assay (Endosafe).
Mice were put under anesthesia, 100 μg MSU crystals (2 mg/mL) were injected into the tibiofemoral knee joints, and the inflammatory parameters were evaluated at different time points (0, 2, 4, 6 and 8 h) after the injection of the MSU crystals by collecting blood samples. Mice were then put to death, both blood samples and joints tissues were collected for further experiments.
Rats were put under anesthesia. Choose the right posterior ankle joint in each group as the puncture point, and flex the ankle joint 90 degrees as much as possible. Inject the MSU solution (100 μL 10 mg/mL MSU) from the inside of the tendon 30–40 degrees downwards into the joint cavity. The above process strictly follows the aseptic operation, with the bulging of the joint capsule as the injection standard. Rats were then put to death at 24 hours after injection, both blood samples and joints tissues were collected for further experiments.
Histopathological Observation of Synovial Tissue of Ankle Joint
At the end of the experiment, the ankle joints were fixed in formalin and embedded in paraffin, samples were then cut into 4μm sections and deparaffinized with xylene, washed with running water for 20 min, stained with hematoxylin for 30 min, washed with running water for 20 min, differentiated with hydrochloric acid and alcohol, stained with eosin for 5 min, finally dehydrated with gradient alcohol, and sealed with resin glue after transparent xylene. Histopathological pictures were then captured in electron microscopy imaging system.
Cytokine and LDH Assay
The concentration of IL-1β was quantified by Human IL-1β kit (RD, cat# DLB50), mouse IL-1β kit (RD, cat# MLB00C), and rat IL-1β kit (RD, cat# RLB00), respectively. All samples were added in triplicate, the protein concentration was calculated from the standard curve. The serum and cultured cells LDH was determined by Lactate Dehydrogenase (LDH) Assay kit (Solarbio, cat# BC0685). All operations are performed in strict accordance with the operating instructions.
Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from whole blood using a whole Blood RNA isolation kit (Simgen), total RNA was isolated from cultured cells using Hipure total RNA mini kit (Magen). Isolated total RNA was reverse transcribed with the BioRT Master Hisensi cDNA strand synthesis kit (BioFlux, cat# BSB40M1). ABI Prism 7500 Sequence Detection System (Applied Biosystems) was used for RT-PCR. SYBR Green PCR Master Mix (Muma, cat# A4004M) was used to perform PCR of rat ACTB and NINJ1, IL-1β, TNF-α, IL-6, IL-18, NLRP3, caspase-1, and ASC. The relative level of gene expression is determined by the comparative threshold cycle method described by the manufacturer, in which the data for each sample is normalized to ACTB constituent genes and expressed as a fold change compared to the control. PCR primers can be found in Supplementary Materials Table 2.
Western Blot
Western blot was processed with a general protocol from Abcam (https://www.abcam.com/protocols/general-western-blot-protocol). Membranes were incubated overnight at 4℃ with the following antibodies: anti–IL-1β antibody (Abcam, cat# ab9722, 1/1000 dilution), anti–NLRP3 antibody (Cell Signaling Technology, cat# D4D8T, 1/1000 dilution), anti–β-actin antibody (Cell Signaling Technology, cat# D6A8, 1/1000 dilution), anti–Ninjurin antibody (BD Biosciences, cat# 610777, 1/500 dilution).
Statistical Analysis
All results are expressed as the mean ± standard error of the mean. GraphPad Prism version 7.04 software was used to perform a one-way analysis of variance on the data obtained in the in vivo experiment, and then Dunnett’s multiple comparison test was performed. P< 0.05 was used as a statistically significant threshold.
Results
Elevated NINJ1 Expression and PMR in Gout Patients
In order to have a comprehensive understanding of NINJ1 function in gout, we divided gout patients into chronic, diapause, and acute groups. We first assessed the level of NINJ1 protein in different patient groups. Western Blot results showed that gout patients possess higher levels of NINJ1 protein than that of healthy control (HC), chronic gout (CG) patients possess even higher levels of NINJ1 protein than acute gout (AG) (), suggesting that NINJ1 may affect gout flare. Because NINJ1 induces PMR during inflammatory cell death, we then analyzed LDH levels, which are a key marker for PMR. We found that both AG and CG patients also have higher levels of LDH than HC (), suggesting that gout patients possess higher level PMR and cell death. NINJ1-induced PMR is associated with gout flare. Moreover, gout in diapause (GD) patients possess a higher NINJ1 protein level and LDH production than that of HC (), which could be the reason for gout recurrence.
