Abstract
Purpose
Acute coronary syndrome (ACS) has a high incidence and mortality rate worldwide, which has a considerable negative impact on the global economy. This study aimed to identify a group of ACS patients at a high risk of recurrent adverse cardiac events using the plasma NLRP3 inflammasome.
Patients and methods
ACS patients admitted to Liaocheng People’s Hospital between June 2021 and March 2022 were enrolled in this study. Patients were divided into low (levels < 3.84 ng/mL) and high (levels ≥ 3.84 ng/mL) groups based on the median NLRP3 inflammasome levels. The patients were divided into three groups according to the Thrombolysis in Myocardial Infarction Risk Score for Secondary Prevention (TRS-2P): low (scores ≤ 2 points), intermediate (scores = 3 points), and high (score ≥ 4 points) risk. We investigated the relationship between NLRP3 inflammasome and laboratory indicators. Additionally, we examined whether the NLRP3 inflammasome was an independent predictor of high TRS-2P and explored the applicability of the plasma NLRP3 inflammasome for predicting high TRS-2P.
Results
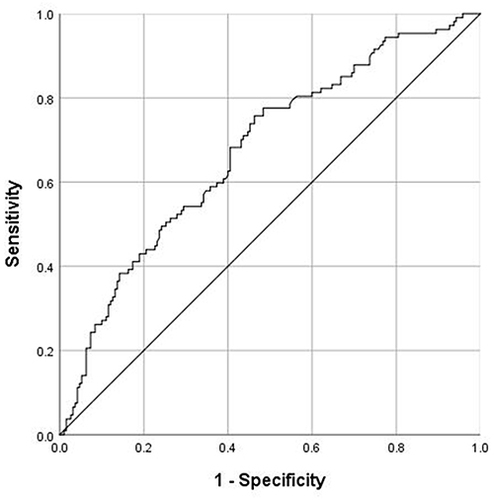
Logistic regression analysis revealed that NLRP3 inflammasome was an independent predictor of high TRS-2P (odds ratio [OR]:2.013; 95% confidence interval [CI]: 1.174–3.452). The area under the receiver operating characteristic curve value of the NLRP3 inflammasome was 0.674 (95% CI: 0.611–0.737; P < 0.001).
Conclusion
NLRP3 inflammasome levels are an independent predictive factor for high TRS-2P levels, which indicates that the NLRP3 inflammasome may help predict the prognosis of ACS patients.
Introduction
Acute coronary syndrome (ACS) is a group of ischemic diseases, including unstable angina (UA), non-ST-elevation myocardial infarction (NSTEMI), and ST-elevation myocardial infarction (STEMI).Citation1 In 2015, approximately 422.7 million cases of coronary artery disease were reported, with 17.92 million deaths causing a great burden on the global economy.Citation2 Percutaneous coronary intervention (PCI) can significantly prevent further necrosis of the myocardium and improve the quality of life of patients.Citation3 Therefore, it is widely used in ACS patients. Using intravascular imaging techniques for PCI guidance reduces the risk of cardiovascular death and adverse events compared those associated with coronary angiography.Citation4 However, the long-term prognosis of ACS patients remains an unsolved problem.Citation5 Numerous prognostic indicators of ACS, including lymphocyte-to-monocyte ratio, neutrophil-to-lymphocyte ratio (NLR), triglyceride-glucose index,Citation6–8 and others, have been reported. However, these biological indicatorsCitation6–8 are easily affected by various factors and do not have an exact cutoff value. Therefore, novel biomarkers for predicting the prognosis of ACS patients are required.
In recent years, an increasing number of studies have shown that inflammation plays an important role in acute myocardial infarction (AMI), and it has a great impact on the prognosis.Citation9,Citation10 NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome, composed of NLRP3, apoptosis speck-like protein (ASC), and pro-caspase 1, plays an important role in the inflammatory stage.Citation11,Citation12 Moreover, studies have shown that the size of myocardial infarction can be reduced, and cardiac function can be preserved by inhibiting the NLRP3 inflammasome in animal models.Citation13–15 In clinical research, the NLRP3 inflammasome is related to the severity and prognosis of ACS patients.Citation16,Citation17 The studies by Afrasyab et al and Peng et al were conducted at the peripheral blood monocyte and platelet levels, respectively. While our study was performed at the plasma level. In addition, we explored the relationship between the NLRP3 inflammasome and thrombolysis in the myocardial infarction risk score for secondary prevention (TRS-2P).
