Abstract
Purpose
Meiotic nuclear division 1 (MND1) is a meiosis-specific protein that promotes lung adenocarcinoma progression. However, its expression and biological function across cancers remain largely unexplored.
Patients and Methods
The expression, prognostic significance, mutation status, and methylation profile of MND1 in various cancers were comprehensively analyzed using the TIMER, GTEX, Kaplan-Meier plotter, cBioPortal, and GSCA databases. Additionally, we constructed a PPI network, enrichment analysis and single-cell transcriptomic sequencing to elucidate the underlying mechanism of MND1. Furthermore, we investigated the association between MND1 expression and drug sensitivity using CellMiner. Moreover, we also explored the correlation between MND1 expression and immune infiltration. Finally, we validated the functional role of MND1 in breast cancer through IHC staining, CCK8, EdU, colony formation, and flow cytometry assays.
Results
MND1 has been reported to be highly expressed in Pan-cancer, High MND1 expression was significantly associated with poor prognosis in cancers. Additionally, MND1 mutation frequency is high in most cancers, and its expression correlates with methylation. Furthermore, MND1 expression significantly correlates with immune checkpoint blockade (ICB) markers, including PD-L1, PD-1, and CTLA-4. The PPI network reveals interactions between MND1 and PSMC3IP, BRCA1, and BRCA2. Enrichment analysis and single-cell sequencing indicate that MND1 positively correlates with cell cycle. ROC curve reveals favorable diagnostic efficacy of MND1 in breast cancer. In vitro, MND1 overexpression promotes breast cancer cell proliferation and increases the expression of key cell cycle regulators (CDK4, CDK6, and cyclin D3), accelerating the G1/S phase transition and leading to abnormal breast cancer cell proliferation. The immunohistochemical analysis revealed a robust expression of MND1 in breast cancer tissues, exhibiting a significant positive correlation with PD-L1 and FOXP3.
Conclusion
MND1 is an oncogene and may serve as a biomarker for cancer prognosis and immunotherapy. Targeting MND1 may be a potential tumor treatment strategy.
Introduction
Meiotic nuclear division 1 (MND1) is a meiosis-specific protein that promotes the repair of homologous chromosome-paired DNA double-strand breaks (DSBs) during meiosis.Citation1–3 Multiple previous studies have found that MND1 can form a complex with homologous pairing protein (HOP2) to promote homologous chromosome pairing and DSB repair during meiosis.Citation1,Citation4,Citation5 HOP2-MND1 heterodimers are essential for homologous recombination in eukaryotes.Citation6,Citation7 In the HOP2-MND1 heterodimeric complex, HOP2 acts as the major DNA-binding subunit, while MND1 is an important Rad51-interacting entity that regulates the ATP and DNA binding of RAD51 to stabilize RAD51 presynaptic filaments and duplex DNA capture, which improves the composition of the synaptic complex.Citation6,Citation8,Citation9 Pezza et al also found that the HOP2-MND1 complex could also stimulate Dmc1 to promote the formation of synaptic complexes on long double-stranded DNA.Citation10
Several studies have demonstrated that meiotic factors can serve as effective targets for tumor therapy and biomonitoring.Citation11,Citation12 In lung adenocarcinoma, Wei et al identified MND1 as a possible diagnostic and prognostic target for LUAD using public datasets.Citation9 In addition, Zhang et al elucidated that MND1 could form a positive feedback loop with Kruppel-like factor 6 (KLF6) and E2F transcription factor 1 (E2F1) to regulate the cell cycle and confer cisplatin (DDP) resistance in LUAD.Citation2 And Bao et al identified MND1 as a prognostic predictor in breast cancer using bioinformatics analysis.Citation13 However, these studies are not thorough and comprehensive. Pan-Cancer analysis can reveal tumor similarities and differences, and it would be of interest to further explore the oncogene profile of MND1 in various cancers.Citation14
More importantly, cancer immunotherapy has made outstanding progress in the treatment of a wide range of cancers, particularly with the use of chimeric antigen receptor T-cell (CART) therapy and immune checkpoint blockade (ICB).Citation15–17 According to a new theory, the tumor microenvironment (TME) plays a key role in initiating and developing human malignancies.Citation18–21 MND1 has been reported to be associated with immune infiltration in renal clear cell carcinoma.Citation11 However, the relationship between MND1 and immune infiltration in other tumors is still largely unknown.
Therefore, we systematically and comprehensively analyzed the role of MND1 in multiple cancers from multiple perspectives, including gene and protein expression, prognosis, mutation, methylation, protein-protein interaction (PPI) networks, regulated signaling pathways, single-cell transcriptome sequencing, and drug response. Additionally, to better understand whether MND1 plays an important role in cancer immunotherapy, we also performed a series of analyses on factors involved in tumor immunity, such as association with immune cells, immune checkpoints, microsatellite instability (MSI), and tumor mutation burden (TMB). More importantly, we also demonstrated through a series of in vitro experiments that MND1 promotes breast cancer cell proliferation by accelerating the G1/S phase transition.
This study provides the first systematic and comprehensive analysis of the various roles of MND1 across cancers to determine the potential usefulness of MDN1 for cancer diagnosis, prognosis and immunotherapy.
Materials and Methods
Data Acquisition and Processing
The mRNA expression levels of MND1 in various tumors were obtained from the Tumor Immune Estimation Resource (TIMER) database (http://www.linkedomics.org) and the GEPIA 2 database (http://gepia2.cancer-pku.cn/#index).Citation22,Citation23 High-throughput RNA sequencing data of paired cancers were downloaded from The Cancer Genome Atlas (TCGA) (https://cancergenome.nih.gov) database. We also extracted high-throughput sequencing data from the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) dataset for breast cancer (GSE45827: 85 breast cancer tissues and 70 normal breast tissues, GSE7904: 43 breast cancer tissues and 19 normal breast tissues, GSE42568: 104 breast cancer tissues and GSE109169: 25 paired breast cancer samples and 12 in stage I, 22 in stage II, and 16 in stage III).Citation24–28
Correlation Analysis of MND1 with Pan-Cancer Prognosis
Clinical information and prognostic data were obtained from the TCGA prognostic study,Citation29 including overall survival (OS), disease-specific survival (DSS), and progression-free interval (PFI). The relationship between MND1 expression and the Pan-Cancer prognosis was explored by univariate Cox regression and Kaplan‒Meier analysis using the “survival” and “survminer” R packages. The significance between high- and low-expression subgroups was determined by log-rank statistical tests. Statistical significance was defined as p< 0.05.
Genetic Mutation Analysis of MND1 Across Cancers
After entering the cBioPortal database (https://www.cbioportal.org/),Citation30 we selected “TCGA Pan Cancer Atlas Studies” in the “Quick select” section and entered “MND1” to search for characteristic genetic alterations in MND1. The frequency of alterations, mutational types, and copy number alterations (CNAs) were observed in all TCGA tumors in the “Cancer Type Summary” module. Mutational locus information for MND1 were displayed in a protein structure or three-dimensional schematic through the “Mutations” module. We also used the “Comparison” module to obtain data on overall survival differences in TCGA cancer patients with or without MND1 genetic alterations.
MND1 Pan-Cancer Methylation Profiles
Gene Set Cancer Analysis (GSCA) (http://bioinfo.life.hust.edu.cn/GSCA/#/) is an integrated platform for genomic, pharmacogenomic, and immunogenomic cancer analyses that provides information about mRNA expression, methylation, immune infiltration, and drug resistance.Citation31 We used GSCA to analyze the MND1 methylation profile across cancers and to determine its relationship with prognosis.
Correlation Analysis Between MND1 and Cancer Immunity
To assess the reliability of the immune score evaluation, we used immuneeconv, an R software package that integrates the six latest algorithms, including TIMER, xCell, MCP-counter, CIBERSORT, EPIC, and quanTIseq. The correlation between MND1 expression and immune cell infiltration in multiple cancers was evaluated using the R packages “ggpubr” and “ggExtra”. Siglec-15 (SIGLEC15), PD-L1 (CD274), TIM-3 (HAVCR2), PD-1 (PDCD1), CTLA4 (CTLA4), LAG3 (LAG3) and PD-L2 (PDCD1LG2) are ICB markers. We used the Wilcox test to evaluate the correlation between MND1 expression and the expression of these genes. Moreover, we also performed an association analysis between MND1 expression and genes related to TMB, MSI, and mismatch repair system (MMR).Citation15,Citation32 All the analysis methods and R package were implemented by R version 4.0.3. The Wilcox test analyzed two-group data. P values less than 0.05 were considered statistically significant.