Figure 1 Elevated NINJ1 expression and PMR in gout patients. (A) Western blot of NINJ1 protein in PBMCs that isolated from different groups. (B) LDH levels in the plasma of different groups. (C) DEGs in MSU stimulated BMDMs. (D) RT-PCR of NINJ1 expression in PBMCs that stimulated with indicated reagents. (E) ELISA test of supernatant IL-1β in PBMCs that stimulated with indicated reagents. (F) Western blot of NINJ1 protein in PBMCs that stimulated with indicated reagents. (G) RT-PCR of NINJ1 expression in IL-1β treated PBMCs. (H) Western blot of NINJ1 protein in IL-1β treated PBMCs. Data are shown as mean± SEM. *** P<0.001, **** P<0.0001.
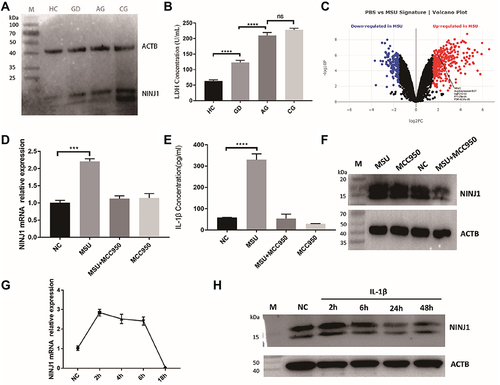
NLRP3 Inflammasome Sourced IL-1β Regulate NINJ1 Expression
We then download raw data (GSE191054) from the Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) database and reanalyzed with limma R package. Through data processing, we finally obtained phosphate-buffered saline (PBS) treated mouse Bone Marrow-Derived Macrophages (BMDMs) samples (3 cases) as the control group and MSU treated BMDMs samples (3 cases) as the treat group, using |log2 fold change (FC)|>2 and adjusted p<0.05 to identify MSU induced differentially expressed gene (MSU-deg). We found that NINJ1 was upregulated in MSU stimulated BMDMs (), suggesting MSU treatment induce NINJ1 expression. We then isolated PBMC from HC and processed it to MSU stimulation with or without sulfonylurea MCC950, a specific NLRP3 inflammasome inhibitor.Citation34 We found that MSU induced NLRP3 inflammasome activation and NINJ1 upregulation (). NLRP3 inflammasome inhibition blocked NINJ1 upregulation (), suggesting that NINJ1 expression is NLRP3 inflammasome dependent. Previous results showed that IL-1β, a key indicator of NLRP3 inflammasome activation, induced NINJ1 expression in human endometriotic stromal cells.Citation35 Our results also showed that NINJ1 is upregulated during IL-1β stimulation in human PBMC (). Altogether, support IL-1β as a regulator of NINJ1 expression, MSU activated NLRP3 inflammasome releasing mature IL-1β to upregulate NINJ1 expression, and this upregulated NINJ1 expression correlated with PMR and gout attack. However, this upregulation is time-dependent, since prolonged incubation of IL-1β inhibits NINJ1 expression (), this prolonged IL-1β incubation-induced NINJ1 downregulation provides a potential mechanism for gout remission.
NINJ1 Knockdown Prevents Gout Flare by Inhibiting NLRP3 Inflammasome Activation in a Rat Model
To understand the function of NINJ1 in gout arthritis, we utilized an MSU crystal-induced gout model by direct injection of MSU crystal suspensions into the rat articular cavity.Citation36 Our previous results showed that rat joint inflammation peaks at 24 h after MSU crystal injection;Citation37 hence, we selected this time point to estimate rat joint inflammatory levels. Our results showed that MSU crystal injection-induced strong inflammation in rat joints compared with the NC group injected with the same volume of saline. Both joint swelling and inflammatory cell infiltration were dramatically increased ().
Figure 2 NINJ1 knockdown inhibits rat gout flare. (A) Images of rat joint swelling and redness, and hematoxylin and eosin staining of rat joint inflammation. Asterisks indicate the infiltrating leukocytes. (B) RT-PCR of NINJ1 expression in rat joints. (C) Western blot of NLRP3, pro-IL-1β, and NINJ1 expression in PBMCs of different groups. (D) Compare of individual inflammatory genes expression between MUS treated WT and NINJ1 knockdown animals in PBMCs. (E) Serum mature IL-1β level quantified by ELISA. ***P<0.001, and ****P<0.0001. Three rats were included in each group, and the experiment was repeated for four times. (M) standard protein ladder.