TRS-2P has been used to predict recurrent cardiovascular events in ACS patients.Citation18,Citation19 We aimed to use the TRS-2P to evaluate the NLRP3 inflammasome as a potential biomarker to better identify patients with a high risk of recurrent adverse cardiac events.
Methods
Participants and Design
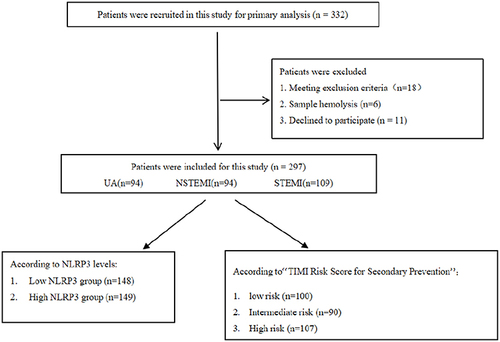
ACS patients who underwent coronary angiography at the Liaocheng People’s Hospital between June 2021 and March 2022 were enrolled. The diagnosis of myocardial infarction was defined according to the fourth universal definition of myocardial infarction,Citation20 and UA pectoris, according to the 2021 AHA/ACC guidelines for evaluating and diagnosing chest pain.Citation21 Patients with infections, cancer, immune system diseases, acute stroke, liver dysfunction, renal insufficiency, and previous cardiac insufficiency were excluded from this study because these diseases may affect the NLRP3 expression in plasma, resulting in experimental bias. A study flowchart is shown in . This study was approved by the Medical Ethics Committee of Liaocheng People’s Hospital (approval number: 2021096). All procedures were performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Data Collection and Laboratory Testing
The demographic characteristics of the patients included age, sex, smoking status, hypertension, type 2 diabetes mellitus (T2DM), and previous medication history, including aspirin, clopidogrel, and statins. Hematological indices were white blood cell (WBC), C-reactive protein (CRP), D-dimer, troponin I (cTnI), type B natriuretic peptide (BNP), low-density lipoprotein cholesterol, and creatinine (Cr). The echocardiographic indices included the left ventricle end-diastolic dimension and left ventricle ejection fraction (LVEF). All data were obtained from the case system of the Liaocheng People’s Hospital.
An enzyme-linked immunosorbent assay (ELISA) was used to determine NLRP3 inflammasome levels. Blood samples were collected in tubes containing ethylenediaminetetraacetic . After collecting the blood samples, they were immediately centrifuged at 1000 rpm for 15 min and stored at −80 °C. When all the samples were collected, they were thawed for ELISA. The plasma NLRP3 inflammasome level was determined strictly following the manufacturer’s instructions (Ml560903-2; Mlbio, China).
TRS-2P
The original version conferred 1 point for each of nine cardiovascular risk factors: heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke, prior coronary artery bypass graft surgery, peripheral vascular disease, estimated glomerular filtration rate < 60 mL·min1·1.73 m2, and current smoking. To stratify risk in ACS patients, the score was adapted to include prior MI as a risk factor to obtain a maximum 10-point score.Citation22 The patients were divided into three groups: low (scores ≤ 2 points), intermediate (scores = 3 points), and high (scores ≥ 4 points) risk.
Statistical Analysis
SPSS software (version 26.0; SPSS NY., USA) was used for the statistical analysis. The Shapiro–Wilk test was used to test whether the data were normally distributed. Non-normal distribution data were expressed as median (interquartile range), which were compared using the Mann–Whitney U-test in two groups. Non-normal distribution data among the three groups were tested using the Kruskal–Wallis H-test. Categorical variables were expressed as frequencies (percentages) and compared using the chi-square test. Pearson’s or Spearman correlation was used to compare the correlation between the NLRP3 inflammasome and other indices. Logistic regression analysis was performed to determine whether NLRP3 inflammasome was an independent predictor of high TRS-2P levels. In the univariate analysis, variables with an unadjusted P-value < 0.05 stepped in the multifactor logistic regression model. To evaluate the fit of the multifactor logistic regression model, Omnibus and Hosmer-Lemeshow tests were performed. The receiver operating characteristic (ROC) curve was used to further explore the applicability of the plasma NLRP3 inflammasome in predicting high TRS-2P. Each test was two-sided, and P-values < 0.05 were considered statistically significant.