Construction of PPI Networks
GeneMANIA (http://www.genemania.org) is an interactive website designed to establish PPI networks and provide predictive assumptions of gene function.Citation33,Citation34 We constructed the PPI of MND1 using the GeneMANIA website in this study.
Enrichment Analysis of MND1-Related Genes
We identified the top 100 MND1-related genes based on the datasets of all TCGA tumors and normal tissues using the “Similar Gene Detection” module of the GEPIA2 database (http://gepia2.cancer-pku.cn/#index).Citation35 Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of these genes were performed using the cluster Profiler package.Citation36
Single-Cell Sequencing Data Analysis
CancerSEA (http://biocc.hrbmu.edu.cn/AdenocarcinomaSEA/home.jsp) is the first single-cell sequencing database to provide different functional states of cancer cells at the single-cell level.Citation37 We downloaded data that correlated MND1 expression with different tumor functional states from CancerSEA and visualized this data on a heatmap. We further downloaded breast and lung cancer single-cell data from CancerSEA (Exp ID: EXP0052 and EXP0066) to analyze the relationships among MND1 expression, the cell cycle, DNA damage, and DNA repair in breast and lung cancers using Spearman’s algorithm.
Association of MND1 Expression and Drug Response
CellMiner (https://discover.nci.nih.gov/cellminer/home.do) is a database and query tool designed for the cancer research community to facilitate the integration and study of molecular and pharmacological data from the NCI-60 cancer cell line, a panel of 60 different human cancer cell lines that have been used by the NCI’s Developmental Therapeutics Program to screen over 100,000 chemical compounds.Citation38 We explored the correlation between the expression of MND1 and different drug responses using the CellMiner database.
Tissue Microarray
The human breast cancer tissue microarray was purchased from Shanghai Outdo Biotech Co, Ltd. (#HBreD090PG01, Shanghai, China) and was used to measure the protein expression of MND1 in breast cancer by immunohistochemistry. A total of 90 paraffin-embedded tissue samples, including 70 breast cancer tissues and 20 paraneoplastic tissues, were used. Another microarray (#HBreD030PG01, Shanghai, China), including 30 breast cancer tissues, was used to analyze the expression of MND1, FOXP3 and PD-L1, and correlation was calculated using the Spearman algorithm. The Ethics Committee of Shanghai Outdo Biotech Co, Ltd approved the study of human subjects. The anti-MND1 antibody was purchased from Beijing Bioss Biotech Co, Ltd. (1:200 dilution, #bs-7799R, Beijing, China), anti-FOXP3 and anti-PD-L1 antibody were purchased from Servicebio (anti-FOXP3: 1:200 dilution, # GB11093; anti-PD-L1: 1:1000 dilution, #GB11339A).
Cell Culture and Transfection
MDA-MB-231 and MCF7 cells were purchased from the bank of the Shanghai Institute of Biological Sciences and the Chinese Academy of Sciences and cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Gibco, Detroit, MI, USA) at 37 °C and 5% CO2. The siRNAs were purchased from GenePharma (Suzhou, China). Plasmid and siRNA transfection experiments were performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The MND1 gene was cloned into a pCMV-N-Flag vector between the 5ʹBamHI-3ʹEcoRI restriction sites. The siRNAs targeting MND1 had the following sequences: siRNA-MDN1#1: 5ʹ- GCUUAGUUGAUGAUGGUAUTT-3ʹ and siRNA-MDN1#2: 5ʹ- GAAACGGCCAAGCAAAUAATT-3ʹ.
Cell Proliferation Assays
The effects of MND1 overexpression or knockdown on cell viability were measured using CCK-8 assays (Cell Counting Kit-8; Dojindo, Japan), clone formation assays, and 5-ethynyl-20-deoxyuridine (EdU) assays.
For CCK8 assays, 48 h after plasmid and siRNA transfection, 96-well plates were inoculated with transformants (2×103 cells/well). At 0, 24, 48, 72, and 96 h after inoculation, 10 µL of CCK8 reagent was added to each well and plates were incubated for 2 h at 37 °C. The absorbance value at 450 nm was then measured (Tecan, Austria).
For clone formation assays, six-well plates were inoculated with transformants (2000 cells/well) and cultured in DMEM supplemented with 20% FBS for 2 weeks. The cells were then washed twice with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 20 min, and stained with 0.1% (MDA-MB-231) and 0.5% (MCF7) crystal violet for 5 min. The number of cell clones in each group was counted.
For EdU assays, the rate of DNA synthesis in breast cancer cells (1×104/well) was measured using an EdU assay kit (Ribobio, China) according to the manufacturer’s instructions. Proliferation activity was assessed by measuring the ratio of EdU-positive cells (red fluorescence) to Hoechst-stained cells (blue fluorescence). Experiments were performed in triplicate.
RNA Isolation and Real-Time Fluorescence Quantitative PCR (qRT‒PCR)
RNA was extracted from cells using a total isolation kit (Vazyme, China) according to the manufacturer’s instructions. RNA concentrations were measured using a Nanodrop One spectrophotometer (Thermo Fisher, USA). The RNA was subsequently reverse-transcribed into cDNA using a cDNA synthesis kit (Vazyme, China). qRT‒PCR was performed using an SYBR Green Master Mix Kit (Vazyme, China) with the following primers: MND1 forward: 5ʹ- TGTGAGAGGATCGGAACTTCT-3ʹ, MND1 reverse: 5ʹ-CACATCGGCCAATTTTAGCTTTC-3ʹ; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward: 5ʹ-GGTGTGAACCATGAGAAGTATGA-3ʹ, GAPDH reverse: 5ʹ-GAGTCCTTCCACGATACCAAAG-3ʹ. The expression level of the GAPDH gene was used for normalization. The real-time PCR amplification conditions were as follows: 95 °C for 30s; 40 cycles of 95 °C for 10s, 60 °C for 30s, followed by collecting fluorescence; and finally melt curve at 95 °C for 15s, 60 °C for 60s, and 95 °C for 15s. Experiments were performed in triplicate.
Western Blotting
Cells transfected with plasmid or siRNA were collected and lysed using radioimmunoprecipitation assay (RIPA) buffer (Absin, China) containing phenylmethylsulfonyl fluoride (Absin, China). Protein concentrations were determined using a BCA protein assay kit (Beyotime, China). For sodium dodecyl sulfate‒polyacrylamide gel electrophoresis, the samples were supplemented with 3×104 ng of protein and imprinted on polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were then blocked with 5% skim milk for 1 h at room temperature and incubated with the respective primary antibodies—MND1 (1:1000 dilution, # 11636-1-AP, Proteintech, USA), cyclin A2 (1:1000 dilution, #4656, Cell Signaling Technology, USA), cyclin B1 (1:1000 dilution, #12231, Cell Signaling Technology, USA), cyclin D3 (1:1000 dilution, #2936, Cell Signaling Technology, USA), cyclin E2 (1:1000 dilution, #4132, Cell Signaling Technology, USA), CDK2 (1:1000 dilution, #18048, Cell Signaling Technology, USA), CDK4 (1:1000 dilution, #12790, Cell Signaling Technology, USA), CDK6 (1:1000 dilution, #3136, Cell Signaling Technology, USA), and b-actin (1:5000 dilution, #ab8226, Abcam, USA)—overnight at 4 °C. The membranes were then incubated with secondary antibodies conjugated to horseradish peroxidase (1:5000 dilution, #7076, #7074, Cell Signaling Technology, USA) for 1 h at room temperature. Signals were detected using a chemiluminescence detection reagent (Millipore, USA).
Flow Cytometry for Cell Cycle Analysis
Forty-eight hours after transfection with either plasmids or siRNA, cells were collected and fixed overnight with 75% alcohol at −20 °C. After washing with PBS, the cells were incubated with propidium iodide (PI)/RNase A solution (#abs50005, Absin, China) at 37 °C for 30 min. Samples were analyzed within 5 h of staining using a flow cytometer (BD, USA), and data were analyzed using FlowJo V10 software.