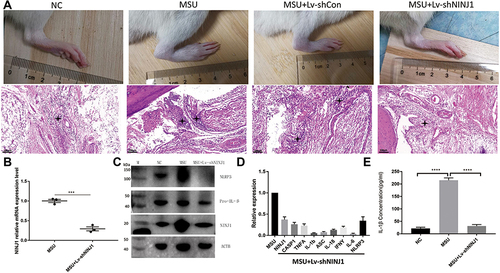
According to the manufacturer’s protocol, lentiviral vectors for NINJ1 knockdown were injected one week prior to disease induction, and RT-PCR and WB confirmed the knockdown efficacy in rat joints and PBMCs, respectively (). Intriguingly, NINJ1 knockdown significantly inhibited rat joint swelling and inflammatory cell infiltration relative to the empty vector control (). Production of inflammatory cytokines such as TNF-α, IFN-γ, and IL-6 was inhibited in NINJ1 knockdown animals (), confirming that NINJ1 knockdown inhibits inflammation. Further work revealed that NINJ1 knockdown inhibited NLRP3 inflammasome activation, and that both NLRP3 and pro-IL-1β protein expression was inhibited (). Moreover, mature IL-1β, a product resulting from NLRP3 inflammasome activity, decreased in NINJ1 knockdown gouty rats (), suggesting that NINJ1 functions as an NLRP3 inflammasome regulator to control gout disease.
NINJ1 Neutralizing Antibody Has Therapeutic Potential in a Murine Gout Model
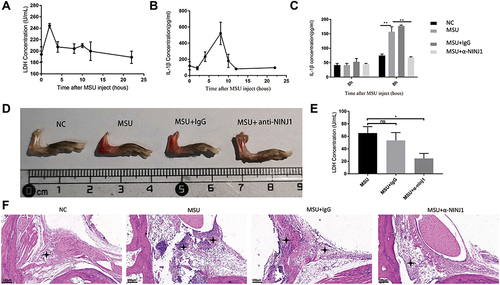
We then wondered whether NINJ1 could be a useful therapeutic target in gout arthritis. To test this, we utilized a commercial neutralizing antibody that targets NINJ1.Citation38 Because this is a mouse antibody, we had to employ a murine model using MSU crystal injection for these experiments. In this case, inflammation and PMR levels peaked 2 h after MSU injection (). The highest IL-1β secretion was observed at 8 hours (), consistent with a pre-inflammatory function of NINJ1 via inducing PMR, responsible for NLRP3 inflammasome over-activation. Therefore, we injected NINJ1 neutralizing antibody before MSU injection and assessed the level of inflammation at 8 h. Mice injected with NINJ1 neutralizing antibody exhibited less joint swelling and redness than controls (). In these mice, joint infiltration by inflammatory cells was also less marked than the IgG control group (). Consistent with this, NINJ1 neutralizing antibody-treated mice had lower IL-1β levels () and LDH increase (). Altogether, these results support the notion that NINJ1 neutralizing antibody inhibits NLRP3 inflammasome activation and the subsequent inflammatory cascade, thus alleviating gout inflammation.
Figure 3 NINJ1 neutralizing antibody prevents murine gout. (A) LDH production after MSU injection into mouse joints at different time points. (B) IL-1beta production after MSU injection into mouse joints at different time points. (C) IL-1β production in different groups. (D) Images of mouse joint swelling and redness in different groups. (E) Mice LDH increase after MSU injection. (F) Histochemical analysis of mouse joint inflammation. Asterisks indicate the infiltrating leukocytes. *P<0.05, **P<0.01. Three mice were included in each group, and the experiment was repeated for four times.
NINJ1 is a Potential Therapeutic Target for Gout Arthritis
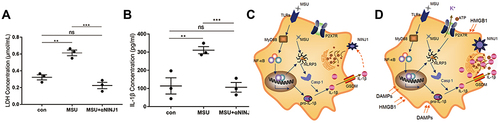
Although targeting NINJ1 inhibited gout flare in both animal models, the potential effects of targeting it in human gout remained unknown. Therefore, we further tested the NINJ1 neutralizing antibody on human samples in vitro. PBMCs from either AG or CG patients were isolated and stimulated with MSU in the presence or absence of NINJ1 neutralizing antibody. Our results showed that NINJ1 neutralizing antibody inhibited LDH production and IL-1β secretion (), thus preventing PMR production and the following inflammation.