Results
Patient Characteristics
This study included 297 patients (94 with UA, 94 with NSTEMI, and 109 with STEMI). The baseline patient characteristics are presented in . The three groups were significantly different in sex, smoking status, hypertension, previous medication history, NLRP3 inflammasome, WBC, and CRP, D-dimer, cTnI, BNP levels, and LVEF (P < 0.05).
Table 1 The Baseline Characteristics of the Patients
In the subgroup analysis, all patients were divided into two groups according to NLRP3 inflammasome levels: the low (NLRP3 levels < 3.84 ng/mL) and high (NLRP3 level ≥3.84 ng/mL) groups. In the high NLRP3 inflammasome group, patients were older, with high WBC, CRP, D-dimer, cTnI, BNP, and Cr levels, and low LVEF levels (). Furthermore, we divided the patients into three subgroups according to TRS-2P (). As the risk increased, the percentage of patients who were smokers, had hypertension and had T2DM increased. Age, NLRP3 inflammasome levels, WBC count and D-dimer, cTnI, and BNP levels increased with increasing scores. However, the LVEF decreased in the high-risk group.
Table 2 Basic Characteristics of Patients by NLRP3 Levels
Table 3 Patients Stratified by “TRS-2P”
Correlation Analysis Between NLRP3 Inflammasome and Other Indicators
NLRP3 inflammasome levels were positively correlated with age (r =0.165, P =0.004), WBC (r = 0.192, P = 0.001), CRP (r = 0.220, P < 0.001), D-dimer (r = 0.219, P < 0.001), cTnI (r = 0.200, P = 0.001), BNP (r = 0.324, P < 0.001), and Cr (r = 0.135, P =0.020) and inversely correlated with LVEF (r = −0.245, P < 0.001) ().
Table 4 Correlation Analysis Between NLRP3 and Laboratory Indicators
Multivariate Logistic Regression Model for Predicting High TRS-2P
Logistic regression analysis was performed to analyze the value of the NLRP3 inflammasome as a potential biomarker for the prediction of high TRS-2P. The results revealed that NLRP3 inflammasome (odds ratio [OR]: 2.013; 95% confidence interval [CI]: 1.174–3.452) and WBC (OR:1.107; 95% CI:1.020–1.202) were independent predictors of high TRS-2P (). The omnibus test showed that the overall model is meaningful (P < 0.001; ). The Hosmer-Lemeshow test indicated that the model has a good fit. (Chi-square:10.933; P > 0.05; ).
Table 5 Logistic Regression Analysis of Predictors of High Risk in ACS Patients
Table 6 Omnibus and Hosmer-Lemeshow Tests
Performance of NLRP3 Inflammasome in the Prediction of High TRS-2P
ROC analysis was conducted to test the performance of the NLRP3 inflammasome in predicting high TRS-2P levels (). The area under the curve value of the NLRP3 inflammasome was 0.674 (95% CI: 0.611–0.737; P < 0.001). The cutoff was 3.51 ng/mL, and the sensitivity and specificity were 75.7% and 70.6%, respectively.
Discussion
This study demonstrated that the plasma level of the NLRP3 inflammasome in AMI patients was higher than that in UA patients, and the level of the NLRP3 inflammasome was correlated with age, WBC count, CRP, D-dimer, BNP levels, and LVEF. To our knowledge, this is the first study to identify plasma NLRP3 inflammasome levels as an independent predictive factor of high TRS-2P. These findings suggest that the NLRP3 inflammasome can be used to predict the prognosis of ACS patients.
Previous studies have shown that the NLRP3 inflammasome is elevated in ACS patients.Citation16,Citation17 However, their study was based on platelet and peripheral monocyte levels. To our knowledge, this is the first study to investigate the plasma NLRP3 inflammasome and link it to TRS-2P. Toldo et alCitation23 have reviewed the mechanisms of NLRP3 inflammasome activation through the kinase and oxidative stress pathways in AMI. In the analysis of AMI subgroups, the STEMI and NSTEMI groups exhibited no significant differences. No studies have compared the expression of the plasma NLRP3 inflammasome in STEMI and NSTEMI patients.