Statistical Analysis
The experimental results were analyzed using GraphPad Prism version 8.4.0 for Mac OS X (GraphPad Software, San Diego, California USA) using the average of three replicates. Student’s t test was used to assess the significance of differences in two-group comparisons. The Kaplan–Meier method was used to evaluate survival across groups, and analysis of variance (ANOVA) was used to assess the statistical significance of the differences between various groups. Spearman’s rank correlation coefficient was used to assess the correlation between the two groups. The alpha level was set at 0.05.
Results
The Expression of MND1 is Significantly Upregulated in Various Types of Cancer
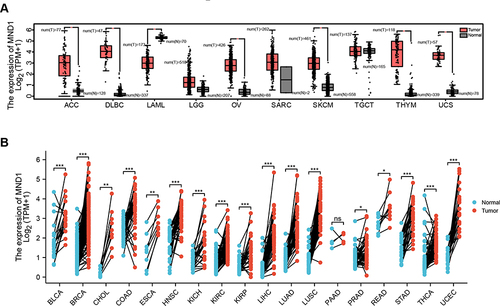
Fang et al,Citation11 Bao et alCitation13 and Tan et alCitation39 found that the MND1 mRNA expression level was significantly elevated in a variety of tumors through the TIMER database, including bladder urothelial carcinomas (BLCA), breast invasive carcinomas (BRCA), cervical squamous cell carcinomas and endocervical adenocarcinomas (CESC), cholangiocarcinoma (CHOL), colon adenocarcinomas (COAD), esophageal carcinomas (ESCA), glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), pheochromocytoma and paraganglioma (PCPG), prostate adenocarcinoma (PRAD), rectum adenocarcinoma (READ), stomach adenocarcinoma (STAD), thyroid carcinoma (THCA) and uterine corpus endometrial carcinoma (UCEC) (). Since some tumors lack normal tissue data in the TIMER database, we further assessed the differences in MND1 expression between tumors and normal tissues using the GTEx dataset in the GEPIA database. The results also revealed that MND1 was highly expressed in adrenocortical carcinoma (ACC), lymphoid neoplasm diffuse large B-cell lymphoma (DLBC), ovarian serous cystadenocarcinoma (OV), skin cutaneous melanoma (SKCM), thymoma (THYM), and uterine carcinosarcoma (UCS) compared to normal tissues, whereas its expression was lower in acute myeloid leukemia (LAML) (). These results were confirmed by transcriptomic data from Pan-Cancer patients in the TCGA database, which revealed that MND1 was highly expressed in BLCA, breast cancer, CHOL, COAD, ESCA, HNSC, KICH, KIRC, KIRP, LIHC, LUAD, LUSC, PRAD, READ, STAD, THCA, and UCEC compared to paired normal tissues ().
Figure 1 MND1 was significantly overexpressed across cancers. (A) The mRNA expression levels of MND1 in ACC, DLBC, LAML, LGG, OV, SARC, SKCM, TGCT, THYM, and UCS by the GEPIA2 database. (B) The mRNA expression levels of MND1 in normal tissues and paired cancer tissues from Pan-Cancer. ns: no significance, *p<0.05, **p<0.01, ***p<0.001.
MND1 is a Prognostic Pan-Cancer Biomarker
To investigate the relationship between MND1 expression and prognosis, including OS, DSS, and PFI in various cancers, we performed a univariate Cox regression analysis using the TCGA database. According to the univariate Cox regression analysis, higher MND1 expression correlated with poorer OS and DSS in ACC, breast cancer, HNSC, KICH, KIRP, LGG, LUAD, and MESO and was related to shorter PFI in ACC, breast cancer, HNSC, KIRP, KIRC, LGG, LUAD, PRAD, and THCA (). Subsequently, Kaplan–Meier survival analysis further proved that the patients with high levels of MND1 had shorter OS, DSS, and PFI in cancers (, Supplementary Figure S1). These results suggested that MND1 might be an effective prognostic indicator in multiple cancers.
Figure 2 High expression of MND1 was significantly associated with poor prognosis across cancers. (A–C) Correlation between MND1 expression and prognosis by univariate Cox regression analysis [OS (A), DSS (B), and PFI (C)]. (D–K) Kaplan‒Meier analysis of the association between MND1 expression and OS [ACC (D), BRCA (E), HNSC (F), MESO (G), KICH (H), KIRP (I), LGG (J), LUAD (K)].
![Figure 2 High expression of MND1 was significantly associated with poor prognosis across cancers. (A–C) Correlation between MND1 expression and prognosis by univariate Cox regression analysis [OS (A), DSS (B), and PFI (C)]. (D–K) Kaplan‒Meier analysis of the association between MND1 expression and OS [ACC (D), BRCA (E), HNSC (F), MESO (G), KICH (H), KIRP (I), LGG (J), LUAD (K)].](/cms/asset/df1730a0-a98a-4872-b703-84b491b92dc3/djir_a_12304414_f0002_c.jpg)
Genetic Alteration Analysis of Pan-Cancer MND1
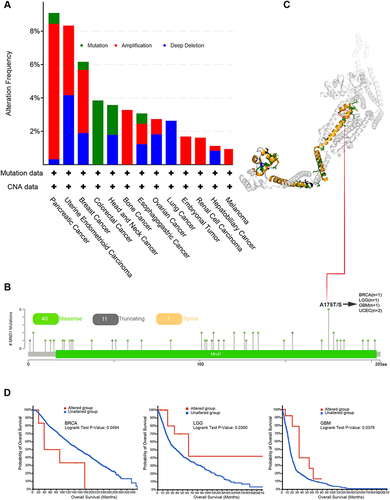
The accumulation of genetic alterations drives the development of cancers.Citation40 Therefore, we explored the genetic alterations in MND1 in different cancers through the cBioPortal database. We found that MND1 had a high alteration frequency in pancreatic cancer, uterine endometrioid carcinoma, and breast cancer (>5%), with the highest alteration frequency that was amplified in pancreatic cancer (>8%) (). Further analysis of the type, sites, and case number of the MND1 genetic alteration showed that missense mutation of MND1 was the main type of genetic alteration. The A (alanine)→ T (threonine) and A (alanine)→ S (serine) transformation at the 175th site of the MND1 protein exhibited the highest mutation frequency in tumors—1 case in breast cancer, 1 case in LGG, 1 case in GBM, and 2 cases in UCEC (). We visualized the A175 site in the 3D structure of the MND1 protein (). In addition, we explored the potential association between all MND1 mutation types and clinical survival outcomes in patients with different types of cancer. We found that in breast cancer, MND1 mutations significantly reduced OS, while in LGG and GBM, MND1 mutations resulted in longer OS (). However, owing to the small number of patients with the mutation, this result must be further validated by large-scale sequencing. In brief, mutations in MND1 may play an important role in the progression and prognosis of multiple types of tumors.
Figure 3 Mutation features of MND1 in different tumors based on TCGA. (A–B) The alteration frequency with MND1 mutation type (A) and mutation site (B) are displayed. (C) The highest alteration frequency site (A175T/S) is shown in the 3D structure of MND1. (D) Correlation between MND1 mutation status and OS in BRCA, LGG, and GBM.
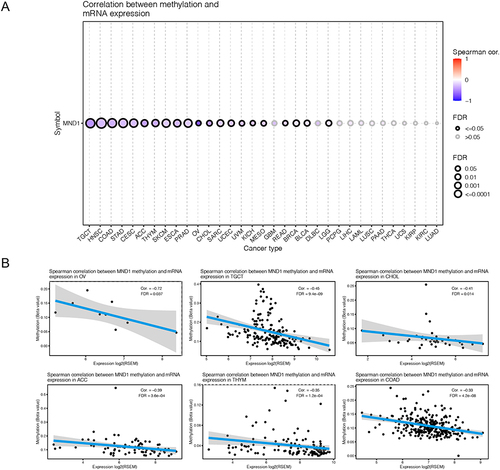
The Pan-Cancer Methylation Profile of MND1
To further investigate the mechanism by which MND1 expression is aberrantly upregulated in multiple cancers, we analyzed the correlation between MND1 mRNA expression and its methylation profile through the GSCA database. We found a significant negative correlation between MND1 methylation and its mRNA expression in the majority of tumors (), such as OV (Cor=−0.72), TGCT (Cor=−0.45), CHOL (Cor=−0.41), ACC (Cor=−0.39), THYM (Cor=−0.35) and COAD (Cor=−0.33), which showed the significant correlation linear relationships (). Additionally, we conducted Kaplan‒Meier survival analysis to investigate the relationship between MDN1 methylation and survival in cancers. However, in the six tumors where MND1 mRNA expression was most associated with methylation, methylation of MND1 was unrelated to patient prognosis (Supplementary Figure S2).