Figure 4 NINJ1 is a potential target for human gout arthritis. (A) LDH levels in different groups. (B) Production of IL-1β from PBMC. (C) MSU activate NLRP3 inflammasome inside of macrophage, which results GSDMD activation and IL-1β secretion. (D) Production of IL-1β upregulate NINJ1 expression on macrophage, NINJ1 then facilitate pyroptosis and PMR, releasing DAMPs including ATP, DNA, LDH, HMGB1 into surroundings. Released DAMPs in turn synergistic with MSU to activate NLRP3 inflammasome leading hyperinflammation and gout flare. ** P<0.01, *** P<0.001.
Discussion
NINJ1 is a cell-surface adhesion molecule that regulates cell migration and attachment. It is widely expressed and upregulated during inflammatory conditions, such as following lipopolysaccharide (LPS), poly(I:C), or TNF-α stimulation.Citation39 Increased oxidative stress, endoplasmic reticulum (ER) stress (ERS) or NF-κB activation also enhances NINJ1 expression.Citation40 NINJ1 is involved in many inflammatory diseases, including diabetes mellitus,Citation24 nervous system inflammatory lesions,Citation22 intestinal inflammatory conditions,Citation23 and experimental autoimmune encephalomyelitis.Citation21 However, these previously established studies on the roles of NINJ1 in inflammation were mainly focused on NINJ1-mediated leukocyte migration, although NINJ1 can also interact with LPS and regulate Toll-like receptor 4 (TLR4) signaling.Citation39,Citation41 No data from these studies are related to PMR.
MSU-induced NLRP3 inflammasome activation plays a central role in gout arthritis, but MSU alone is insufficient to trigger gout in humans because only a small fraction of hyperuricemia patients develop overt clinical gout. Our results showed that NINJ1 was only upregulated during the acute inflammatory phase in gouty patients. Both AG and CG, MSU induce NINJ1 upregulation in an NLRP3 inflammasome-dependent manner, suggesting that NINJ1 plays a key role in gout flare. NINJ1-induced PMR promotes NLRP3 inflammasome activation and inflammation amplification, as both NINJ1 knockdown and NINJ1 neutralizing antibody inhibited NLRP3 inflammasome activation and gout flare, supporting the notion that during gout development, elements that induce cell death are also pathological factors for gout, and that cell death is the key step for inflammatory disease. Consistent with this view, our previous results revealed that ATP, a product of cell death, facilitates gout flare through the P2X7 receptor.Citation4,Citation5 Further work may reveal other key factors involved in cell death and synergistic with MSU to induce human gout.
NLRP3 inflammasome is a multicomponent cellular platform responsible for various PAMPs and DAMPs detection. MSU activated NLRP3 inflammasome releasing mature IL-1β that immediately promote NINJ1 expression, however, this directly release of IL-1β is insufficient to induce gout flare, upregulated NINJ1 then introduce PMR and cell death releasing abundant DAMPs, which in turn activate NLRP3 inflammasome, triggering abundant IL-1β release leading hyper inflammation and gout flare (). Once activated, gout patients maintain a relatively higher NINJ1 expression than healthy control (). Although there is no inflammation, the maintained NINJ1 made a lower threshold for gout happening again and possibly explains the recurrence of gout. As inflammation progresses, prolonged IL-1β then downregulates NINJ1 expression and together with other inhibitory mechanisms to control gout flare, leading to self-remission.
The limitations of this study include the fact that although NINJ1 is mainly expressed in inflammatory cells, such as monocytes and neutrophils, it is also expressed in other cell types, including T cells and B cells.Citation42 NINJ1-induced cell death also regulates the homeostasis of these other cells, and targeting NINJ1 might lead to side effects. Moreover, appropriately-regulated inflammation is essential for adaptive immune responses to clear pathogens and maintain health, so targeting NINJ1 might increase infections. At present, there are mainly three NINJ1 inhibitory strategies, namely, small molecule inhibitors, neutralizing antibodies, and peptide mimics.Citation26 Recently, antibody engineering has progressed dramatically, and artificial antibody constructs have become mainstays in immune therapy. These approaches may allow the generation of bispecific antibodies that target only NINJ1 and inflammatory cells simultaneously to minimize side effects on other cells.