Couchie et alCitation24 found that human plasma thioredoxin-80 levels increased with age and promoted the activation of the NLRP3 inflammasome through the Akt2/mechanistic target of the rapamycin-C1/70S6K pathway. A recent study also revealed that serum concentrations of pro-inflammatory cytokines, including IL-1α/β, TNF-α, IL-6, and NLRP3, significantly increased with age.Citation25 This was consistent with our study, wherein patients in the high NLRP3 group were older. Because this was a retrospective study with a small sample size, the relationship between age and the NLRP3 inflammasome may be stronger if a larger sample size is assessed. Our study shows that the level of NLRP3 inflammasome is higher in AMI than that of UA and positively correlated with WBC and CRP levels, which is consistent with previous studies.Citation16,Citation17 NLRP3 inflammasome activation in AMI is mediated by a dual-signal model.Citation26 Various endogenous molecules promote the expression of the NLRP3 gene and lead to the recombination of NLRP3, which mediates apoptosis and inflammatory response.Citation27 In our study, WBC and CRP were associated with NLRP3 inflammasome. To date, we have shown that the NLRP3 inflammasome can increase the expression of CRP via the caspase-1/IL-6 pathway.Citation28 Bian et alCitation29 found CRP increased the expression of NLRP3 via the NF-κB pathway. Therefore, we speculate that NLRP3 and CRP interact with each other. In recent years, an increasing number of clinical studies have shown that D-dimer is related to the prognosis of ACS patients.Citation30–32 In our study, patients with high TRS-2P had high D-dimer levels, which was consistent with previous studies.Citation30–32 In addition, NLRP3 inflammasome levels positively correlated with D-dimer levels, suggesting that NLRP3 inflammasome levels may be associated with the prognosis of patients with ACS. Previous studies have shown that uric acid is important in inflammation and is associated with mortality in ACS patients.Citation33,Citation34 However, our study observed no correlation between uric acid and TRS-2P. This might have been due to the small sample size and sampling errors.
Furthermore, TRS-2P has been used to predict recurrent cardiovascular events in patients with ACS.Citation18,Citation19 In animal experiments, NLRP3 inflammasome has been associated with cardiac remodeling and function. A study including an experimental mouse model revealed that a specific NLRP3 inhibitor, MCC950, can alleviate fibrosis and improve cardiac function by suppressing early inflammatory responses post-MI.Citation35 Similarly, Zhao et al also demonstrated that MCC950 could attenuate cardiac remodeling by inhibiting cardiac hypertrophy, fibrosis, and inflammation.Citation36 As another specific NLRP3 inhibitor, OLT1177 has also been widely studied. In a large non-reperfused anterior MI mouse model, it preserved β-adrenergic responsiveness and prevented left ventricular diastolic dysfunction.Citation37 Researchers have recently identified many substances related to NLRP3 inflammasome. Nie et alCitation38 unearthed that inhalation of H2 can ameliorate myocardial infarction-induced cardiac remodeling and fibrosis in rats with MI. In another study, the LuQi Formula inhibited the activation of the NLRP3/ASC/caspase-1/IL-1β cascade, decreasing inflammatory and delayed ventricular remodeling.Citation39 In summary, the NLRP3 inflammasome has been confirmed to potentiate the cardiac inflammatory response and cardiac remodeling, which lays a foundation for its use in predicting the prognosis of ACS patients. Per current clinical research, drugs suppress the NLRP3 inflammasome directly or indirectly via IL-1 activity. In a randomized trial involving patients with chronic coronary disease, the risk of cardiovascular events was significantly lower among those who received 0.5 mg of colchicine once daily than among those who received a placebo.Citation40 A LoDoCo2 biomarker sub-study found that colchicine can reduce the extracellular vesicle NLRP3 protein levels.Citation41 