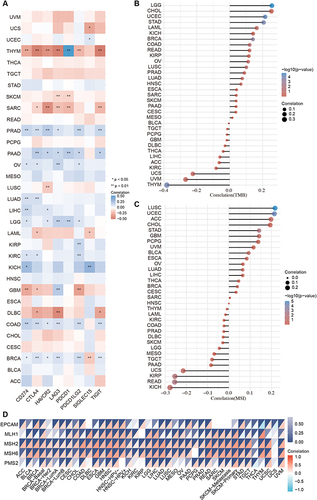
MND1 is Significantly Correlation with Immune Infiltration Levels in Cancers
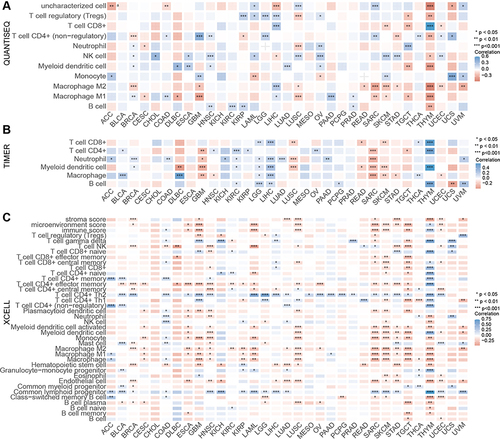
To further systematically evaluate the correlation between MND1 expression and tumor immunity, we assessed the relationship between MND1 expression and immune infiltration in 33 tumors by three different algorithms (QUANTISEQ, TIMER, and XCELL algorithms). High levels of MND1 expression in tumor tissues were accompanied by higher levels of regulatory T cells (Tregs), NK cells, CD4+ Th2 T cells, neutrophils, and common lymphoid progenitors; relatively low levels of MND1 expression in tumor tissues were accompanied by higher levels of CD4+ effector memory T cells, M2 macrophages, M1 macrophages, NK T cells, and monocytes ().
Figure 5 MND1 has significant correlation with immune infiltration in cancers. (A–C) The correlation between MND1 and immune infiltration levels in multiple tumor tissues was analyzed via the QUANTISEQ (A), TIMER (B), and XCELL (C) algorithms. *p<0.05, **p<0.01, ***p<0.001.
Immune checkpoint blockades (ICBs) are used to control and clear tumors by blocking abnormally activated immunosuppressive pathways, reactivating T-cell immune efficacy, and restoring and enhancing systemic antitumor immune responses.Citation41 Therefore, we analyzed the correlation between MND1 expression and immune checkpoint marker expression in cancers. In short, the expression of MND1 was positively correlated with the transcription level of PD-L1, CTLA4, TIM-3, LAG3, PD-1, PD-L2, Siglec-15, and TIGIT in various cancers except for THYM, SARC, and DLBC (). MSI is associated with an increased risk of cancers with specific clinicopathological features, including TMB and an increase in the number of lymphocytes entering the tumor. In particular, TMB is a potential biomarker for predicting ICB response.Citation32,Citation42,Citation43 The expression of MND1 was closely correlated with TMB and MSI in various cancers ( and ). In addition, MMR deficiency commonly results in MSI and is associated with patient outcomes.Citation44 Interestingly, we found that MND1 expression significantly correlation correlated with that of MMR-related genes, including EPCAM, MLH1, MSH2, MSH6, and PMS2, in various cancers ().
Figure 6 MND1 expression was significantly correlation correlated with ICB-related genes. (A) The correlation between MND1 and immune checkpoint-related genes. (B–C) The correlation between MND1 expression and TMB (B) and MSI (C). (D) Correlation between MND1 expression and MMR-related genes across cancers. *p<0.05, **p<0.01.
Pan-Cancer PPI Network of MND1 and Enrichment Analysis
We then used GeneMANIA’s online program to create a PPI network for MND1 to investigate the possible role of MND1 in cancer carcinogenesis. As shown in , the highest correlation occurred between MND1 and proteasome 26S subunit ATPase 3 interacting protein (PSMC3IP), a breast cancer-associated gene that can regulate the extrinsic apoptotic pathway in breast cancer cell lines.Citation45 We also found a significant correlation between MND1 and the breast cancer susceptibility genes BRCA1 and BRCA2, indicating that MND1 may have a more important role in breast cancer (). MND1 was also significant correlation correlated with the cell cycle marker CDK4 and the DNA damage marker H2AX.
Figure 7 Pan-Cancer PPI network of MND1 and enrichment analysis. (A) A PPI network for MND1 was constructed using GeneMANIA’s online program. (B) BP, CC, MF, and KEGG enrichment analysis of MND1-interacting proteins.
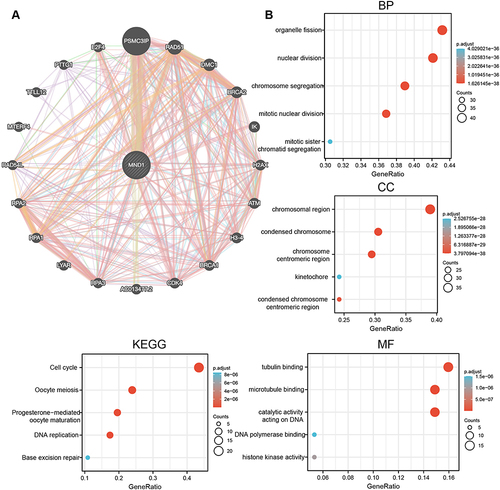
In the GO analysis of the MND1-interacting proteins, the biological processes (BP) were mainly related to organelle fission, nuclear division, chromosome nuclear division, and mitotic nuclear division. The enriched cellular component (CC) ontology contained chromosomal regions and condensed chromosomes. The molecular function (MF) results showed that tubulin binding and microtubule binding were most significantly enriched (). KEGG enrichment analysis revealed that the major enriched pathways were cell cycle, oocyte meiosis, progesterone-mediated oocyte maturation, DNA republication, and base excision repair (). These results are consistent with the previous findings of Bao et alCitation13 and Fang et alCitation11 and further demonstrate that MND1 may play an oncogenic role in tumors by affecting the cell cycle, nuclear division, and other processes, whereas it may play an important function in breast cancer mainly by interacting with genes such as BRCA1 and BRCA2.
Single-Cell Transcriptomic Sequence Analysis of MND1 Expression
Single-cell transcriptomic sequencing can reveal the heterogeneity of tumor cells and monitor the progress of tumor development at the single-cell level.Citation46 Therefore, we analyzed MND1 expression across cancers using single-cell transcriptomics data and explored the relationship between MND1 expression and tumor functional status using the Cancer SEA database. MND1 expression was positively associated with the cell cycle, DNA damage, and DNA repair in most tumors, especially in breast cancer (cell cycle Cor=0.519, DNA damage Cor=0.471, DNA repair=0.449) and LUAD (cell cycle Cor=0.617, DNA damage Cor=0.458, DNA repair=0.549) (). We also found that MND1 expression was negatively associated with invasion in OV (Cor=−0.538) but positively associated with quiescence (Cor=0.529) ().
Figure 8 The expression of MND1 in single-cell sequencing and its correlation with the functional status of LUAD and breast cancer. (A) Heatmap showing the correlation between MND1 and different tumor statuses based on the CancerSEA database. (B) Correlation between MND1 expression and significant functional statuses in LUAD (up) and breast cancer (down). ***p<0.001.