Altogether, these results strongly suggest that NINJ1 crosstalk with NLRP3 inflammasome and plays a key role in gout inflammation. Thus, NINJ1 may be a potential therapeutic target for human gout arthritis. Both inhibitors and humanized antibodies that target NINJ1 can be used in clinical treatment, and the clinical development of these drugs is awaited. We conclude that broadly targeting NINJ1 to inhibit NLRP3 inflammasome over-activation should control gout flare, but might increase infections and have other unknown risks. More exquisite targeting strategies will be needed to reduce these side effects and bring NINJ1 targeting to gout treatment in the clinic in the future. Moreover, NINJ1 may also be a target of choice in other inflammatory diseases characterized by excessive inflammation.
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Hefei Mujing Biotechnology Co., LTD for their support of reagents.
Additional information
Funding
References
- Dalbeth N, Gosling AL, Gaffo A, Abhishek A. Gout. Lancet. 2021;397(10287):1843–1855. doi:10.1016/s0140-6736(21)00569-9
- Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. doi:10.1038/s41572-019-0115-y
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi:10.1038/nature04516
- Tao JH, Cheng M, Tang JP, et al. Single nucleotide polymorphisms associated with P2X7R function regulate the onset of gouty arthritis. PLoS One. 2017;12(8):e0181685. doi:10.1371/journal.pone.0181685
- Tao JH, Zhang Y, Li XP. P2X7R: a potential key regulator of acute gouty arthritis. Semin Arthritis Rheum. 2013;43(3):376–380. doi:10.1016/j.semarthrit.2013.04.007
- Demarco B, Danielli S, Fischer FA, Bezbradica JS. How pyroptosis contributes to inflammation and fibroblast-macrophage cross-talk in rheumatoid arthritis. Cells. 2022;11(8):1307. doi:10.3390/cells11081307
- Roškar S, Hafner-Bratkovič I. The role of inflammasomes in osteoarthritis and secondary joint degeneration diseases. Life. 2022;12(5):731. doi:10.3390/life12050731
- He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi:10.1038/cr.2015.139
- Mitroulis I, Kambas K, Ritis K. Neutrophils, IL-1β, and gout: is there a link? Semin Immunopathol. 2013;35(4):501–512. doi:10.1007/s00281-013-0361-0
- Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discov. 2021;20(5):384–405. doi:10.1038/s41573-021-00154-z
- Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol. 2016;26(13):R568–R572. doi:10.1016/j.cub.2016.02.019
- Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127. doi:10.1038/s41423-021-00740-6
- Desai J, Steiger S, Anders HJ. Molecular pathophysiology of gout. Trends Mol Med. 2017;23(8):756–768. doi:10.1016/j.molmed.2017.06.005
- Mulay SR, Desai J, Kumar SV, et al. Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun. 2016;7:10274. doi:10.1038/ncomms10274
- Araki T, Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17(2):353–361. doi:10.1016/s0896-6273(00)80166-x
- Araki T, Zimonjic DB, Popescu NC, Milbrandt J. Mechanism of homophilic binding mediated by ninjurin, a novel widely expressed adhesion molecule. J Biol Chem. 1997;272(34):21373–21380. doi:10.1074/jbc.272.34.21373
- Kim JW, Moon AR, Kim JH, et al. Up-Regulation of ninjurin expression in human hepatocellular carcinoma associated with cirrhosis and chronic viral hepatitis. Mol Cells. 2001;11(2):151–157.
- Toyama T, Sasaki Y, Horimoto M, et al. Ninjurin1 increases p21 expression and induces cellular senescence in human hepatoma cells. J Hepatol. 2004;41(4):637–643. doi:10.1016/j.jhep.2004.06.027
- Cardoso CC, Martinez AN, Guimarães PE, et al. Ninjurin 1 asp110ala single nucleotide polymorphism is associated with protection in leprosy nerve damage. J Neuroimmunol. 2007;190(1–2):131–138. doi:10.1016/j.jneuroim.2007.07.015
- Lee HJ, Ahn BJ, Shin MW, Jeong JW, Kim JH, Kim KW. Ninjurin1 mediates macrophage-induced programmed cell death during early ocular development. Cell Death Differ. 2009;16(10):1395–1407. doi:10.1038/cdd.2009.78