However, we do not recommend increasing the dose of colchicine during acute inflammatory reaction because its side effects increase with its increasing dose, including myelosuppression, neuromuscular toxicity, liver damage, and dermatologic issue.Citation42 A systematic review and meta-analysis of randomized trials reported that low-dose colchicine could reduce the risk of MACE in patients with coronary disease.Citation43 In addition to colchicine, other drugs that indirectly inhibit IL-1 by suppressing the NLRP3 pathway, such as anakinra and canakinumab, are also under clinical research. In thetwo clinical studies,Citation44,Citation45 STEMI patients treated with anakinra (an IL-1 blocker) exhibited reduced CRP levels and reduced incidence of heart failure. NLRP3 inflammasome, as an upstream factor of IL-1 and CRP,Citation23 may play an important role in the prognosis of STEMI patients. A clinical trial on canakinumab suggested that canakinumab can reduce the occurrence of MACE, accompanied by a decrease in IL-6 and CRP levels.Citation46 In conclusion, NLRP3 inflammasome has a great impact on the prognosis of ACS patients, which has laid the foundation for distinguishing high-risk patients and administering early intervention. However, the mechanisms by which NLRP3 inflammasome affects prognosis remain unclear. The NLRP3 inflammasome can affect cardiac function, but its activation alone does not affect systolic cardiac dysfunction.Citation26 Activating NLRP3 causes its downstream IL-1β and IL-18, leading to cardiac dysfunction.Citation47,Citation48 A recent study indicated that the inhibition of the NLRP3/ IL-1β pathway can alleviate the cardiac inflammatory response, improve cardiac contractility, and attenuate cardiomyopathy in sepsis.Citation49 Therefore, mechanisms other than the classical mechanism require further study.
In this study, NLRP3 inflammasomes were significantly associated with higher TRS-2P levels, supporting the NLRP3 inflammasome as a potential biomarker for predicting the prognosis of ACS patients. However, further studies are needed to clarify this association.
Our study had several limitations. First, it was a single-center study with a small sample size. Multi-center studies with larger sample sizes may be needed in the future. Second, the plasma NLRP3 inflammasome level was not monitored dynamically. Finally, the study only involved Chinese patients and should be conducted in other ethnic groups. Further research is needed to completely understand the mechanisms by which the NLRP3 inflammasome affects cardiac function and prognosis of patients with ACS.
Conclusions
Plasma NLRP3 inflammasome levels are an independent predictive factor for high TRS-2P, indicating that the NLRP3 inflammasome may be used to predict the prognosis of ACS patients.
Abbreviations
NLRP3, NACHT, LRR, and PYD domain-containing protein 3; ACS, Acute coronary syndrome; UA, unstable angina; NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; NLR, neutrophil-to-lymphocyte ratio; ASC, apoptosis speck-like protein; TRS-2P, Thrombolysis in Myocardial Infarction Risk Score for Secondary Prevention; T2DM, type 2 diabetes mellitus; WBC, white blood cell; CRP, C-reactive protein; cTnI, troponin I; BNP, type B natriuretic peptide; Cr, creatinine; LVEF, left ventricle ejection fraction; ELISA, enzyme-linked immunosorbent assay; ROC, receiver operating characteristic; OR, odds ratio; CI, confidence interval.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee of the Liaocheng People’s Hospital. All procedures were performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest associated with this study.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Additional information
Funding
References
- Hedayati T, Yadav N, Khanagavi J. Non-ST-segment acute coronary syndromes. Cardiol Clin. 2018;36(1):37–52. doi:10.1016/j.ccl.2017.08.003
- Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. doi:10.1016/j.jacc.2017.04.052
- Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381(15):1411–1421. doi:10.1056/NEJMoa1907775