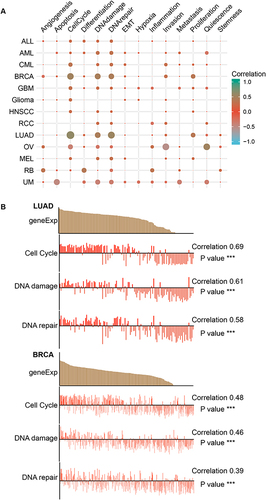
Subsequently, we selected breast and lung cancer single-cell datasets to further validate the correlation between MND1 expression and the cell cycle, DNA damage and DNA repair. In LUAD (ExpID: EXP0066), MND1 expression had significant correlation with cell cycle progression (correlation=0.69), DNA damage (correlation=0.61), and DNA repair (correlation=0.58) (). There was also a significant correlation between MND1 expression and cell cycle progression (Correlation=0.48), DNA damage (Correlation=0.46) and DNA repair (Correlation=0.39) in breast cancer (ExpID: EXP0052) (). These results further support the association of MND1 with cell cycle, DNA damage and DNA repair processes, particularly in breast and lung cancers, which was consistent with the results obtained from the PPI network and functional enrichment analysis of MND1.
MND1 is Highly Expressed in Breast Cancer and is an Independent Prognostic Indicator
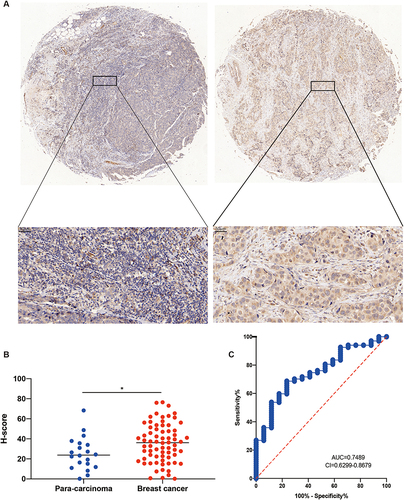
The previous Pan-Cancer studies have demonstrated the potential oncogenic function of MND1 in various tumors, particularly breast cancer and LUAD. Bao et alCitation13 identified MND1 as a prognostically relevant gene in breast cancer through a weighted gene co-expression network, but there is still a lack of exploration of the biological function of MND1 in breast cancer, so we selected breast cancer to verify the oncogene role of MND1. Three independent external GEO datasets (GSE42587, GSE7904, and GSE42568) were selected to analyze MND1 transcript levels in cancerous and adjacent tissues in breast cancer patients. MND1 transcription levels in breast cancer were significantly higher than those in adjacent tissues (Supplementary Figure S3A–C). The results were also verified in breast cancer tissues and paired noncancerous tissues, regardless of TCGA or GEO dataset (Supplementary Figure S3D and E). We also analyzed MND1 protein expression levels using a breast cancer tissue microarray. MND1 protein expression was significantly higher in breast cancer than adjacent tissues ( and ). We also analyzed the diagnostic potential of MND1 in breast cancer using immunohistochemical data and found that MND1 has a promising diagnostic potential in breast cancer (AUC=0.74) (). As shown in , the expression of MND1 was also significantly correlated with tumor T stage, pathologic stage, age, histological type, PR, ER, etc. Receiver operating characteristic (ROC) curve analysis showed that MND1 was a potential diagnostic biomarker for breast cancer (AUC=0.919, Supplementary Figure S3F).
Table 1 Correlation Between MND1 Expression and Clinical Features in Breast Cancer Patients
Figure 9 Protein expression of MND1 in clinical breast cancer samples. (A) Representative pictures of MDN1 expression in 70 breast cancer tissues and 20 adjacent tissues from tissue chips. (B) Statistical analysis of H score for MND1 protein expression in breast cancer tissues and adjacent tissues in the lower (H-SCORE=∑(pi×i)=(percentage of weak intensity ×1)+(percentage of moderate intensity ×2)+(percentage of strong intensity ×3)). (C) The diagnostic efficacy of MND1 was assessed in 70 breast cancer tissues and 20 adjacent tissues, based on the analysis of immunohistochemical data using the ROC curve. *p<0.05.
The GEO dataset was used to validate the prognostic value of MND1 in breast cancer. The results proved that increased MND1 expression was significantly negatively correlated with OS, relapse-free survival (RFS), distant metastasis-free survival (DMFS), and post-progression survival (PPS) in patients with breast cancer from the GSE20685 dataset (Supplementary Figure S3G–3J). More importantly, according to univariate and multivariate Cox regression analyses found that MND1 is an independent prognosis factor together with T4 (univariate method), N stages (univariate method), M1 (both method), stages III/IV (univariate method), and >60 years old (both method) (). Taken together, these results suggest that MND1 may be a promising biological target for the diagnosis and assessing the prognosis of breast cancer patients.
Table 2 Univariate and Multivariate Cox Regression Analyses of the Clinical Characteristics Associated with Overall Survival in Breast Cancer in the Cancer Genome Atlas (TCGA)
MND1 Promotes Breast Cancer Cell Proliferation by Regulating the Cell Cycle
To validate the effect of MND1 on the cell cycle and cell proliferation in breast cancer cells, we measured the proliferative phenotypes and the expression of cell cycle regulators in breast cancer cells. As shown in the CCLE database (https://depmap.org/portal/ccle/), the expression level of MND1 was the lowest in MCF7 cells and the highest in MDA-MB-231 cells (Supplementary Figure S3K); therefore, we selected MCF7 for MND1 overexpression experiments and MDA-MB-231 for MND1 knockdown experiments. MND1 overexpression in MCF7 cells and silencing in MDA-MB-231 cells were confirmed through qRT‒PCR and western blotting ( and ). Cell proliferation was measured using CCK8, EdU, and colony formation assays. The results were consistent and demonstrated that MND1 overexpression promoted the proliferation of MCF7 cells, whereas MND1 knockdown using siRNA inhibited the proliferation of MDA-MB-231 cells ().
Figure 10 MND1 promoted breast cancer cell proliferation in vitro. (A–B) The efficiency of MND1 overexpression in MCF7 cells and MND1 knockdown in MDA-MB-231 cells at the mRNA and protein levels was validated by RT‒qPCR (A) and Western blotting (B). (C) CCK-8 assays measure cell proliferation kinetics after overexpression in MCF7 cells (left) and knockdown in MDA-MB231 cells (right). (D) Colony number was counted from three replicates for each condition in MCF7 cells (up) and MCF7 knockdown in MDA-MB-231 cells (down). (E) Relative EdU positive cells were also plotted in MCF7 cells (left) and MND1 knockdown in MDA-MB-231 cells (right). **p<0.05, **p<0.01, ***p<0.001. CCK-8, Cell Counting Kit 8. EdU, 5-ethynyl-20-deoxyuridine.
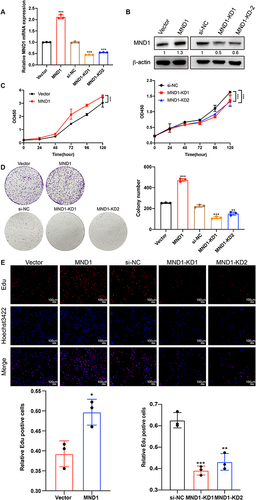
We then investigated the role of MND1 in cell cycle progression and its underlying molecular mechanisms. Flow cytometry experiments revealed that overexpression of MND1 decreased the proportion of G1-phase cells and increased the proportion of S-phase cells in MCF7 cells; conversely, knockdown of MND1 inhibited G1/S-phase transitions (). Since MND1-KD1 had a better inhibitory effect compared to MND1-KD2, we chose MND1-KD1 for the following experiments. Western blot analysis revealed that MND1 overexpression increased the expression levels of the G1-phase regulators CDK4, CDK6, and cyclin D3; the G1/S transition regulators CDK2 and cyclin E2; the S phase regulator cyclin A2; and the M phase regulator cyclin B1. Conversely, MND1 silencing produced opposite results to those of MND1 overexpression (). Taken together, these findings indicate that MND1 is a positive regulator of cell cycle progression. Inhibition of MND1 expression not only induces cell cycle arrest at the G1-S transition but also reduces the proliferation ability of breast cancer cells. Furthermore, MND1 was also significantly associated with immune infiltration in breast cancer.
Figure 11 MND1 promoted cell cycle progression and correlated with immune infiltration in breast cancer. (A) The proportions of MND1-overexpressing cells and MND1-knockdown cells in the G1, S and G2-M phases of the cell cycle were analyzed by flow cytometry, representative cell cycle distribution graphs are shown above and statistical analysis graphs are shown below. (B) The relative expression levels of cell cycle markers were examined by Western blotting after MND1 overexpression in MCF7 cells and MDN1 silencing in MDA-MB-231 cells. (C) Immunohistochemical staining analysis of MND1, PD-L1, and FOXP3 expression in 30 breast cancer tissues shows representative slides to the left, and Spearman calculates the correlation. ns: no significance, *p<0.05, **p<0.01, ***p<0.001.