- Ahn BJ, Lee HJ, Shin MW, Choi JH, Jeong JW, Kim KW. Ninjurin1 is expressed in myeloid cells and mediates endothelium adhesion in the brains of EAE rats. Biochem Biophys Res Commun. 2009;387(2):321–325. doi:10.1016/j.bbrc.2009.07.019
- Ifergan I, Kebir H, Terouz S, et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol. 2011;70(5):751–763. doi:10.1002/ana.22519
- Jung HJ, Kang JH, Pak S, Lee K, Seong JK, Oh SH. Detrimental role of nerve injury-induced protein 1 in myeloid cells under intestinal inflammatory conditions. Int J Mol Sci. 2020;21(2). doi:10.3390/ijms21020614
- Wang X, Qin J, Zhang X, et al. Functional blocking of Ninjurin1 as a strategy for protecting endothelial cells in diabetes mellitus. Clin Sci. 2018;132(2):213–229. doi:10.1042/cs20171273
- Lee HJ, Ahn BJ, Shin MW, Choi JH, Kim KW. Ninjurin1: a potential adhesion molecule and its role in inflammation and tissue remodeling. Mol Cells. 2010;29(3):223–227. doi:10.1007/s10059-010-0043-x
- Jeon S, Kim TK, Jeong SJ, et al. Anti-inflammatory actions of soluble ninjurin-1 ameliorate atherosclerosis. Circulation. 2020;142(18):1736–1751. doi:10.1161/circulationaha.120.046907
- Kayagaki N, Kornfeld OS, Lee BL, et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591(7848):131–136. doi:10.1038/s41586-021-03218-7
- McKenzie BJ, Wechalekar MD, Johnston RV, Schlesinger N, Buchbinder R. Colchicine for acute gout. Cochrane Database Syst Rev. 2021;8(8):Cd006190. doi:10.1002/14651858.CD006190.pub3
- Bou-Salah L, Benarous K, Linani A, et al. Anti-inflammatory drugs as new inhibitors to xanthine oxidase: in vitro and in silico approach. Mol Cell Probes. 2021;58:101733. doi:10.1016/j.mcp.2021.101733
- Linani A, Benarous K, Bou-Salah L, Yousfi M. The inhibitory kinetics of vitamins B9, C, E, and D3 on bovine xanthine oxidase: gout treatment. Chem Biol Interact. 2022;359:109922. doi:10.1016/j.cbi.2022.109922
- Rashidi M, Simpson DS, Hempel A, et al. The pyroptotic cell death effector gasdermin D is activated by gout-associated uric acid crystals but is dispensable for cell death and IL-1β release. J Immunol. 2019;203(3):736–748. doi:10.4049/jimmunol.1900228
- Neogi T, Jansen TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789–1798. doi:10.1136/annrheumdis-2015-208237
- Neogi T, Jansen TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67(10):2557–2568. doi:10.1002/art.39254
- Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–255. doi:10.1038/nm.3806
- Miyashita M, Koga K, Takeuchi A, et al. Expression of nerve injury-induced protein1 (Ninj1) in endometriosis. Reprod Sci. 2019;26(8):1105–1110. doi:10.1177/1933719118806395
- Coderre TJ, Wall PD. Ankle joint urate arthritis in rats provides a useful tool for the evaluation of analgesic and anti-arthritic agents. Pharmacol Biochem Behav. 1988;29(3):461–466. doi:10.1016/0091-3057(88)90004-4
- Dai XJ, Tao JH, Fang X, et al. Changes of Treg/Th17 ratio in spleen of acute gouty arthritis rat induced by MSU crystals. Inflammation. 2018;41(5):1955–1964. doi:10.1007/s10753-018-0839-y
- Yin GN, Choi MJ, Kim WJ, et al. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc Natl Acad Sci U S A. 2014;111(26):E2731–E2740. doi:10.1073/pnas.1403471111
- Jennewein C, Sowa R, Faber AC, et al. Contribution of Ninjurin1 to Toll-like receptor 4 signaling and systemic inflammation. Am J Respir Cell Mol Biol. 2015;53(5):656–663. doi:10.1165/rcmb.2014-0354OC
- Toma L, Sanda GM, Raileanu M, Stancu CS, Niculescu LS, Sima AV. Ninjurin-1 upregulated by TNFα receptor 1 stimulates monocyte adhesion to human TNFα-activated endothelial cells; benefic effects of amlodipine. Life Sci. 2020;249:117518. doi:10.1016/j.lfs.2020.117518
- Shin MW, Bae SJ, Wee HJ, et al. Ninjurin1 regulates lipopolysaccharide-induced inflammation through direct binding. Int J Oncol. 2016;48(2):821–828. doi:10.3892/ijo.2015.3296
- Ahn BJ, Le H, Shin MW, et al. Ninjurin1 enhances the basal motility and transendothelial migration of immune cells by inducing protrusive membrane dynamics. J Biol Chem. 2014;289(32):21926–21936. doi:10.1074/jbc.M113.532358