- Buccheri S, Franchina G, Romano S, et al. Clinical Outcomes Following Intravascular Imaging-Guided Versus Coronary Angiography–Guided Percutaneous Coronary Intervention With Stent Implantation. JACC Cardiovasc Interv. 2017;10(24):2488–2498. doi:10.1016/j.jcin.2017.08.051
- de Carvalho LP, Gao F, Chen Q, et al. Long-term prognosis and risk heterogeneity of heart failure complicating acute myocardial infarction. Am J Cardiol. 2015;115(7):872–878. doi:10.1016/j.amjcard.2015.01.010
- Quan X-Q, Wang R-C, Zhang Q, Zhang C-T, Sun L. The predictive value of lymphocyte-to-monocyte ratio in the prognosis of acute coronary syndrome patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2020;20(1):338. doi:10.1186/s12872-020-01614-x
- Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14(5):573–577. doi:10.1586/14779072.2016.1154788
- Wang L, Cong H-L, Zhang J-X, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. doi:10.1186/s12933-020-01054-z
- Westman PC, Lipinski MJ, Luger D, et al. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J Am Coll Cardiol. 2016;67(17):2050–2060. doi:10.1016/j.jacc.2016.01.073
- Ruparelia N, Godec J, Lee R, Chai JT. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J. 2015;36(29):1923–1934. doi:10.1093/eurheartj/ehv195
- Zhang WJ, Chen SJ, Zhou SC, Wu SZ, Wang H. Inflammasomes and Fibrosis. Front Immunol. 2021;12:643149. doi:10.3389/fimmu.2021.643149
- Haneklaus M, O’Neill LAJ. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265(1):53–62. doi:10.1111/imr.12285
- Toldo S, Mauro AG, Cutter Z, et al. The NLRP3 Inflammasome Inhibitor, OLT1177 (Dapansutrile), Reduces Infarct Size and Preserves Contractile Function After Ischemia Reperfusion Injury in the Mouse. J Cardiovasc Pharmacol. 2019;73(4):215–222. doi:10.1097/FJC.0000000000000658
- Yang F, Qin Y, Wang Y, et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int J Biol Sci. 2019;15(5):1010–1019. doi:10.7150/ijbs.29680
- Feng H, Mou SQ, Li WJ, et al. Resveratrol Inhibits Ischemia-Induced Myocardial Senescence Signals and NLRP3 Inflammasome Activation. Oxid Med Cell Longev. 2020;2020:2647807. doi:10.1155/2020/2647807
- Afrasyab A, Qu P, Zhao Y, et al. Correlation of NLRP3 with severity and prognosis of coronary atherosclerosis in acute coronary syndrome patients. Heart Vessels. 2016;31(8):1218–1229. doi:10.1007/s00380-015-0723-8
- Peng H, Wu H, Zhang G, et al. Expression and Clinical Prognostic Value of Platelet NLRP3 in Acute Coronary Syndrome. Int J Gen Med. 2020;13:791–802. doi:10.2147/IJGM.S275481
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi:10.1161/CIR.0000000000000625
- Puymirat E, Bonaca M, Fumery M, et al. Atherothrombotic risk stratification after acute myocardial infarction: the Thrombolysis in Myocardial Infarction Risk Score for Secondary Prevention in the light of the French Registry of Acute ST Elevation or non-ST Elevation Myocardial Infarction registries. Clin Cardiol. 2019;42(2):227–234. doi:10.1002/clc.23131
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. doi:10.1016/j.jacc.2018.08.1038
- Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368–e454.
- Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation. 2019;139(11):1384–1395. doi:10.1161/CIRCULATIONAHA.118.037778
- Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15(4):203–214. doi:10.1038/nrcardio.2017.161
- Couchie D, Vaisman B, Abderrazak A, et al. Human Plasma Thioredoxin-80 Increases With Age and in ApoE −/− Mice Induces Inflammation, Angiogenesis, and Atherosclerosis. Circulation. 2017;136(5):464–475. doi:10.1161/CIRCULATIONAHA.117.027612
- Lliberos C, Liew SH, Zareie P, La Gruta NL, Mansell A, Hutt K. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci Rep. 2021;11(1):278. doi:10.1038/s41598-020-79488-4
- Toldo S, Mezzaroma E, McGeough MD, et al. Independent roles of the priming and the triggering of the NLRP3 inflammasome in the heart. Cardiovasc Res. 2015;105(2):203–212. doi:10.1093/cvr/cvu259
- Shen S, Wang Z, Sun H, Ma L. Role of NLRP3 Inflammasome in Myocardial Ischemia-Reperfusion Injury and Ventricular Remodeling. Med Sci Monit. 2022;28:e934255. doi:10.12659/MSM.934255