Additionally, we observed a significant and positive correlation between MND1 and CD274 as well as FOXP3 in 30 breast cancer patients (MND1: CD274, R=0.572; MND1: FOXP3, R=0.626) (), which aligns with the findings from TCGA analysis. These results suggest that MND1 may serve as a potential immunological biomarker associated with the response to immune checkpoint blockade therapy.
MND1 Expression and Drug Response
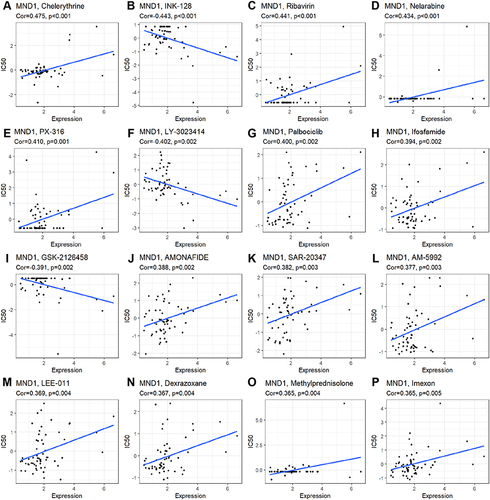
Finally, we also investigated the response to targeted therapy and chemotherapy based on the level of MND1 using the CellMiner database. MND1 expression was positively correlated with sensitivity to chelerythrine, ribavirin, nelarabine, PX-316, palbociclib, ifosfamide, ammonafide, SAR-20347, AM-5992, LEE-011, dexrazoxane, methylprednisolone, and imexon ( and ) and negatively correlated with sensitivity to INK-128, LY-3023414, and GSK-2126458 ( and ).
Figure 12 Correlation between MND1 expression and IC50 of multiple chemotherapy chemicals using the CellMiner database. (A) Chelerythrine, (B) INK-1, (C) Ribavirin, (D) Nelarabine, (E) PX-316, (F) LY-3023414, (G) Palbocicilib, (H) Ifosfamide, (I) GSK-21266458, (J) AMONAFIDE, (K) SAR-20347, (L) AM-5992, (M) LEE-011, (N) Dexrazoxane, (O) Methylprednisolone, (P) Lmexon.
Discussion
In this study, we systematically and comprehensively analyzed the expression, prognosis, mutation, immune infiltration, and drug sensitivity concerning MND1 and explored its underlying molecular Pan-Cancer mechanisms using the PPI network and single-cell sequencing (). The pro-proliferation effect of MND1 was further validated in breast cancer.
Table 3 Summary of the Results of the MND1 Pan-Cancer Analysis
We found that MND1 was abnormally overexpressed in different types of tumors through unpaired and paired comparisons based on multiple databases. Abnormally high expression of MND1 correlated with poor OS, DSS, and PFI in multiple cancers. According to the available studies, MND1 is highly expressed in STAD,Citation47 BRCA,Citation13 LUAD,Citation2 KIRC,Citation11 LIHCCitation39 and other tumors and is associated with patient prognosis, while our pan-cancer study found that, in addition to the reported tumors, MND1 is also highly expressed in ACC, HNSC, LGG and other tumors, which is related to the poor prognosis of the patients, suggesting that MND1 may play an important role in a wide range of tumors. This indicates that MND1 may play an important role in a variety of tumors, and further in-depth studies are needed to explore the role of MND1 in these tumors. In addition, we found that MND1 protein and mRNA were significantly elevated in breast cancer tissues, and overexpression of MND1 could promote the proliferation and metastasis of breast cancer cells, while knockdown inhibited it, as shown in the in vitro proliferation and metastasis assay. More importantly, we found that MND1 has promising diagnostic efficacy in breast cancer through real tissue samples, which is consistent with the TCGA data, indicating that MND1 has the potential to be a diagnostic target for breast cancer. The forthcoming study will primarily focus on the significance of blood MND1 expression in facilitating early breast cancer diagnosis, through the collection of a substantial number of blood samples from patients with breast cancer.
DNA methylation, a common epigenetic modification, and its abnormalities typically affect gene expression.Citation48,Citation49 MND1 expression correlated with methylation in several tumors, such as OV, TGCT, CHOL, ACC, THYM, and COAD. This may explain the aberrant expression of MND1 in these tumors. In LGG, patients with MND1 hypermethylation had worse OS (data not shown). However, the methylation of MND1 was largely unrelated to patient prognosis in six cancers where MND1 expression was most associated with methylation. The reason for this may be attributed to the fact that while methylation level is a crucial determinant of gene transcription, it is not the sole factor governing this process. Gene transcription is also regulated by histones, transcription factors, enhancers, and other epigenetic modification elements. Additionally, it should be noted that the limited number of patient cases available for survival analysis and potential individual patient differences could potentially impact the results obtained. Therefore, further studies are required to validate the association between MND1 methylation level and prognosis.
PPI networks of MND1 using the GeneMANIA program predicted that MND1 may interact with PSMC3IP, RADiation sensitive51 (RAD51), Disrupted Meiotic cDNA1 (DMC1), and others. PSMC3IP is proposed to be associated with breast cancer susceptibility.Citation50 Additionally, Capdevila-Busquets et al reported that PSMC3IP could modulate the apoptotic pathway in TNBC and ER+ breast cancer.Citation45 RAD51 is associated with aggressive cancer biology, cancer cell proliferation, and poor survival in breast cancer patients.Citation51 Interestingly, we observed potential interactions of MND1 with BRCA1 or BRCA2 from the PPI network. Therefore, we speculate that MND1 may regulate important biological activities of breast cancer cells by binding to PSMC3IP, RAD51, BRCA1 or BRCA2.
In addition, MND1 may also interact with CDK4, which is a key regulatory protein of the cell cycle.Citation52 KEGG enrichment analysis and single-cell sequencing analysis also revealed that MND1 is closely associated with the cell cycle in LUAD and breast cancer. We further demonstrated that MND1 overexpression elevated the expression of positive cell cycle regulators, such as CDK2, CDK4, and CDK6, and accelerated the G1/S phase transition. As reported, the CDK4/6-cyclin D complex drives the G1 to S phase transition by phosphorylating and inactivating retinoblastoma protein (RB).Citation53 During the S phase, cyclin A2 is restricted to the nucleus, and during the S/G2 phase transition, a portion of cyclin A2 is transferred to the cytoplasm to phosphorylate Aurora Borealis (Bora), which activates polo-like kinase 1 (PLK1) and promotes the cell cycle.Citation54 We will further detect the phosphorylation levels of Rb, PLK1, AURKB and other proteins in subsequent studies to further understand the molecular mechanism of cell cycle regulation by MND1. By increasing the expression of these proteins, MND1 is involved in different phases of the cell cycle and thus promotes the proliferation of breast cancer cells. Increasing evidence suggests that BRCA1 is involved in all phases of the cell cycle and regulates orderly events during cell cycle progression.Citation55,Citation56 We speculate that the involvement of MND1 in different phases of the cell cycle may be related to its interaction with BRCA1.
Tumor immunotherapy has achieved remarkable clinical success in cancer treatment in the last decade.Citation57 The application of ICB has started a new phase of tumor treatment, and ICB is now a popular therapy for tumor treatment.Citation58 However, its clinical application is greatly limited because of poor response rates and severe adverse effects.Citation59 Therefore, the discovery of novel immunological biomarkers is of great importance for the diagnosis and treatment of cancers. In the present study, we found that MND1 was significantly and positively correlated with ICB markers such as PD-L1, PD-1, and CTLA-4 in some cancers, including PRAD, PAAD, and LGG. PD-L1 (encoded by CD274), PD-1 (encoded by PDCD1), and CTLA-4 are the major suppressor checkpoint signals that control T-cell activity, and high levels of PD-L1 expression are found in almost all types of cancer; PD-L1/PD-1 signaling can be exploited to evade T-cell immunity.Citation41,Citation60 Blocking the PD-L1/PD-1 pathway has consistently shown significant antitumor effects in patients with advanced cancer.Citation41
In addition, by immune infiltration analysis, MND1 expression was found to be significantly positively correlated with regulatory T cells (Tregs) infiltration and negatively correlated with infiltration of tumor-killing cells such as CD4+ T cells and NK cells in certain tumors. Tregs possess the ability to suppress the activity of effector T cells and other immune cells, which play crucial roles in maintaining peripheral tolerance and preventing detrimental immune responses.Citation61 Enhanced functionality and increased infiltration of Tregs within the tumor immune microenvironment impede the anti-tumor immune response while facilitating tumor angiogenesis and growth.Citation62 Collectively, MND1 was found to correlate with both ICB markers as well as some immune cells, from which we hypothesized that MND1 may be a promising immune target in tumors.
Abnormal meiotic and nuclear division processes are the underlying cause of the malignant proliferation of tumor cells, and important regulators of this process (eg, SYCP3, REC8, etc). are also common tumor indicator targets; however, whether MND1, as such an important regulator, can be a drug target and its sensitivity and resistance to multiple drugs are still unreported.Citation12
Using the CellMiner database, we found that MND1 may be associated with multidrug resistance, including resistance to palbociclib, ifosfamide, nelarabine, and others. Palbociclib, a selective inhibitor of CDK4 and CDK6, can be used in the treatment of HR-positive and HER2-negative breast cancer and hepatocellular carcinoma.Citation63–65 Ifosfamide is an alkylating chemotherapeutic agent with activity against a wide range of tumors, including those of breast cancer, LUAD and others.Citation66 Nelarabine is a nucleoside analog that can be used for research on T-cell acute lymphoblastic leukemia.Citation67 In addition, it has been found that the MND1/FOXA1/TKT axis can regulate oxaliplatin sensitivity through PI3K/AKT signaling.Citation47 Therefore, high MND1 expression may be associated with chemotherapy drug resistance. Targeting MND1 may be a potential strategy for chemoresistance.
In addition, some inhibitors may have a promising inhibitory effect on MND1, such as INK-128, an ATP-dependent mTOR1/2 inhibitor;Citation68 LY-3023414, which selectively inhibits class I PI3K isoforms;Citation69 and GSK-2126458, a highly selective inhibitor of PI3K.Citation70 Similarly, Hu et al reported that in gastric cancer, MND1 was able to regulate oxaliplatin sensitivity in gastric cancer with FOXA1, TKT through PI3K/AKT signaling.Citation47 Whether MND1 can also regulate the sensitivity of chemotherapeutic agents by modulating PI3K signaling in other tumors besides gastric cancer still needs to be studied in depth.
Conclusion
Ultimately, our findings demonstrate that MND1 is a potential biomarker for the diagnosis and prognosis of multiple cancers. MND1 may be an immunological biomarker associated with the infiltration of various immune cells. In addition, MND1 is a breast cancer oncogene whose high expression accelerates the G1/S transition and promotes breast cancer cell proliferation.
Ethics Approval
Research involving human subjects complied with all relevant national regulations, and institutional policies and is by the tenets of the Helsinki Declaration and has been approved by the Ethics Committee of Qingyuan People’s Hospital (IRB-2022-010).
Disclosure
The authors declare that they have no competing interests in this work.
Acknowledgments
The authors thank TCGA, Timer, GSCA, and Cancer SEA for their open-access databases. And we are grateful to Dr Yaru Liang from the Department of Laboratory Medicine, The Affiliated Qingyuan Hospital (Qingyuan People’s Hospital), Guangzhou Medical University for her contribution in data collection.
Additional information
Funding
References
- Kang H-A, Shin H-C, Kalantzi A-S, et al. Crystal structure of Hop2-Mnd1 and mechanistic insights into its role in meiotic recombination. Nucleic Acids Res. 2015;43(7):3841–3856. doi:10.1093/nar/gkv172
- Zhang Q, Shi R, Bai Y, et al. Meiotic nuclear divisions 1 (MND1) fuels cell cycle progression by activating a KLF6/E2F1 positive feedback loop in lung adenocarcinoma. Cancer Commun. 2021;41(6):492–510. doi:10.1002/cac2.12155
- Zelceski A, Francica P, Lingg L, et al. MND1 and PSMC3IP control PARP inhibitor sensitivity in mitotic cells. Cell Rep. 2023;42(5):112484. doi:10.1016/j.celrep.2023.112484
- Tsubouchi H, Roeder GS. The Mnd1 protein forms a complex with hop2 to promote homologous chromosome pairing and meiotic double-strand break repair. Mol Cell Biol. 2002;22(9):3078–3088. doi:10.1128/MCB.22.9.3078-3088.2002
- Lee W, Iwasaki H, Tsubouchi H, Li H-W. Hop2-Mnd1 and Swi5-Sfr1 stimulate Dmc1 filament assembly using distinct mechanisms. Nucleic Acids Res. 2023;51(16):8550–8562. doi:10.1093/nar/gkad561
- Bugreev DV, Huang F, Mazina OM, et al. HOP2-MND1 modulates RAD51 binding to nucleotides and DNA. Nat Commun. 2014;5(1):4198. doi:10.1038/ncomms5198
- Tsubouchi H. The Hop2-Mnd1 complex and its regulation of homologous recombination. Biomolecules. 2023;13(4):662. doi:10.3390/biom13040662
- Chi P, San Filippo J, Sehorn MG, Petukhova GV, Sung P. Bipartite stimulatory action of the Hop2-Mnd1 complex on the Rad51 recombinase. Genes Dev. 2007;21(14):1747–1757. doi:10.1101/gad.1563007
- Wei J, Meng G, Wu J, Zhang Q, Zhang J. Genetic network and gene set enrichment analyses identify MND1 as potential diagnostic and therapeutic target gene for lung adenocarcinoma. Sci Rep. 2021;11(1):9430. doi:10.1038/s41598-021-88948-4
- Pezza RJ, Voloshin ON, Vanevski F, Camerini-Otero RD. Hop2/Mnd1 acts on two critical steps in Dmc1-promoted homologous pairing. Genes Dev. 2007;21(14):1758–1766. doi:10.1101/gad.1562907
- Fang J, Zhen J, Gong Y, et al. MND1 functions as a potential prognostic biomarker associated with cell cycle and immune infiltration in kidney renal clear cell carcinoma. Aging. 2022;14(18):7416–7442. doi:10.18632/aging.204280
- McFarlane RJ, Wakeman JA. Meiosis-like Functions in Oncogenesis: a New View of Cancer. Cancer Res. 2017;77(21):5712–5716. doi:10.1158/0008-5472.CAN-17-1535
- Bao Z, Cheng J, Zhu J, et al. Using weighted gene co-expression network analysis to identify increased MND1 expression as a predictor of poor breast cancer survival. Int J Gen Med. 2022;15:4959–4974. doi:10.2147/IJGM.S354826
- Zhang L, Li X, Zhang J, Xu G. Prognostic implication and oncogenic role of PNPO in Pan-Cancer. Front Cell Dev Biol. 2021;9:763674. doi:10.3389/fcell.2021.763674
- Thorsson V, Gibbs DL, Brown SD, et al. The Immune Landscape of Cancer. Immunity. 2018;48(4):812–830.e14. doi:10.1016/j.immuni.2018.03.023
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335–3337. doi:10.1172/JCI83871
- Ren L, Yi J, Yang Y, et al. Systematic pan-cancer analysis identifies APOC1 as an immunological biomarker which regulates macrophage polarization and promotes tumor metastasis. Pharmacol Res. 2022;183:106376. doi:10.1016/j.phrs.2022.106376
- Hui L, Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368(1):7–13. doi:10.1016/j.canlet.2015.07.039
- Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25(4):198–213. doi:10.1016/j.tcb.2014.11.006
- Fang H, Sheng S, Chen B, et al. A Pan-Cancer analysis of the oncogenic role of cell division cycle-associated protein 4 (CDCA4) in human tumors. Front Immunol. 2022;13:826337. doi:10.3389/fimmu.2022.826337
- Borlongan MC, Saha D, Wang H. Tumor microenvironment: a niche for cancer stem cell immunotherapy. Stem Cell Rev Rep. 2024;20(1):3–24. doi:10.1007/s12015-023-10639-6
- Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–W514. doi:10.1093/nar/gkaa407
- Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
- Gruosso T, Mieulet V, Cardon M, et al. Chronic oxidative stress promotes H2AX protein degradation and enhances chemosensitivity in breast cancer patients. EMBO Mol Med. 2016;8(5):527–549. doi:10.15252/emmm.201505891