- Sethwala AM, Goh I, Amerena JV. Combating Inflammation in Cardiovascular Disease. Heart Lung Circ. 2021;30(2):197–206. doi:10.1016/j.hlc.2020.09.003
- Bian F, Yang X-Y, Xu G, Zheng T, Jin S. CRP-Induced NLRP3 Inflammasome Activation Increases LDL Transcytosis Across Endothelial Cells. Front Pharmacol. 2019;10:40. doi:10.3389/fphar.2019.00040
- Zhang X, Wang S, Liu J, et al. D-dimer and the incidence of heart failure and mortality after acute myocardial infarction. Heart. 2021;107(3):237–244. doi:10.1136/heartjnl-2020-316880
- Chen R, Liu C, Zhou P, et al. Prognostic Value of D-dimer in patients with acute coronary syndrome treated by percutaneous coronary intervention: a retrospective cohort study. Thromb J. 2021;19(1):30. doi:10.1186/s12959-021-00281-y
- Koch V, Booz C, Gruenewald LD, et al. Diagnostic performance and predictive value of D-dimer testing in patients referred to the emergency department for suspected myocardial infarction. Clin Biochem. 2022;104:22–29. doi:10.1016/j.clinbiochem.2022.02.003
- Maloberti A, Biolcati M, Ruzzenenti G, et al. The Role of Uric Acid in Acute and Chronic Coronary Syndromes. J Clin Med. 2021;10(20):20. doi:10.3390/jcm10204750
- Rebora P, Centola M, Morici N, et al. Uric acid associated with acute heart failure presentation in Acute Coronary Syndrome patients. Eur J Intern Med. 2022;99:30–37. doi:10.1016/j.ejim.2022.01.018
- Gao R, Shi H, Chang S, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol. 2019;74:105575. doi:10.1016/j.intimp.2019.04.022
- Zhao M, Zhang J, Xu Y, et al. Selective Inhibition of NLRP3 Inflammasome Reverses Pressure Overload-Induced Pathological Cardiac Remodeling by Attenuating Hypertrophy, Fibrosis, and Inflammation. Int Immunopharmacol. 2021;99:108046. doi:10.1016/j.intimp.2021.108046
- Aliaga J, Bonaventura A, Mezzaroma E, et al. Preservation of Contractile Reserve and Diastolic Function by Inhibiting the NLRP3 Inflammasome with OLT1177® (Dapansutrile) in a Mouse Model of Severe Ischemic Cardiomyopathy Due to Non-Reperfused Anterior Wall Myocardial Infarction. Molecules. 2021;26(12):12. doi:10.3390/molecules26123534
- Nie C, Zou R, Pan S. Hydrogen gas inhalation ameliorates cardiac remodelling and fibrosis by regulating NLRP3 inflammasome in myocardial infarction rats. J Cell Mol Med. 2021;25(18):8997–9010. doi:10.1111/jcmm.16863
- Zhang X, Zhao D, Feng J, et al. LuQi Formula Regulates NLRP3 Inflammasome to Relieve Myocardial-Infarction-Induced Cardiac Remodeling in Mice. Evid Based Complement Alternat Med. 2021;2021:5518083. doi:10.1155/2021/5518083
- Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383(19):1838–1847. doi:10.1056/NEJMoa2021372
- Silvis MJM, Fiolet ATL, Opstal TSJ, et al. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: a LoDoCo2 biomarker substudy. Atherosclerosis. 2021;334:93–100. doi:10.1016/j.atherosclerosis.2021.08.005
- Deftereos SG, Beerkens FJ, Shah B, et al. Colchicine in Cardiovascular Disease: in-Depth Review. Circulation. 2022;145(1):61–78. doi:10.1161/CIRCULATIONAHA.121.056171
- Fiolet ATL, Opstal TSJ, Mosterd A, et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2021;42(28):2765–2775. doi:10.1093/eurheartj/ehab115
- Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111(10):1394–1400. doi:10.1016/j.amjcard.2013.01.287
- Abbate A, Trankle CR, Buckley LF, et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment–Elevation Myocardial Infarction. J Am Heart Assoc. 2020;9(5):e014941. doi:10.1161/JAHA.119.014941
- Ridker PM, Libby P, MacFadyen JG, et al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J. 2018;39(38):3499–3507. doi:10.1093/eurheartj/ehy310
- Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315(6):H1553–H1568. doi:10.1152/ajpheart.00158.2018
- Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ Res. 2020;126(9):1260–1280. doi:10.1161/CIRCRESAHA.120.315937
- Busch K, Kny M, Huang N, et al. Inhibition of the NLRP3/IL-1β axis protects against sepsis-induced cardiomyopathy. J Cachexia Sarcopenia Muscle. 2021;12(6):1653–1668. doi:10.1002/jcsm.12763