- Richardson AL, Wang ZC, De Nicolo A, et al. X chromosomal abnormalities in basal-like human breast cancer. Cancer Cell. 2006;9(2):121–132. doi:10.1016/j.ccr.2006.01.013
- Clarke C, Madden SF, Doolan P, et al. Correlating transcriptional networks to breast cancer survival: a large-scale coexpression analysis. Carcinogenesis. 2013;34(10):2300–2308. doi:10.1093/carcin/bgt208
- Egelston CA, Guo W, Tan J, et al. Tumor-infiltrating exhausted CD8+ T cells dictate reduced survival in premenopausal estrogen receptor-positive breast cancer. JCI Insight. 2022;7(3). doi:10.1172/jci.insight.153963
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. doi:10.1093/nar/30.1.207
- Liu J, Lichtenberg T, Hoadley KA, et al. An integrated TCGA Pan-Cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173(2):400–416.e11. doi:10.1016/j.cell.2018.02.052
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi:10.1158/2159-8290.CD-12-0095
- Liu C-J, F-F H, Xia M-X, Han L, Zhang Q, Guo A-Y. GSCALite: a web server for gene set cancer analysis. Bioinformatics. 2018;34(21):3771–3772. doi:10.1093/bioinformatics/bty411
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017;2017. doi:10.1200/PO.17.00073
- Franz M, Rodriguez H, Lopes C, et al. GeneMANIA update 2018. Nucleic Acids Res. 2018;46(W1):W60–W64. doi:10.1093/nar/gky311
- Warde-Farley D, Donaldson SL, Comes O, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(suppl_2):W214–W220. doi:10.1093/nar/gkq537
- Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–W560. doi:10.1093/nar/gkz430
- Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi:10.1089/omi.2011.0118
- Yuan H, Yan M, Zhang G, et al. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 2019;47(D1):D900–D908. doi:10.1093/nar/gky939
- Reinhold WC, Sunshine M, Liu H, et al. CellMiner: a web-based suite of genomic and pharmacologic tools to explore transcript and drug patterns in the NCI-60 cell line set. Cancer Res. 2012;72(14):3499–3511. doi:10.1158/0008-5472.CAN-12-1370
- Tan K, Wang K, Zhao A, et al. Meiotic nuclear divisions 1 promotes proliferation and metastasis in hepatocellular carcinoma and is a potential diagnostic and therapeutic target gene. Med Oncol. 2022;40(1):14. doi:10.1007/s12032-022-01875-w
- Iranzo J, Martincorena I, Koonin EV. Cancer-mutation network and the number and specificity of driver mutations. Proc Natl Acad Sci USA. 2018;115(26):E6010–E6019. doi:10.1073/pnas.1803155115
- Cha J-H, Chan L-C, C-W L, Hsu JL, Hung M-C. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76(3):359–370. doi:10.1016/j.molcel.2019.09.030
- Izzi V, Davis MN, Naba A. Pan-Cancer analysis of the genomic alterations and mutations of the matrisome. Cancers. 2020;12(8):2046. doi:10.3390/cancers12082046
- Sahin IH, Akce M, Alese O, et al. Immune checkpoint inhibitors for the treatment of MSI-H/MMR-D colorectal cancer and a perspective on resistance mechanisms. Br J Cancer. 2019;121(10):809–818. doi:10.1038/s41416-019-0599-y
- van Velzen MJM, Derks S, van Grieken NCT, Haj Mohammad N, van Laarhoven HWM. MSI as a predictive factor for treatment outcome of gastroesophageal adenocarcinoma. Cancer Treat Rev. 2020;86:102024. doi:10.1016/j.ctrv.2020.102024
- Capdevila-Busquets E, Badiola N, Arroyo R, Alcalde V, Soler-López M, Aloy P. Breast cancer genes PSMC3IP and EPSTI1 play a role in apoptosis regulation. PLoS One. 2015;10(1):e0115352. doi:10.1371/journal.pone.0115352
- Zhang Y, Wang D, Peng M, et al. Single-cell RNA sequencing in cancer research. J Exp Clin Cancer Res. 2021;40(1):81. doi:10.1186/s13046-021-01874-1
- Hu X, Zhou S, Li H, et al. FOXA1/MND1/TKT axis regulates gastric cancer progression and oxaliplatin sensitivity via PI3K/AKT signaling pathway. Cancer Cell Int. 2023;23(1):234. doi:10.1186/s12935-023-03077-4
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–492. doi:10.1038/nrg3230
- Manoochehri M, Borhani N, Gerhäuser C, et al. DNA methylation biomarkers for noninvasive detection of triple-negative breast cancer using liquid biopsy. Int J Cancer. 2023;152(5):1025–1035. doi:10.1002/ijc.34337
- Yang Z, Peng M, Cheng L, et al. GT198 expression defines mutant tumor stroma in human breast cancer. Am J Pathol. 2016;186(5):1340–1350. doi:10.1016/j.ajpath.2016.01.006
- Wu R, Patel A, Tokumaru Y, et al. High RAD51 gene expression is associated with aggressive biology and with poor survival in breast cancer. Breast Cancer Res Treat. 2022;193(1):49–63. doi:10.1007/s10549-022-06552-0
- Fassl A, Geng Y, Sicinski P. CDK4 and CDK6 kinases: from basic science to cancer therapy. Science. 2022;375(6577):eabc1495. doi:10.1126/science.abc1495
- Gao X, Leone GW, Wang H. Cyclin D-CDK4/6 functions in cancer. Adv Cancer Res. 2020;148:147–169.
- Silva Cascales H, Burdova K, Middleton A, et al. Cyclin A2 localises in the cytoplasm at the S/G2 transition to activate PLK1. Life Sci Alliance. 2021;4(3):e202000980. doi:10.26508/lsa.202000980
- Deng C-X. BRCA1: cell cycle checkpoint, genetic instability, DNA damage response and cancer evolution. Nucleic Acids Res. 2006;34(5):1416–1426. doi:10.1093/nar/gkl010
- Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95(11):866–871. doi:10.1111/j.1349-7006.2004.tb02195.x
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398(10304):1002–1014. doi:10.1016/S0140-6736(21)01206-X
- Xu H, Van der Jeught K, Zhou Z, et al. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J Clin Invest. 2021;131(10). doi:10.1172/JCI146832
- Zhou X, Yao Z, Bai H, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22(9):1265–1274. doi:10.1016/S1470-2045(21)00333-8
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi:10.1084/jem.192.7.1027
- Mittal S, Brown NJ, Holen I. The breast tumor microenvironment: role in cancer development, progression and response to therapy. Expert Rev Mol Diagn. 2018;18(3):227–243. doi:10.1080/14737159.2018.1439382
- Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38(1):541–566. doi:10.1146/annurev-immunol-042718-041717
- Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–1438. doi:10.1158/1535-7163.1427.3.11
- Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi:10.1186/bcr2419
- Bollard J, Miguela V, Ruiz de Galarreta M, et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut. 2017;66(7):1286–1296. doi:10.1136/gutjnl-2016-312268
- Helal M. Prenatal effects of transplacental exposure to ifosfamide in rats. Biotech Histochem. 2016;91(5):357–368. doi:10.1080/10520295.2016.1176253
- Lonetti A, Cappellini A, Bertaina A, et al. Improving nelarabine efficacy in T cell acute lymphoblastic leukemia by targeting aberrant PI3K/AKT/mTOR signaling pathway. J Hematol Oncol. 2016;9(1):114. doi:10.1186/s13045-016-0344-4
- Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR mediated anti-cancer drug discovery. Drug Discov Today Ther Strateg. 2009;6(2):47–55. doi:10.1016/j.ddstr.2009.12.001
- Wei L, Chintala S, Ciamporcero E, et al. Genomic profiling is predictive of response to cisplatin treatment but not to PI3K inhibition in bladder cancer patient-derived xenografts. Oncotarget. 2016;7(47):76374–76389. doi:10.18632/oncotarget.13062
- Knight SD, Adams ND, Burgess JL, et al. Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med Chem Lett. 2010;1(1):39–43. doi:10.1021/ml900028r